Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications
Abstract
:1. Introduction
2. Brief Overview of the Actions of Antidepressants and Antipsychotics
3. Repurposing of Antidepressant and Antipsychotic Drugs
4. Candida albicans, a Common Target of Antidepressants and Antipsychotics
5. Possible Antimicrobial Activities of Antidepressants and Antipsychotics
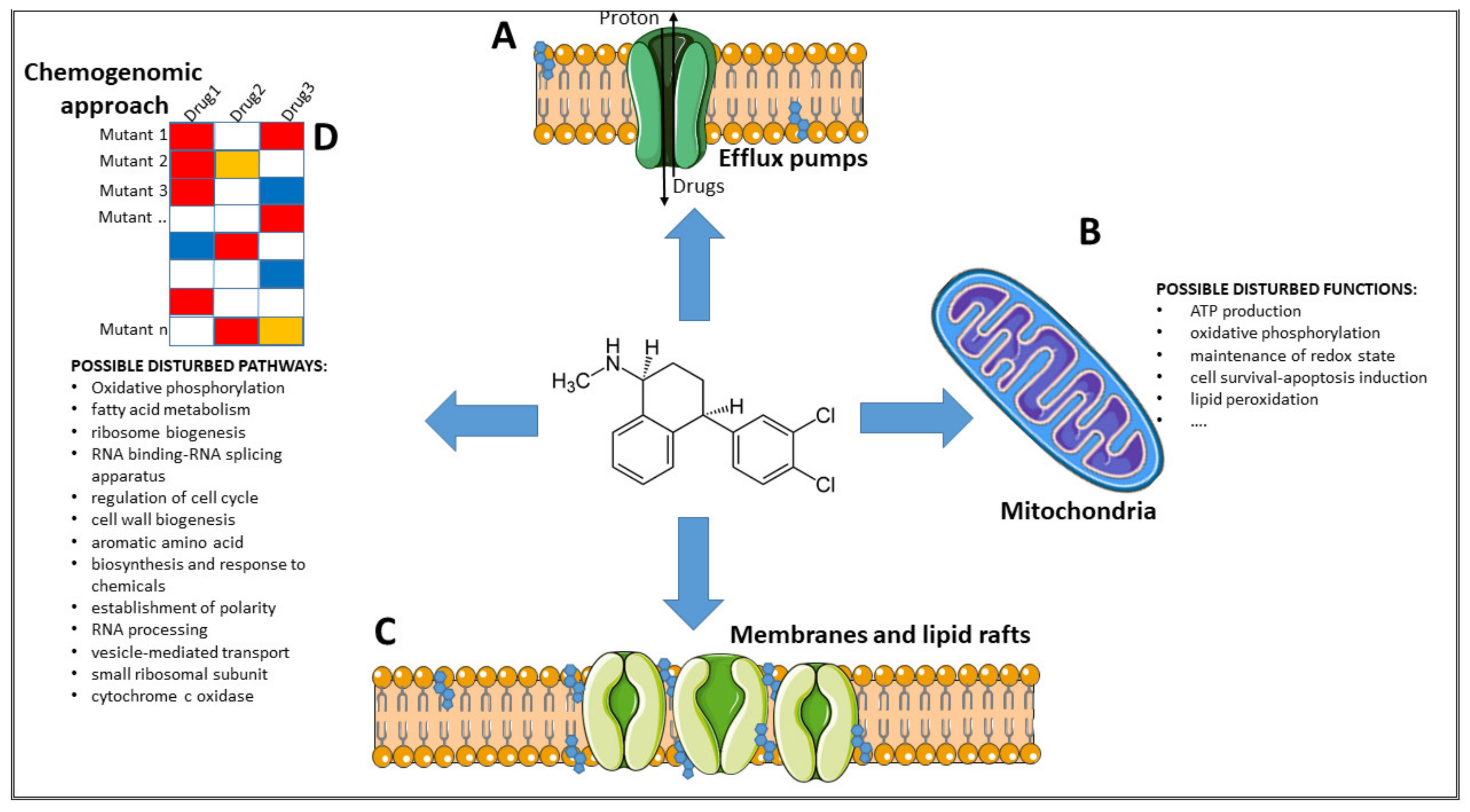
6. Possible Mechanisms of Action
6.1. Inhibition of Efflux Pumps
6.2. Inhibition of Mitochondria Activity
6.3. Interference with Membrane Integrity
6.4. Possible Disturbed Pathways
7. Implications of the Antimicrobial Activity of Antidepressants in Human Gut Ecology
8. Conclusions
- Clearly identify the microbial molecular target(s) of each potential new drug, especially in bacteria
- Describe the connection between the administration of these drugs and their influence on the gut microbiome, after a short and/or prolonged administration, and in the frame of the gut–brain axis
- Pinpoint the concentration at which these molecules would be active as antimicrobials, a parameter that should be equal to, or even lower than, the one used to treat the original disease
- Identify new uses, such as investigate the possibility of topical application
- Test whether, when combined with other known antimicrobials, they potentiate the overall effects of the latter and at the same time, allow lowering antimicrobials’ global concentration and their possible correlated toxic effect
- Better understand which of these drugs may have an acridine-like effect on DNA and which are more active at the level of mitochondrial functions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yssel, A.E.J.; Vanderleyden, J.; Steenackers, H.P. Repurposing of nucleoside- and nucleobase-derivative drugs as antibiotics and biofilm inhibitors. J. Antimicrob. Chemother. 2017, 72, 2326–2333. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; Mcallister-Hollod, L.; Nadle, J.; et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections for the Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team*. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spellberg, B.; Powers, J.H.; Brass, E.P.; Miller, L.G.; Edwards, J.E. Trends in antimicrobial drug development: Implications for the future. Clin. Infect. Dis. 2004, 38, 1279–1286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konreddy, A.K.; Rani, G.U.; Lee, K.; Choi, Y. Recent Drug-Repurposing-Driven Advances in the Discovery of Novel Antibiotics. Curr. Med. Chem. 2018, 26, 5363–5388. [Google Scholar] [CrossRef] [PubMed]
- Altay, O.; Mohammadi, E.; Lam, S.; Turkez, H.; Boren, J.; Nielsen, J.; Uhlen, M.; Mardinoglu, A. Current Status of COVID-19 Therapies and Drug Repositioning Applications. iScience 2020, 23, 101303. [Google Scholar] [CrossRef] [PubMed]
- Viveiros Rosa, S.G.; Santos, W.C. Clinical trials on drug repositioning for COVID-19 treatment. Rev. Panam. Salud Publica/Pan Am. J. Public Health 2020, 44, e40. [Google Scholar] [CrossRef]
- Gysi, D.M.; Do Valle, Í.; Zitnik, M.; Ameli, A.; Gan, X.; Varol, O.; Ghiassian, S.D.; Patten, J.J.; Davey, R.A.; Loscalzo, J.; et al. Network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, 1–11. [Google Scholar] [CrossRef]
