Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations
Abstract
:1. Introduction
2. Results
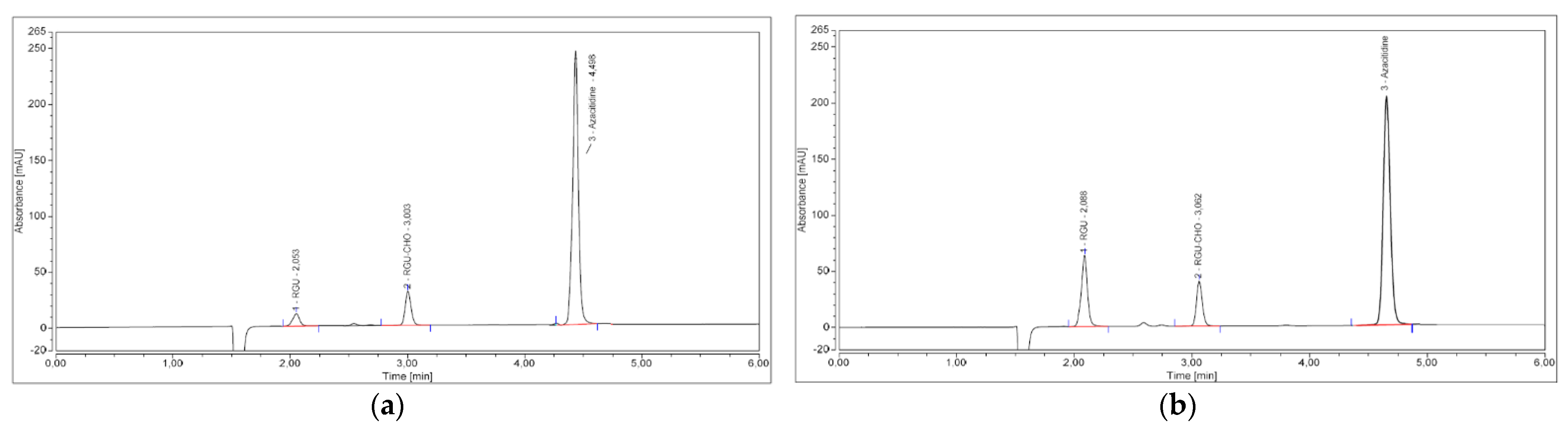
2.1. HPLC–UV Analysis
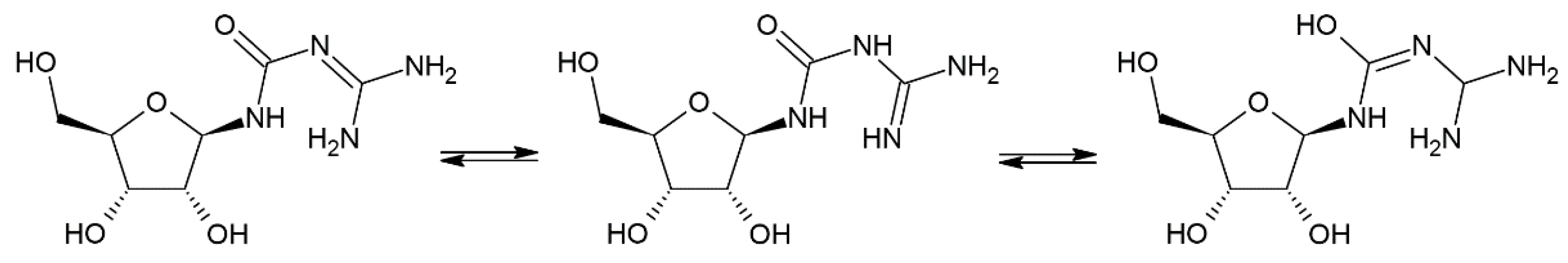
2.2. Forced Degradation
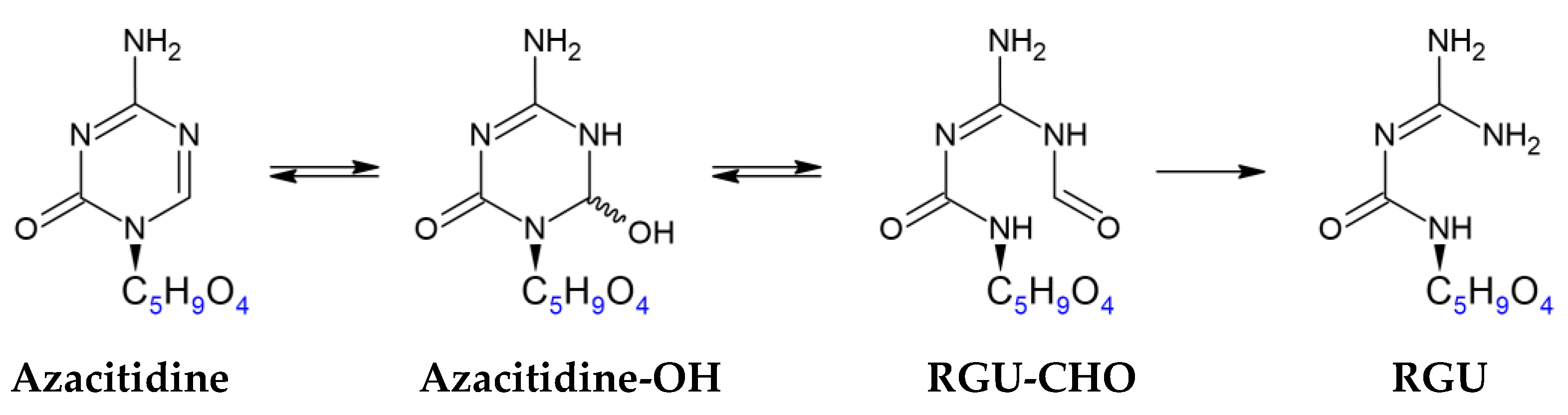
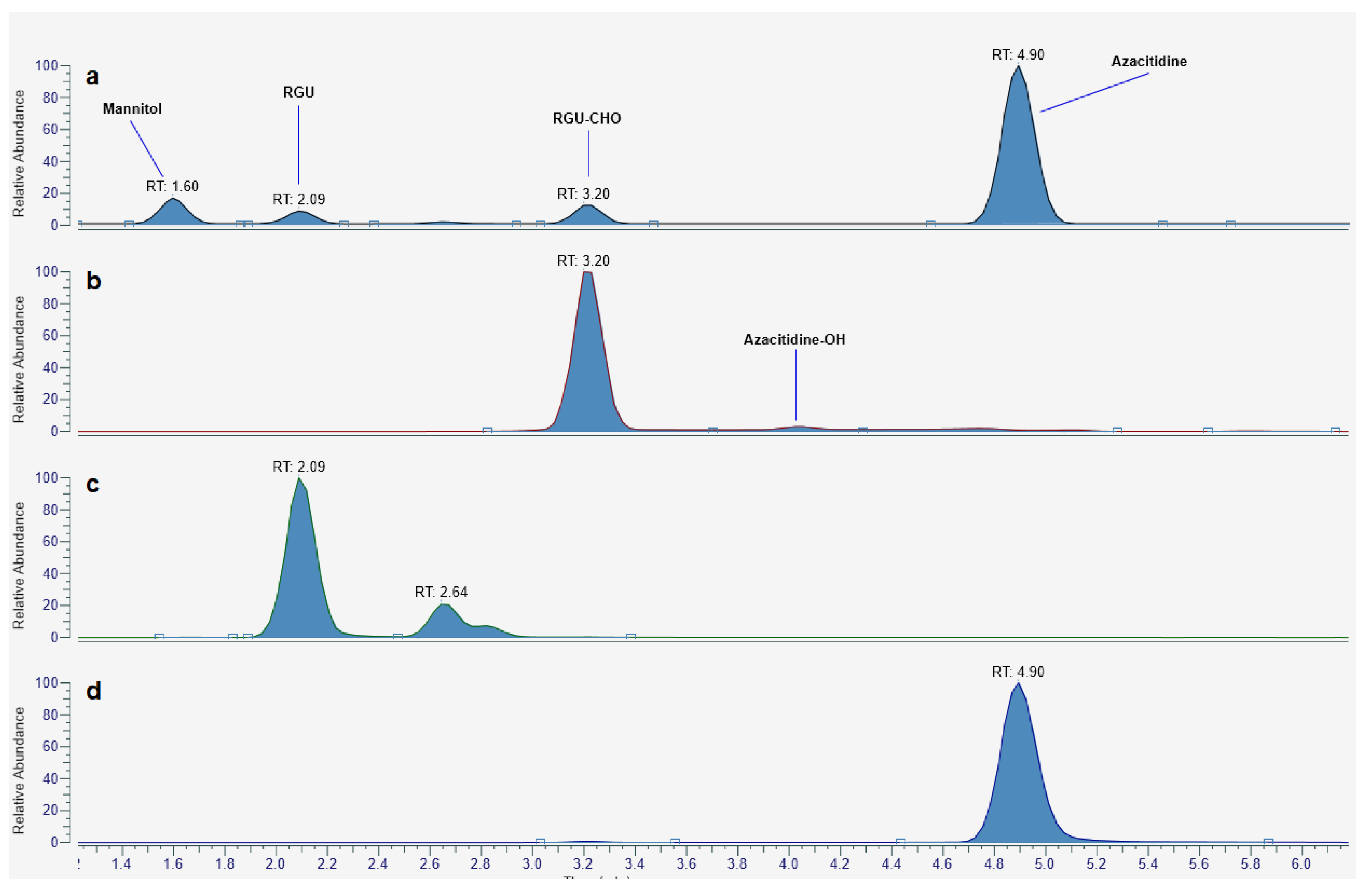
2.3. UHPLC-HRMS Analysis
2.4. Chemical Stability
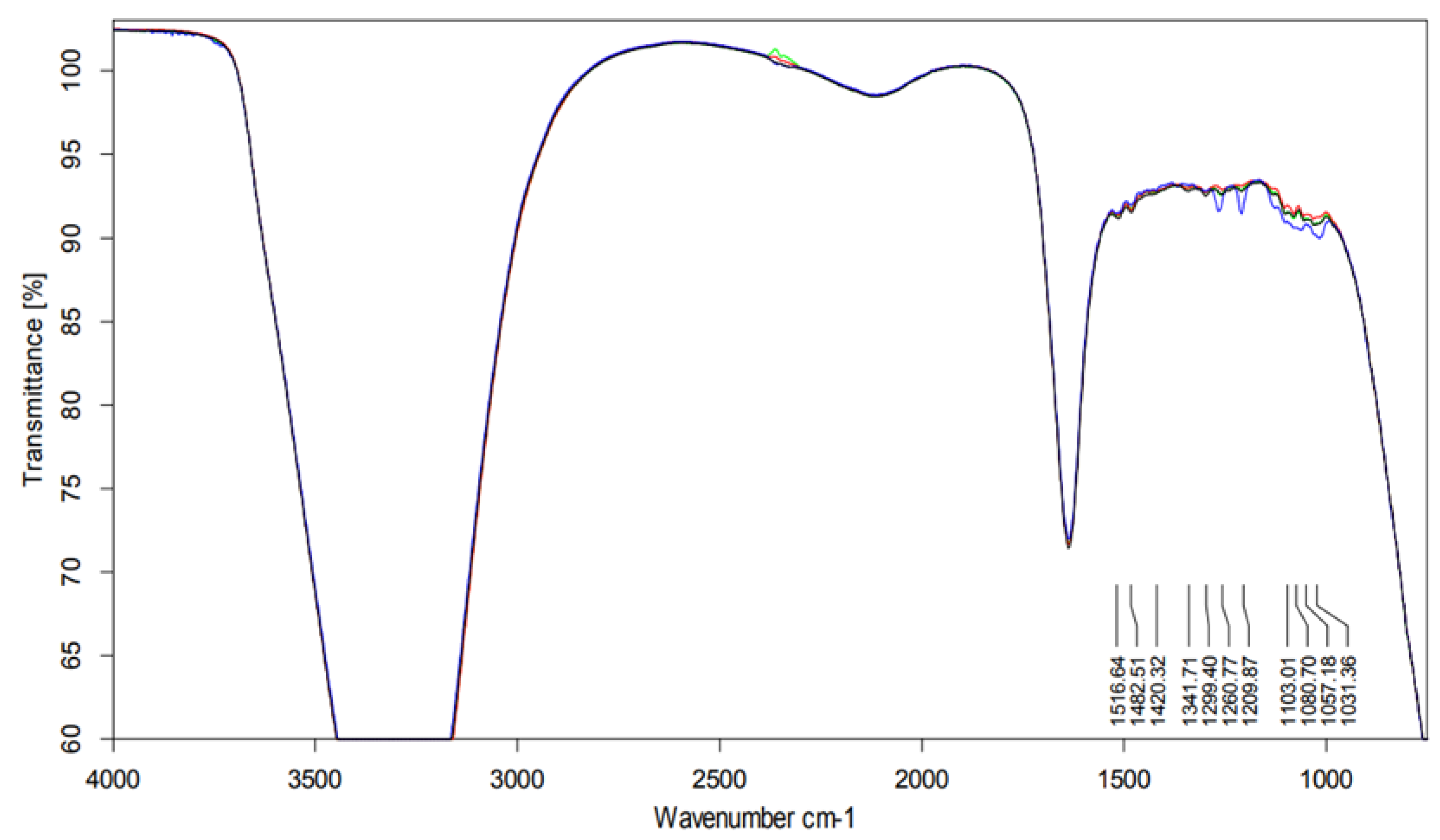
2.4.1. Infrared Spectroscopy Analysis