- Krysan, D.J.; Didone, L. A high-throughput screening assay for small molecules that disrupt yeast cell integrity. J. Biomol. Screen. 2008, 13, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Siles, S.A.; Srinivasan, A.; Pierce, C.G.; Lopez-Ribot, J.L.; Ramasubramanian, A.K. High-throughput screening of a collection of known pharmacologically active small compounds for identification of candida albicans biofilm inhibitors. Antimicrob. Agents Chemother. 2013, 57, 3681–3687. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.C.; DiDone, L.; Jobson, J.; Sofia, M.K.; Krysan, D.; Dunman, P.M. Adenylate kinase release as a high-throughput-screening-compatible reporter of bacterial lysis for identification of antibacterial agents. Antimicrob. Agents Chemother. 2013, 57, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Giaever, G.; Nislow, C. The yeast deletion collection: A decade of functional genomics. Genetics 2014, 197, 451–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giaever, G.; Shoemaker, D.D.; Jones, T.W.; Liang, H.; Winzeler, E.A.; Astromoff, A.; Davis, R.W. Genomic profiling of drug sensitivities via induced haploinsufficiency. Nat. Genet. 1999, 21, 278–283. [Google Scholar] [CrossRef]
- Hillenmeyer, M.E.; Fung, E.; Wildenhain, J.; Pierce, S.E.; Hoon, S.; Lee, W.; Proctor, M.; Onge, R.P.S.; Tyers, M.; Koller, D.; et al. The chemical genomic portrait of yeast: Uncovering a phenotype for all genes. Science 2008, 320, 362–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roemer, T.; Jiang, B.; Davison, J.; Ketela, T.; Veillette, K.; Breton, A.; Tandia, F.; Linteau, A.; Sillaots, S.; Marta, C.; et al. Large-scale essential gene identification in Candida albicans and applications to antifungal drug discovery. Mol. Microbiol. 2003, 50, 167–181. [Google Scholar] [CrossRef]
- Hillenmeyer, M.E.; Ericson, E.; Davis, R.W.; Nislow, C.; Koller, D.; Giaever, G. Systematic analysis of genome-wide fitness data in yeast reveals novel gene function and drug action. Genome Biol. 2010, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, M.A.; Brown, E.D. Unconventional screening approaches for antibiotic discovery. Ann. N. Y. Acad. Sci. 2015, 1354, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Koromina, M.; Pandi, M.T.; Patrinos, G.P. Rethinking Drug Repositioning and Development with Artificial Intelligence, Machine Learning, and Omics. Omi. A J. Integr. Biol. 2019, 23, 539–548. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Chong, C.R.; Sullivan, D.J. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef]
- Foletto, V.S.; da Rosa, T.F.; Serafin, M.B.; Bottega, A.; Hörner, R. Repositioning of non-antibiotic drugs as an alternative to microbial resistance: A systematic review. Int. J. Antimicrob. Agents 2021, 58, 106380. [Google Scholar] [CrossRef]
- He, S.; Lin, B.; Chu, V.; Hu, Z.; Hu, X.; Xiao, J.; Wang, A.Q.; Schweitzer, C.J.; Li, Q.; Imamura, M.; et al. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci. Transl. Med. 2015, 7, 282ra49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyerhoff, A.U.S. Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin. Infect. Dis. 1999, 28, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Magill, A.J.; Parise, M.E.; Arguin, P.M. Doxycycline for malaria chemoprophylaxis and treatment: Report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 2011, 84, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, T.; Wang, S.; Zhao, Y.; Zhu, R.; Wang, W.; Sun, Y. Haloperidol, a sigma receptor 1 antagonist, promotes ferroptosis in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 491, 919–925. [Google Scholar] [CrossRef]
- Dold, M.; Samara, M.T.; Li, C.; Tardy, M.; Leucht, S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst. Rev. 2015, 1, CD009831. [Google Scholar] [CrossRef]
- Georgiadis, M.O.; Karoutzou, O.; Foscolos, A.S.; Papanastasiou, I. Sigma receptor (σR) ligands with antiproliferative and anticancer activity. Molecules 2017, 22, 1408. [Google Scholar] [CrossRef] [Green Version]
- Hoertel, N.; Sánchez-Rico, M.; Vernet, R.; Beeker, N.; Jannot, A.S.; Neuraz, A.; Salamanca, E.; Paris, N.; Daniel, C.; Gramfort, A.; et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: Results from an observational study. Mol. Psychiatry 2021, 1–14. [Google Scholar] [CrossRef]
- Creeden, J.F.; Imami, A.S.; Eby, H.M.; Gillman, C.; Becker, K.N.; Reigle, J.; Andari, E.; Pan, Z.K.; O’Donovan, S.M.; McCullumsmith, R.E.; et al. Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomed. Pharmacother. 2021, 138, 111437. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Baron, S.A.; Rolain, J.M. Drug repurposing to fight colistin and carbapenem-resistant bacteria. Front. Cell. Infect. Microbiol. 2019, 9, 193. [Google Scholar] [CrossRef]
- Mukherjee, D.; Wu, M.L.; Teo, J.W.P.; Dick, T. Vancomycin and clarithromycin show synergy against Mycobacterium abscessus in vitro. Antimicrob. Agents Chemother. 2017, 61, e01298-17. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Weingarten, R.A.; Xu, M.; Southall, N.; Dai, S.; Shinn, P.; Sanderson, P.E.; Williamson, P.R.; Frank, K.M.; Zheng, W. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Shen, P.; Bao, Z.; Zhou, K.; Zheng, B.; Ji, J.; Guo, L.; Huang, C.; Xiao, Y. In vitro antibacterial activity of fosfomycin combined with other antimicrobials against KPC-producing Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2017, 50, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, Serotonin and Tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowen, P.J. Serotonin and depression: Pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci. 2008, 29, 433–436. [Google Scholar] [CrossRef]
- Brasseur, R. Clinical trial with bromperidol in psychotic states. Acta Psychiatr. Belg. 1978, 78, 110–117. [Google Scholar]
- Nestoros, J.N.; Suranyl-Cadotte, B.E.; Spees, R.C.; Schwartz, G.; Vasavan Nair, N.P. Diazepam in high doses is effective in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 1982, 6, 513–516. [Google Scholar] [CrossRef]
- Currier, G.W.; Chou, J.C.Y.; Feifel, D.; Bossie, C.A.; Turkoz, I.; Mahmoud, R.A.; Gharabawi, G.M. Acute treatment of psychotic agitation: A randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J. Clin. Psychiatry 2004, 65, 386–394. [Google Scholar] [CrossRef]
- Kousgaard, S.J.; Licht, R.W.; Nielsen, R.E. Effects of Intramuscular Midazolam and Lorazepam on Acute Agitation in Non-Elderly Subjects—A Systematic Review. Pharmacopsychiatry 2017, 50, 129–135. [Google Scholar] [CrossRef]
- Khan, H.J.; Rohondia, S.O.; Othman Ahmed, Z.S.; Zalavadiya, N.; Dou, Q.P. Increasing opportunities of drug repurposing for treating breast cancer by the integration of molecular, histological, and systemic approaches. In Drug Repurposing in Cancer Therapy; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 121–172. [Google Scholar]