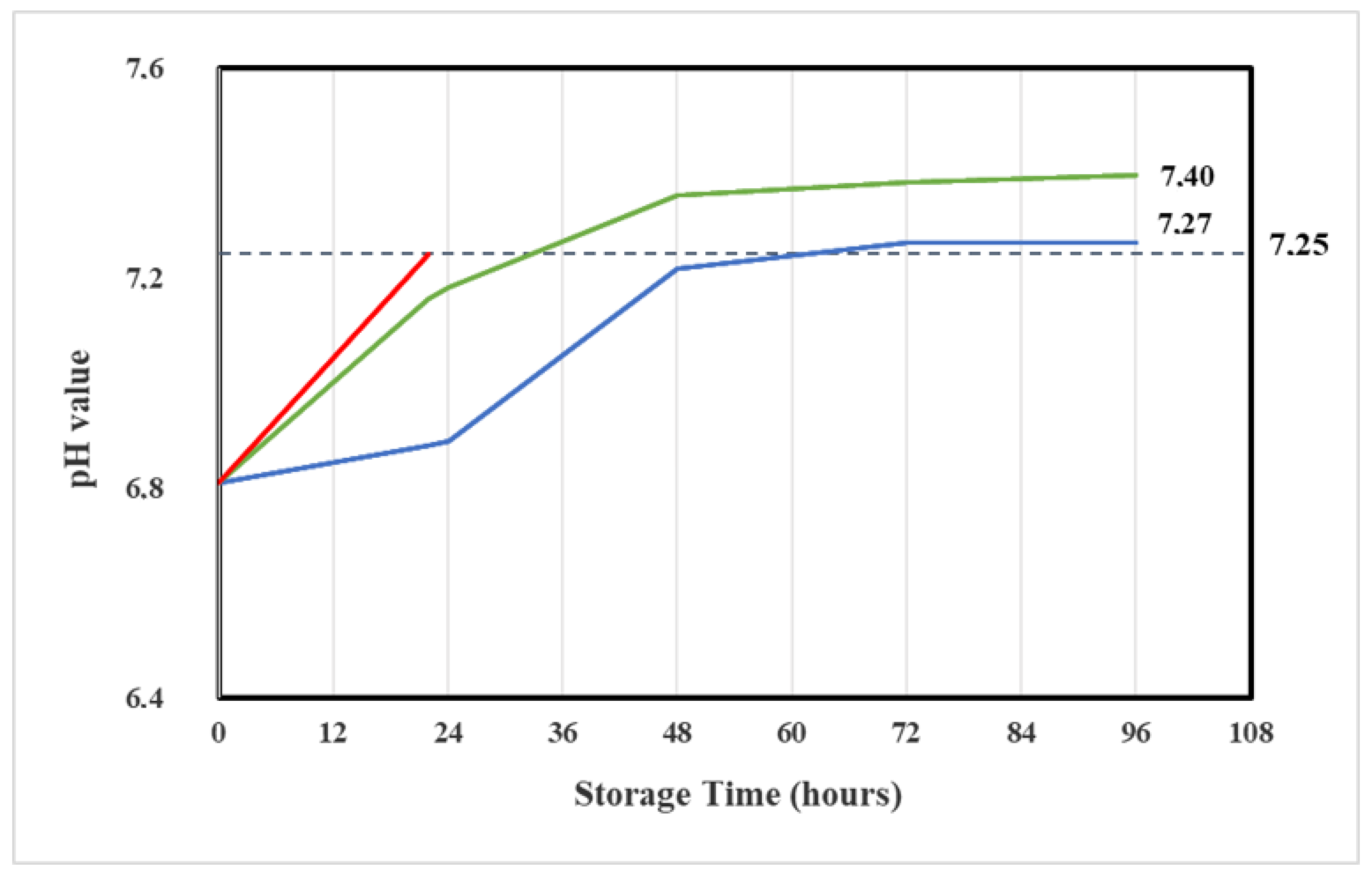
2.4.2. pH Determination
2.5. Physical Stability
2.5.1. Visual Examination
2.5.2. Subvisual Examination
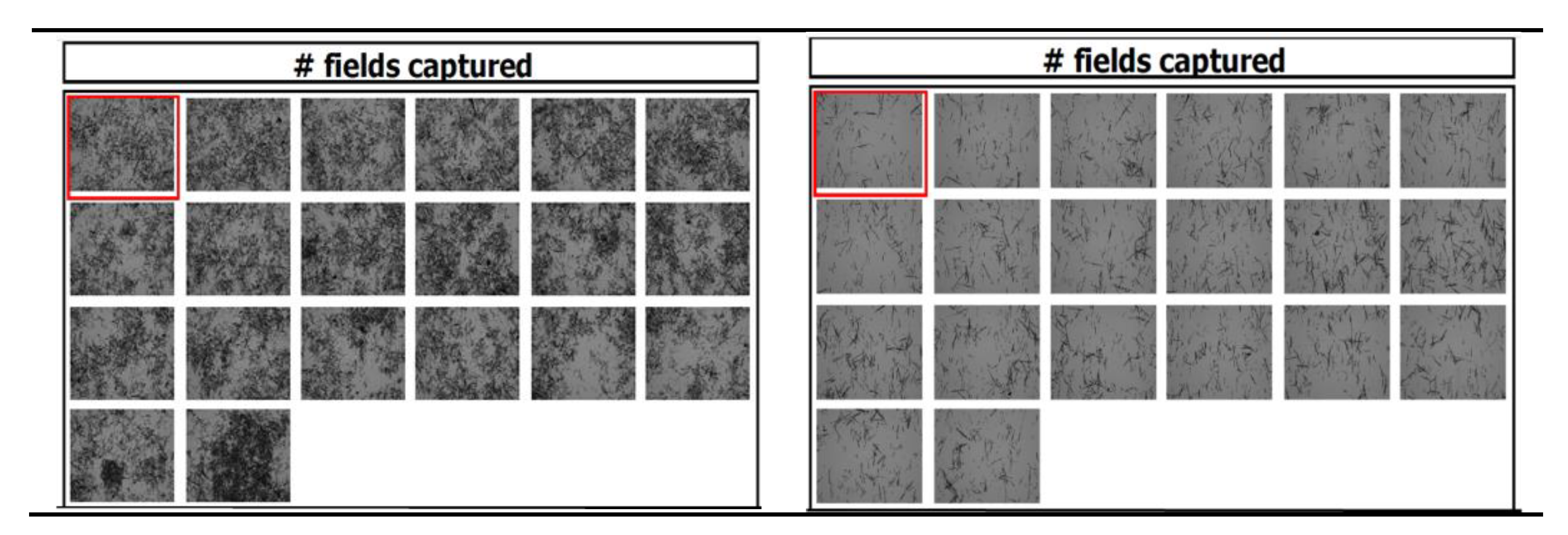
Microscopic Observation
Particle and Size Counting
2.6. Sterility Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Design of the Stability Study
4.2.1. Number and Analysis of Samples
- One was stored refrigerated (2–8 °C) in the original container (according to SPC) placed in a light-protecting plastic bag (hereinafter condition A);
- One was transferred into a polypropylene syringe closed by a red cap, placed in a light-protecting plastic bag, and stored refrigerated (2–8 °C) (hereinafter condition B);
- One was transferred into a polypropylene syringe closed by a red cap, placed in a light-protecting plastic bag, and stored refrigerated (2–8 °C) between two refrigerant gel packs (hereinafter condition C).
4.2.2. Temperature
4.3. Stability Study
4.3.1. Chemical Stability Analysis
Stability Limits
HPLC–UV Analysis
Forced Degradation Study
UHPLC-HRMS Analysis
Infrared Spectroscopy Analysis
pH Determination
4.3.2. Physical Stability Analysis
Visual Examination
Subvisual Examination
Microscopic Observation
Particle and Size Counting
4.3.3. Microbiological Stability Analysis
Sterility Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardin, C.; Astier, A.; Vulto, A.; Sewell, G.; Vigneron, J.; Trittler, R.; Daouphars, M.; Paul, M.; Trojniak, M.; Pinguet, F. Guidelines for the practical stability studies of anticancer drugs: A European consensus conference. Recommandations pour les essais de stabilité pratique des médicaments anticancéreux: Une conférence de consensus européenne. Ann. Pharm. Françaises 2011, 69, 221–231. [Google Scholar] [CrossRef]
- Gøtzsche, P.C.; Jørgensen, A.W. Opening up data at the European Medicines Agency. Br. Med. J. 2011, 342, 1–4. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency; Committee for Proprietary Medicinal Products (CPMP). Note for Guidance in In-Use Stability Testing of Human Medicinal Product; The European Agency for the Evaluation of Medicinal Products: London, UK, 2001.
- Kastango, E.S.; Bradshaw, B.D. USP chapter 797: Establishing a practice standard for compounding sterile preparations in pharmacy. Am. J. Health Syst. Pharm. 2004, 61, 1928–1938. [Google Scholar] [CrossRef]
- European Medicines Agency. Summary of Product Characteristics: Azacitidine. 2013. Available online: https://www.ema.europa.eu/en/documents/product-information/vidaza-epar-product-information_en.pdf (accessed on 5 May 2021).
- Argemì, A.; Saurina, J. Study of the degradation of 5-azacytidine as a model of unstable drugs using a stopped-flow method and further data analysis with multivariate curve resolution. Talanta 2007, 74, 176–182. [Google Scholar] [CrossRef]
- Cheung, Y.-W.; Vishnuvajjala, B.R.; Morris, L.N.; Flora, K.P. Stability of azacitidine in infusion fluids. Am. J. Hosp. Pharm. 1984, 41, 1156–1159. [Google Scholar] [CrossRef]
- Notari, R.E.; DeYoung, J.L. Kinetics and Mechanisms of Degradation of the Antileukemic Agent 5-Azacytidine in Aqueous Solutions. J. Pharm. Sci. 1975, 64, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.K.; Giannini, D.D.; Staroscik, J.A.; Sadee, W. 5-Azacytidine hydrolysis kinetics measured by high-pressure liquid chromatography and 13C-NMR spectroscopy. J. Pharm. Sci. 1979, 68, 807–812. [Google Scholar] [CrossRef]
- Hartigh, J.D.; Brandenburg, H.C.R.; Vermeij, P. Stability of azacitidine in lactated Ringer’s injection frozen in polypropylene syringes. Am. J. Hosp. Pharm. 1989, 46, 2500–2505. [Google Scholar] [CrossRef]
- Balouzet, C.; Chanat, C.; Jobard, M.; Brandely-Piat, M.-L.; Chast, F. Stability of 25 mg/mL Azacitidine Suspensions Kept in Fridge after Freezing. Pharm. Technol. Hosp. Pharm. 2017, 2, 11–16. [Google Scholar] [CrossRef]
- Legeron, R.; Xuereb, F.; Djabarouti, S.; Saux, M.-C.; Breilh, D. Chemical stability of azacitidine suspensions for injection after cold-chain reconstitution of powder and storage. Am. J. Health Syst. Pharm. 2013, 70, 2137–2142. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.M.; Cosgriff, T.M.; Modi, S.S.; Gersh, R.H.; Hainsworth, J.D.; Cohn, A.L.; McIntyre, H.J.; Fernando, I.J.; Backstrom, J.T.; Beach, C.L. Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J. Clin. Oncol. 2009, 27, 1850–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenaux, P.; Bowen, D.; Gattermann, N.; Hellström-Lindberg, E.; Hofmann, W.-K.; Pfeilstöcker, M.; Sanz, G.; Santini, V. Practical use of azacitidine in higher-risk myelodysplastic syndromes: An expert panel opinion. Leuk. Res. 2010, 34, 1410–1416. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomized, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Garcia, R.; de Miguel, D.; Bailen, A.; González, J.R.; Sanz, G.; Falantes, J.F.; Andreu, R.; Tormo, M.; Duarte, R.F.; Figureueredo, A. Different clinical results with the use of different dosing schedules of azacitidine in patients with myelodysplastic syndrome managed with community-based practice: Effectiveness and safety data from the Spanish azacitidine compassionate use registry. Blood 2009, 114, 2773. [Google Scholar] [CrossRef]
- Stabilis®. Available online: https://www.stabilis.org (accessed on 5 May 2021).
- Vigneron, J.; Astier, A.; Trittler, R.; Hecq, J.D.; Daouphars, M.; Larsson, I.; Pourroy, B.; Pinguet, F. SFPO and ESOP recommendations for the practical stability of anticancer drugs: An update. Ann. Pharm. Françaises 2013, 71, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Astier, A.; Pinguet, F.; Vigneron, J. The SFPO stability group members. The practical stability of anticancer drugs: SFPO and ESOP recommendations. Eur. J. Oncol. Pharm. 2010, 4, 4–10. [Google Scholar]
- Walker, S.E.; Charbonneau, L.F.; Law, S.; Earle, C. Stability of azacitidine in sterile water for injection. Can. J. Hosp. Pharm. 2012, 65, 352–359. [Google Scholar] [CrossRef]
- Duriez, A.; Vigneron, J.H.; Zenier, H.A.; May, I.; Demoré, B.M. Stability of Azacitidine Suspensions. Ann. Pharmacother. 2011, 45, 546. [Google Scholar] [CrossRef]
- European Medicines Agency. The European Medicines Agency’s Scientific Guidelines on the Stability of Drug Substances and Drug Products Help Medicine Developers Prepare Marketing Authorization Applications for Human Medicines. 2019. Available online: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/quality/quality-stability (accessed on 16 June 2021).
- Lin, K.-T.; Momparler, R.L.; Rivard, G.E. High-Performance Liquid Chromatographic Analysis of Chemical Stability of 5-Aza-2′-deoxycytidine. J. Pharm. Sci. 1981, 70, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Beisler, J.A. Isolation, Characterization, and Properties of a Labile Hydrolysis Product of the Antitumor Nucleoside, 5-Azacytidine. J. Med. Chem. 1978, 21, 204–208. [Google Scholar] [CrossRef]
- European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019.
- United States Pharmacopoeia USP, 39th ed.; USP: North Bethesda, MD, USA, 2016.
- International Conference of Harmonization of Technical Requirements for Pharmaceuticals for Human Use. Guidelines for Stability. 2011. Available online: https://www.ich.org/products/guidelines (accessed on 5 May 2021).