- Macdonald, G.J.; Bartolomé, J.M. A decade of progress in the discovery and development of “atypical” antipsychotics. In Progress in Medicinal Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2010; Volume 49, pp. 37–80. ISBN 9780123812926. [Google Scholar]
- Włodarczyk, A.; Szarmach, J.; Cubała, W.J.; Wiglusz, M.S. Benzodiazepines in combination with antipsychotic drugs for schizophrenia: GABA-ergic targeted therapy. Psychiatr. Danub. 2017, 29, S345–S348. [Google Scholar]
- Clouse, R.E. Antidepressants for irritable bowel syndrome. Gut 2003, 52, 598–599. [Google Scholar] [CrossRef] [Green Version]
- Macer, B.J.D.; Prady, S.L.; Mikocka-Walus, A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflamm. Bowel Dis. 2017, 23, 534–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, A.C.; Lacy, B.E.; Harris, L.A.; Quigley, E.M.M.; Moayyedi, P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, P.W.; Wyatt, K.M.; Jones, P.W.; O’Brien, P.M.S. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: A systematic review. Lancet 2000, 356, 1131–1136. [Google Scholar] [CrossRef]
- Marjoribanks, J.; Brown, J.; O’Brien, P.M.S.; Wyatt, K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst. Rev. 2013, 6, CD001396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Jenewein, I.; Ledochowski, M. Antifungal properties of selective serotonin reuptake inhibitors against Aspergillus species in vitro. J. Antimicrob. Chemother. 2001, 48, 775–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Galomo, K.S.; González, G.M.; Montoya, A.M.; Becerril-García, M.A.; Solís-Villegas, E.M.; Villanueva-Lozano, H.; Robledo-Leal, E.R.; Treviño-Rangel, R.d.J. In vivo evaluation of the antifungal activity of sertraline against Aspergillus fumigatus. J. Antimicrob. Chemother. 2018, 74, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Gu, W.; Guo, D.; Zhang, L.; Xu, D.; Sun, S. The synergistic effect of azoles and fluoxetine against resistant Candida albicans strains is attributed to attenuating fungal virulence. Antimicrob. Agents Chemother. 2016, 60, 6179–6188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, A.; Sinha, C.; Jena, A.K.; Ghosh, S.; Samanta, A. An investigation on in vitro and in vivo antimicrobial properties of the antidepressant: Amitriptyline hydrochloride. Braz. J. Microbiol. 2010, 41, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.A.; Fitts, E.C.; Kirtley, M.L.; Ponnusamy, D.; Peniche, A.G.; Dann, S.M.; Motin, V.L.; Chauhan, S.; Rosenzweig, J.A.; Sha, J.; et al. New role for FDA-approved drugs in combating antibiotic-resistant bacteria. Antimicrob. Agents Chemother. 2016, 60, 3717–3729. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, S.Y.L.; Garzan, A.; Dennis, E.K.; Shrestha, S.K.; Garneau-Tsodikova, S. Repurposing antipsychotic drugs into antifungal agents: Synergistic combinations of azoles and bromperidol derivatives in the treatment of various fungal infections. Eur. J. Med. Chem. 2017, 139, 12–21. [Google Scholar] [CrossRef]