- Vieillard, V.; Paul, M.; Lim, H.; Astier, A. Physical Stability of Diluted Azacytidine Suspensions stored at +4 °C and −20 °C: Preliminary Results. 2010. Available online: www.sfpo.com/IMG/pdf/vidaza.pdf (accessed on 16 June 2021).
- Bakshi, M.; Singh, S. Development of stability-indicating assay methods-critical review. J. Pharm. Biomed. Anal. 2002, 28, 1011–1040. [Google Scholar] [CrossRef]
- Williams, L.A.; Hastings, M.B. Identifying the criteria of a valid stability study. Int. J. Pharm. Compd. 2009, 13, 32–36. [Google Scholar] [PubMed]
- Trissel, L.A. Trissel’s Stability of Compounded Formulations, 3rd ed.; APhA: Washington, DC, USA, 2005; p. 512. [Google Scholar]
- Hong, D.D.; Shah, M. Development and validation of HPLC stability-indicating assays. In Drug Stability: Principles and Practices; Carstensen, J.T., Rhodes, C.T., Eds.; Marcel Dekker: New York, NY, USA, 2000; pp. 329–384. [Google Scholar]
- Xu, Q.A.; Trissel, L.A. Stability Indicating HPLC Methods for Drug Analysis; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- USP Pending Azacitidine Monograph, November 30, 2017. Available online: http://www.usppf.com/pf/pub/data/v435/MON_IPR_435_m1115.html#MON_IPR_435_m1115 (accessed on 5 May 2021).
- European Pharmacopoeia. Test: 2.9.37. Optical Microscopy; European Pharmacopoeia: Strasbourg, France, 2019; Volume 10. [Google Scholar]
- European Pharmacopoeia. Test: 2.9.19. Particulate Contamination: Sub-Visible Particles; European Pharmacopoeia: Strasbourg, France, 2019; Volume 10. [Google Scholar]
- European Pharmacopoeia. Test: 2.2.1. Clarity and Degree of Opalescence of Liquids; European Pharmacopoeia: Strasbourg, France, 2019; Volume 10. [Google Scholar]
- European Pharmacopoeia. Test: 2.7.24. Flow Cytometry; European Pharmacopoeia: Strasbourg, France, 2019; Volume 10. [Google Scholar]
- Remple, K.; Stone, L. Assessment of GFP Expression and Viability Using the Tali Image-Based Cytometer. J. Vis. Exp. 2011, 57, 3659. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. Test: 2.6.1. Sterility; European Pharmacopoeia: Strasbourg, France, 2019; Volume 10. [Google Scholar]

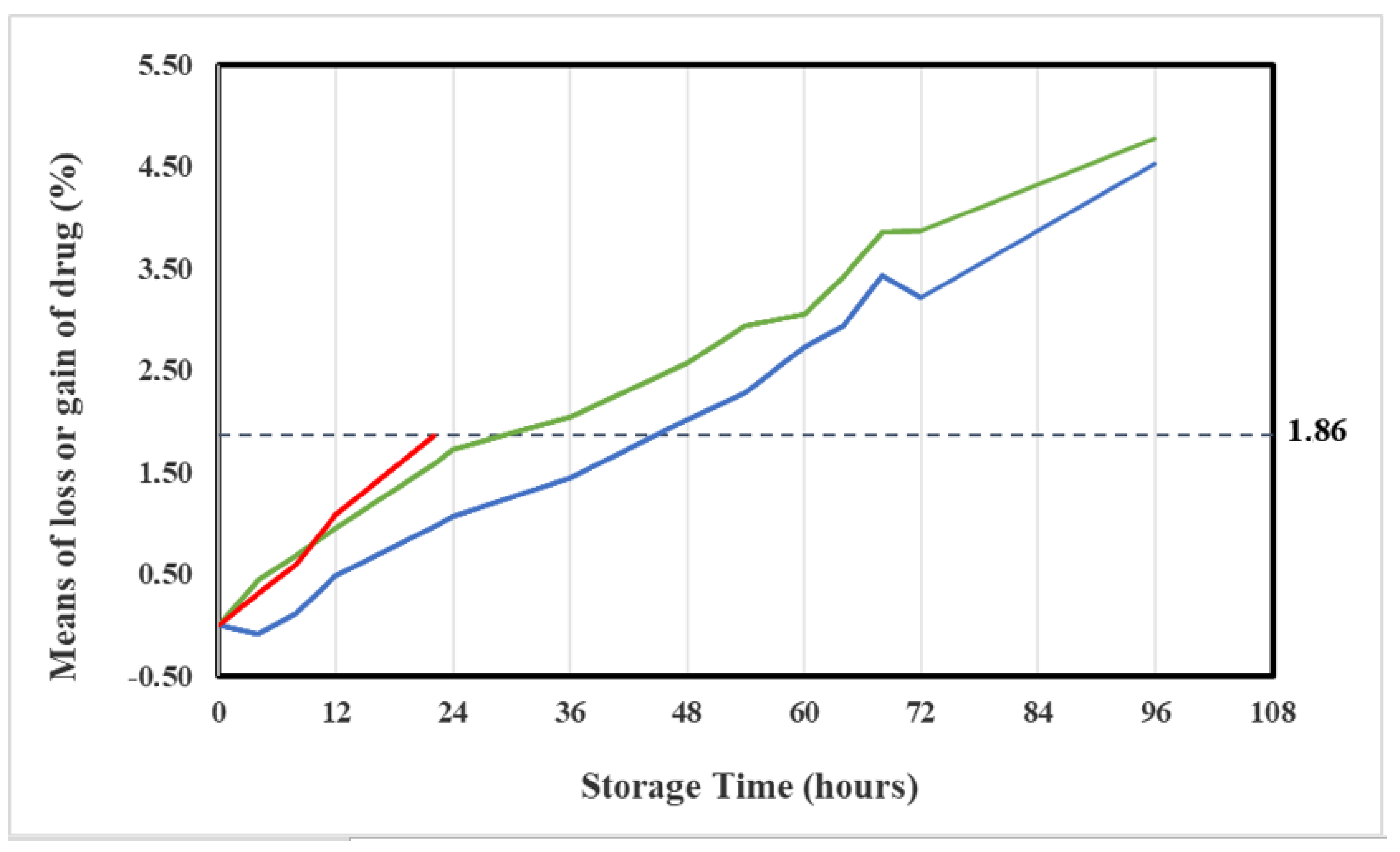

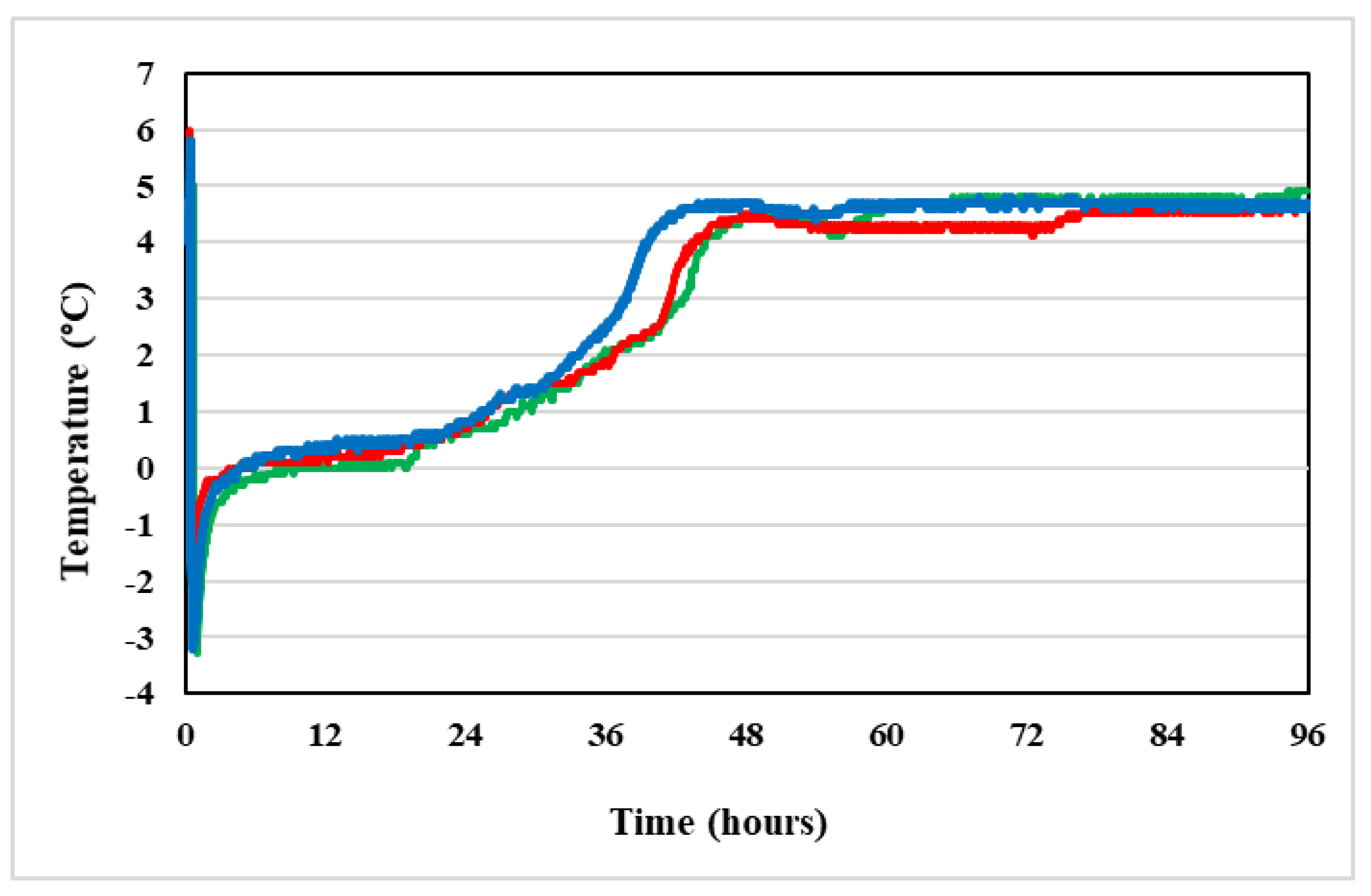
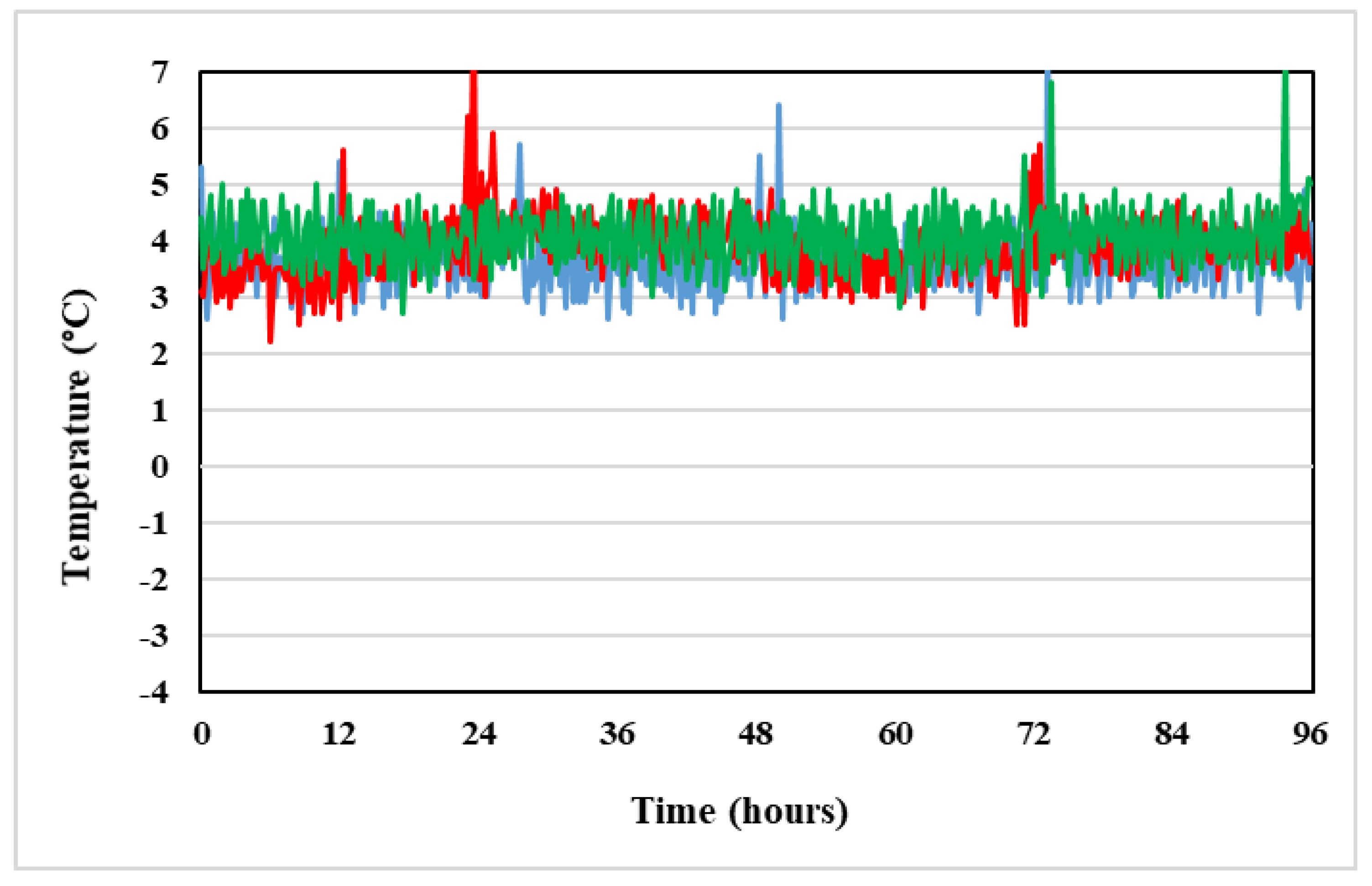
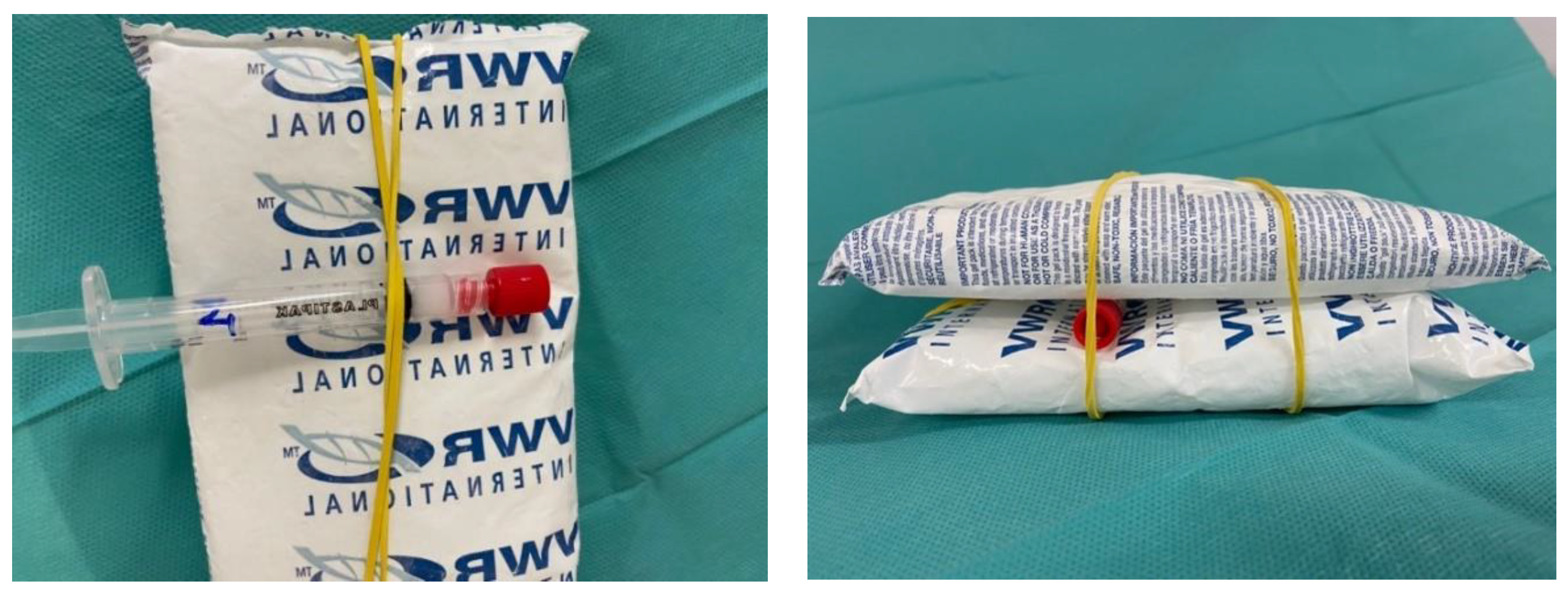
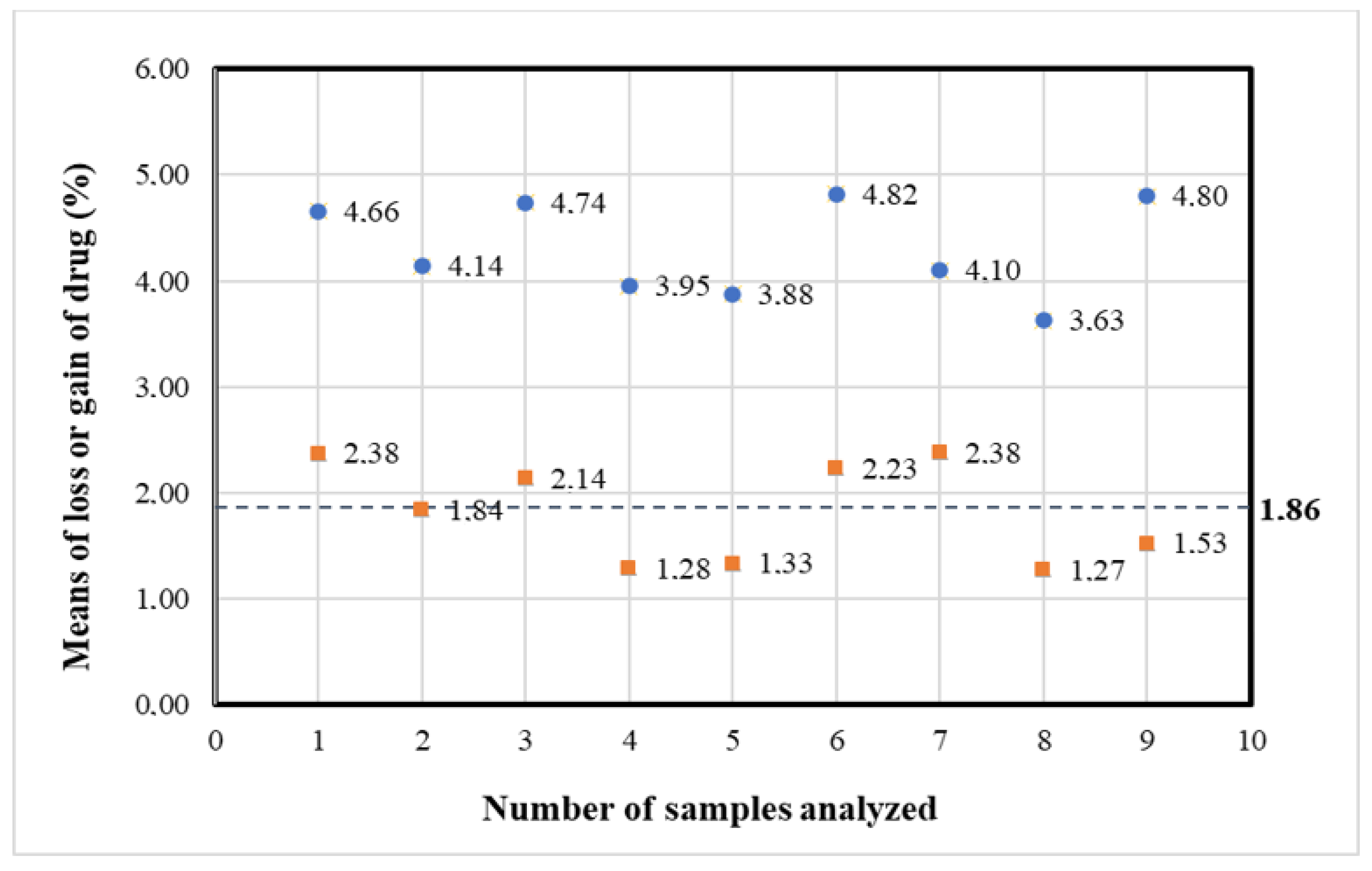
| Storage Time (h) | Vidaza® (25 mg/mL) Stored into Condition A | Vidaza® (25 mg/mL) Stored into Condition B | Vidaza® (25 mg/mL) Stored into Condition C | |||
|---|---|---|---|---|---|---|
| Mean ± SD Drug Concentration (mg/mL) b | Mean Loss or Gain of Drug (%) c | Mean ± SD Drug Concentration (mg/mL) b | Mean Loss or Gain of Drug (%) c | Mean ± SD Drug Concentration (mg/mL) b | Mean Loss or Gain of Drug (%) c | |
| 0 | 24.33 ± 0.11 | - d | 24.33 ± 0.11 | - d | 24.33 ± 0.11 | - d |
| 4 | 24.25 ± 0.11 | −0.30 | 24.22 ± 0.03 | −0.45 | 24.35 ± 0.09 | 0.08 |
| 8 | 24.18 ± 0.14 | −0.61 | 24.16 ± 0.04 | −0.69 | 24.30 ± 0.05 | −0.12 |
| 12 | 24.06 ± 0.19 | −1.09 | 24.10 ± 0.04 | −0.95 | 24.14 ± 0.07 | −0.79 |
| 22 | 23.87 ± 0.09 | −1.86 | ||||
| 24 | 23.91 ± 0.03 | −1.73 | 24.07 ± 0.04 | −1.07 | ||
| 36 | 23.83 ± 0.10 | −2.04 | 23.98 ± 0.04 | −1.46 | ||
| 48 | 23.70 ± 0.08 | −2.57 | 23.81 ± 0.08 | −2.12 | ||
| 54 | 23.62 ± 0.04 | −2.94 | 23.77 ± 0.05 | −2.29 | ||
| 60 | 23.59 ± 0.10 | −3.05 | 23.66 ± 0.08 | −2.74 | ||
| 64 | 23.50 ± 0.13 | −3.41 | 23.62 ± 0.07 | −2.93 | ||