- Moraes, D.C.; Ferreira-Pereira, A. Insights on the anticandidal activity of non-antifungal drugs. J. Mycol. Med. 2019, 29, 253–259. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Dierich, M.P.; Fuchs, D.; Semenitz, E.; Ledochowski, M. Antifungal activity against Candida species of the selective serotonin-reuptake inhibitor, sertraline. Clin. Infect. Dis. 2001, 33, 135–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tekintaş, Y.; Temel, A.; Ateş, A.; Eraç, B.; Metin, D.Y.; Hilmioğlu Polat, S.; Hoşgör Limoncu, M. Antifungal and antibiofilm activities of selective serotonin reuptake inhibitors alone and in combination with fluconazole. Turkish J. Pharm. Sci. 2020, 17, 667–672. [Google Scholar] [CrossRef]
- Chen, J.; Korostyshevsky, D.; Lee, S.; Perlstein, E.O. Accumulation of an antidepressant in vesiculogenic membranes of yeast cells triggers autophagy. PLoS ONE 2012, 7, e34024. [Google Scholar] [CrossRef] [Green Version]
- Zhai, B.; Wu, C.; Wang, L.; Sachs, M.S.; Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012, 56, 3758–3766. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcìa-Rodrìguez, J.A. Antimicrobial activity of psychotropic drugs. Selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.-H.; Hussain, S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. 2015, 22, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyżek, P.; Franiczek, R.; Krzyżanowska, B.; Łaczmański, Ł.; Migdał, P.; Gościniak, G. In vitro activity of sertraline, an antidepressant, against antibiotic-susceptible and antibiotic-resistant Helicobacter pylori strains. Pathogens 2019, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.S.; Gaspar, C.A.; Palmeira-de-Oliveira, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Anti-Candida activity of fluoxetine alone and combined with fluconazole: A synergistic action against fluconazole-resistant strains. Antimicrob. Agents Chemother. 2014, 58, 4224–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karine de Sousa, A.; Rocha, J.E.; Gonçalves de Souza, T.; Sampaio de Freitas, T.; Ribeiro-Filho, J.; Melo Coutinho, H.D. New roles of fluoxetine in pharmacology: Antibacterial effect and modulation of antibiotic activity. Microb. Pathog. 2018, 123, 368–371. [Google Scholar] [CrossRef]
- Zuo, J.; Quinn, K.K.; Kye, S.; Cooper, P.; Damoiseaux, R.; Krogstad, P. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob. Agents Chemother. 2012, 56, 4838–4844. [Google Scholar] [CrossRef] [Green Version]
- Foletto, V.S.; Serafin, M.B.; Bottega, A.; da Rosa, T.F.; de S. Machado, C.; Coelho, S.S.; Hörner, R. Repositioning of fluoxetine and paroxetine: Study of potential antibacterial activity and its combination with ciprofloxacin. Med. Chem. Res. 2020, 29, 556–563. [Google Scholar] [CrossRef]
- Costa Silva, R.A.; da Silva, C.R.; de Andrade Neto, J.B.; da Silva, A.R.; Campos, R.S.; Sampaio, L.S.; do Nascimento, F.B.S.A.; da Silva Gaspar, B.; da Cruz Fonseca, S.G.; Josino, M.A.A.; et al. In vitro anti-Candida activity of selective serotonin reuptake inhibitors against fluconazole-resistant strains and their activity against biofilm-forming isolates. Microb. Pathog. 2017, 107, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Ennis, C.L.; Hartooni, N.; Johnson, A.D.; Lohse, M.B. A selective serotonin reuptake inhibitor, a proton pump inhibitor, and two calcium channel blockers inhibit candida albicans biofilms. Microorganisms 2020, 8, 756. [Google Scholar] [CrossRef]