| 68 | 23.39 ± 0.14 | −3.85 | 23.50 ± 0.06 | −3.43 | ||
| 72 | 23.39 ± 0.07 | −3.87 | 23.55 ± 0.05 | −3.21 | ||
| 96 | 23.17 ± 0.06 | −4.77 | 23.23 ± 0.08 | −4.52 | ||
| Storage Time (h) | Vidaza® (25 mg/mL) Stored in Condition A | Vidaza® (25 mg/mL) Stored in Condition B | Vidaza® (25 mg/mL) Stored in Condition C |
|---|---|---|---|
| Mean ± SD pH | Mean ± SD pH | Mean ± SD pH | |
| 0 | 6.81 ± 0.06 | 6.81 ± 0.06 | 6.81 ± 0.06 |
| 22 | 7.25 ± 0.08 | ||
| 24 | 7.18 ± 0.10 | 6.86 ± 0.06 | |
| 48 | 7.36 ± 0.11 | 7.22 ± 0.02 | |
| 72 | 7.34 ± 0.11 | 7.27 ± 0.03 | |
| 96 | 7.40 ± 0.10 | 7.27 ± 0.03 |
| Storage Time (h) | Vidaza® (25 mg/mL) Stored in Condition A | Vidaza® (25 mg/mL) Stored in Condition B | Vidaza® (25 mg/mL) Stored in Condition C | |||
|---|---|---|---|---|---|---|
| Average Particles Size (µm) | # of Particles Counted | Average Particles Size (µm) | # of Particles Counted | Average Particles Size (µm) | # of Particles Counted | |
| 0 | 18 ± 2.0 | 1182 ± 52 | 18 ± 2 | 1182 ± 52 | 18 ± 2 | 1182 ± 52 |
| 22 | 21 ± 1.5 | 2466 ± 86 | ||||
| 24 | 21 ± 0.6 | 1184 ± 102 | 21 ± 2.0 | 475 ± 27 | ||
| 48 | 22 ± 2.0 | 1371 ± 42 | 23 ± 2.0 | 997 ± 39 | ||
| 72 | 22 ± 3.8 | 1552 ± 77 | 24 ± 0.5 | 2116 ± 165 | ||
| 96 | 24 ± 2.5 | 3025 ± 143 | 24 ± 0.0 | 2435 ± 103 | ||
| Storage Time (h) | Vidaza® (25 mg/mL) Stored in Condition A | Vidaza® (25 mg/mL) Stored in Condition C | ||||||
|---|---|---|---|---|---|---|---|---|
| Loss or Gain of Drug (%) c lot 0F324A | Loss or Gain of Drug (%) c lot 0H333A | Loss or Gain of Drug (%) c lot 0I348A | Mean Loss or Gain of Drug (%)c | Loss or Gain of Drug (%) c lot 0F324A | Loss or Gain of Drug (%) c lot 0H333A | Loss or Gain of Drug (%) c lot 0I348A | Mean Loss or Gain of Drug (%)c | |