- Holmes, A.R.; Keniya, M.V.; Ivnitski-Steele, I.; Monk, B.C.; Lamping, E.; Sklar, L.A.; Cannon, R.D. The monoamine oxidase A inhibitor clorgyline is a broad-spectrum inhibitor of fungal ABC and MFS transporter efflux pump activities which reverses the azole resistance of Candida albicans and Candida glabrata clinical isolates. Antimicrob. Agents Chemother. 2012, 56, 1508–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burin, R.; Shah, D.H. Phenelzine and Amoxapine Inhibit Tyramine and d-Glucuronic Acid Catabolism in Clinically Significant Salmonella in A Serotype-Independent Manner. Pathogens 2021, 10, 469. [Google Scholar] [CrossRef]
- Caldara, M.; Marmiroli, N. Tricyclic antidepressants inhibit Candida albicans growth and biofilm formation. Int. J. Antimicrob. Agents 2018, 52, 500–505. [Google Scholar] [CrossRef]
- Caldara, M.; Marmiroli, N. Known antimicrobials versus nortriptyline in Candida albicans: Repositioning an old drug for new targets. Microorganisms 2020, 8, 742. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Lee, J.H.; Lee, J. Aripiprazole repurposed as an inhibitor of biofilm formation and sterol biosynthesis in multidrug-resistant Candida albicans. Int. J. Antimicrob. Agents 2019, 54, 518–523. [Google Scholar] [CrossRef]
- Ramón-García, S.; Ng, C.; Anderson, H.; Chao, J.D.; Zheng, X.; Pfeifer, T.; Av-Gay, Y.; Roberge, M.; Thompson, C.J. Synergistic drug combinations for tuberculosis therapy identified by a novel high-throughput screen. Antimicrob. Agents Chemother. 2011, 55, 3861–3869. [Google Scholar] [CrossRef] [Green Version]
- Kathwate, G.H.; Shinde, R.B.; Karuppayil, S.M. Antiepileptic drugs inhibit growth, dimorphism, and biofilm mode of growth in human pathogen Candida albicans. Assay Drug Dev. Technol. 2015, 13, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Ayoglu, H.; Kulah, C.; Turan, I. Antimicrobial effects of two anaesthetic agents: Dexmedetomidine and midazolam. Anaesth. Intensive Care 2008, 36, 681–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keleş, G.T.; Kurutepe, S.; Tok, D.; Gazi, H.; Dinç, G. Comparison of antimicrobial effects of dexmedetomidine and etomidate-lipuro with those of propofol and midazolam. Eur. J. Anaesthesiol. 2006, 23, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J.; Volz, P.A. Ecology of Candida albicans gut colonization: Inhibition of Candida adhesion, colonization, and dissemination from the gastrointestinal tract by bacterial antagonism. Infect. Immun. 1985, 49, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Kumamoto, C.A. Candida biofilms. Curr. Opin. Microbiol. 2002, 5, 608–611. [Google Scholar] [CrossRef]
- Kumamoto, C.A. Inflammation and gastrointestinal Candida colonization. Curr. Opin. Microbiol. 2011, 14, 386–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weig, M.; Gross, U.; Muhlschlegel, F. Clinical aspects and pathogenesis of Candida infection. Trends Microbiol. 1998, 6, 468–470. [Google Scholar] [CrossRef]
- Chandra, J.; Kuhn, D.M.; Mukherjee, P.K.; Hoyer, L.L.; McCormick, T.; Ghannoum, M.A. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J. Bacteriol. 2001, 183, 5385–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramage, G.; Mowat, E.; Jones, B.; Williams, C.; Lopez-Ribot, J. Our Current Understanding of Fungal Biofilms. Crit. Rev. Microbiol. 2009, 35, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.P. Nosocomial Candidemia: Risk Factors and Attributable Mortality. Clin. Infect. Dis. 1995, 20, 1531–1534. [Google Scholar] [CrossRef]
- Lepak, A.; Andes, D. Fungal Sepsis: Optimizing Antifungal Therapy in the Critical Care Setting. Crit. Care Clin. 2011, 27, 123–147. [Google Scholar] [CrossRef]
- Anderson, J.B. Evolution of antifungal-drug resistance: Mechanisms and pathogen fitness. Nat. Rev. Microbiol. 2005, 3, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, J.; d’Enfert, C. Candida albicans biofilms: Building a heterogeneous, drug-tolerant environment. Curr. Opin. Microbiol. 2013, 16, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Mechanism of Candida pathogenesis: Revisiting the vital drivers. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1797–1819. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [Green Version]
- Mollinedo, F. Lipid raft involvement in yeast cell growth and death. Front. Oncol. 2012, 2, 140. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Jia, C.; Dong, Y.; Zhang, B.; Xiao, C.; Chen, Y.; Wang, Y.; Li, X.; Wang, L.; Zhang, B.; et al. Candida albicans autophagy, no longer a bystander: Its role in tolerance to ER stress-related antifungal drugs. Fungal Genet. Biol. 2015, 81, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; Sangalli-Leite, F.; de Lacorte Singulani, J.; de Paula e Silva, A.C.A.; Costa-Orlandi, C.B.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Searching new antifungals: The use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 2016, 123, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhein, J.; Morawski, B.M.; Hullsiek, K.H.; Nabeta, H.W.; Kiggundu, R.; Tugume, L.; Musubire, A.; Akampurira, A.; Smith, K.D.; Alhadab, A.; et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: An open-label dose-ranging study. Lancet Infect. Dis. 2016, 16, 809–818. [Google Scholar] [CrossRef] [Green Version]
- Villanueva-Lozano, H.; Treviño-Rangel, R.d.J.; González, G.M.; Hernández-Rodríguez, P.A.; Camacho-Ortiz, A.; Castillo-Reyna, L.; Galindo-Alvarado, S.G.; Martínez-Reséndez, M.F. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: A single Mexican center experience. Infection 2018, 46, 25–30. [Google Scholar] [CrossRef]
- Verma, N.A.; Zheng, X.T.; Harris, M.U.; Cadichon, S.B.; Malm-Aldana, H.; Khetsuriani, N.; Oberste, M.S.; Shulman, S.T. Outbreak of life-threatening coxsackievirus B1 myocarditis in neonates. Clin. Infect. Dis. 2009, 49, 759–763. [Google Scholar] [CrossRef]
- Wilson, T.J.; Blackledge, M.S.; Vigueira, P.A. Resensitization of methicillin-resistant Staphylococcus aureus by amoxapine, an FDA-approved antidepressant. Heliyon 2018, 4, e00501. [Google Scholar] [CrossRef] [Green Version]
- Gillard, K.; Miller, H.B.; Blackledge, M.S. Tricyclic amine antidepressants suppress β-lactam resistance in methicillin-resistant Staphylococcus aureus (MRSA) by repressing mRNA levels of key resistance genes. Chem. Biol. Drug Des. 2018, 92, 1822–1829. [Google Scholar] [CrossRef]
- Bonde, M.; Højland, D.H.; Kolmos, H.J.; Kallipolitis, B.H.; Klitgaard, J.K. Thioridazine affects transcription of genes involved in cell wall biosynthesis in methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 318, 168–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Gaur, N.; Gaur, M.; Komath, S. Efflux Pumps in Drug Resistance of Candida. Infect. Disord.-Drug Targets 2008, 6, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.P.; Xu, Y.H.; Wang, Z.X.; Fang, Y.P.; Shen, J.L. Overexpression of MexAB-OprM efflux pump in carbapenem-resistant Pseudomonas aeruginosa. Arch. Microbiol. 2016, 198, 565–571. [Google Scholar] [CrossRef]