| 0 | - d | - d | - d | - d | - d | - d | ||
| 4 | 0.06 | −0.41 | −0.57 | −0.30 | 0.24 | 0.12 | −0.11 | 0.08 |
| 8 | 0.09 | −0.89 | −1.02 | −0.61 | −0.01 | −0.23 | −0.12 | −0.12 |
| 12 | −0.24 | −1.25 | −1.77 | −1.09 | −0.99 | −0.57 | −0.79 | −0.79 |
| 22 | −1.81 | −1.92 | −1.84 | −1.86 | ||||
| 24 | −1.21 | −1.31 | −0.70 | −1.07 | ||||
| 36 | −1.71 | −1.36 | −1.29 | −1.46 | ||||
| 48 | −2.38 | −1.84 | −2.14 | −2.12 | ||||
| 54 | −2.47 | −2.14 | −2.25 | −2.29 | ||||
| 60 | −3.07 | −2.49 | −2.65 | −2.74 | ||||
| 64 | −3.35 | −2.77 | −2.67 | −2.93 | ||||
| 68 | −3.34 | −3.47 | −3.47 | −3.43 | ||||
| 72 | −3.54 | −3.22 | −2.88 | −3.21 | ||||
| 96 | −5.15 | −4.77 | −4.40 | −4.77 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iudicello, A.; Genovese, F.; Strusi, V.; Dominici, M.; Ruozi, B. Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations. Pharmaceuticals 2021, 14, 943. https://doi.org/10.3390/ph14090943
Iudicello A, Genovese F, Strusi V, Dominici M, Ruozi B. Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations. Pharmaceuticals. 2021; 14(9):943. https://doi.org/10.3390/ph14090943
Chicago/Turabian StyleIudicello, Antonella, Filippo Genovese, Valentina Strusi, Massimo Dominici, and Barbara Ruozi. 2021. "Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations" Pharmaceuticals 14, no. 9: 943. https://doi.org/10.3390/ph14090943
APA StyleIudicello, A., Genovese, F., Strusi, V., Dominici, M., & Ruozi, B. (2021). Development and Validation of a New Storage Procedure to Extend the In-Use Stability of Azacitidine in Pharmaceutical Formulations. Pharmaceuticals, 14(9), 943. https://doi.org/10.3390/ph14090943