- Nikaido, H.; Basina, M.; Nguyen, V.; Rosenberg, E.Y. Multidrug efflux pump AcrAB of Salmonella typhimurium excretes only those β-lactam antibiotics containing lipophilic side chains. J. Bacteriol. 1998, 180, 4686–4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marmiroli, N.; Tedeschi, F.; Sabatini, M.A.; Truzzi, G.; Ferrari, C.; Puglisi, P.P. Relationship between growth inhibition and mitochondrial function in petite-negative yeasts. II. Effects of central nervous system drugs upon pathogenic and non-pathogenic Candida species. Biol. Cell 1985, 53, 75–79. [Google Scholar] [CrossRef]
- Uesono, Y.; Araki, T.; Toh-e, A. Local Anesthetics, Antipsychotic Phenothiazines, and Cationic Surfactants Shut Down Intracellular Reactions through Membrane Perturbation in Yeast. Biosci. Biotechnol. Biochem. 2008, 72, 2884–2894. [Google Scholar] [CrossRef] [PubMed]
- Uesono, Y.; Toh-e, A.; Kikuchi, Y.; Terashima, I. Structural analysis of compounds with actions similar to local anesthetics and antipsychotic phenothiazines in yeast. Yeast 2011, 28, 391–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldara, M.; Graziano, S.; Gullì, M.; Cadonici, S.; Marmiroli, N. Off-target effects of neuroleptics and antidepressants on saccharomyces cerevisiae. Toxicol. Sci. 2017, 156, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Ericson, E.; Gebbia, M.; Heisler, L.E.; Wildenhain, J.; Tyers, M.; Giaever, G.; Nislow, C. Off-target effects of psychoactive drugs revealed by genome-wide assays in yeast. PLoS Genet. 2008, 4, e1000151. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Luan, X.; Gao, C.; Jiang, Y. Recent progress of acridine derivatives with antitumor activity. Prog. Chem. 2012, 24, 1497–1505. [Google Scholar]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. CMGH 2019, 7, 709–728. [Google Scholar] [CrossRef] [Green Version]
- Özoğul, F. Production of biogenic amines by Morganella morganii, Klebsíella pneumoniae and Hafnia alvei using a rapid HPLC method. Eur. Food Res. Technol. 2004, 219, 465–469. [Google Scholar] [CrossRef]
- Shishov, V.A.; Kirovskaya, T.A.; Kudrin, V.S.; Oleskin, A.V. Amine neuromediators, their precursors, and oxidation products in the culture of Escherichia coli k-12. Appl. Biochem. Microbiol. 2009, 45, 494–497. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef]
- Inserra, A.; Rogers, G.B.; Licinio, J.; Wong, M.L. The Microbiota-Inflammasome Hypothesis of Major Depression. BioEssays 2018, 40, 1800027. [Google Scholar] [CrossRef] [Green Version]
- Nikolova, V.L.; Cleare, A.J.; Young, A.H.; Stone, J.M. Updated Review and Meta-Analysis of Probiotics for the Treatment of Clinical Depression: Adjunctive vs. Stand-Alone Treatment. J. Clin. Med. 2021, 10, 647. [Google Scholar] [CrossRef]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef]
- Akkasheh, G.; Kashani-Poor, Z.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akbari, H.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z.; Esmaillzadeh, A. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition 2016, 32, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut–Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]

| Drug | Microbial Target | Concentration Effective on Microbes | Physiological Effects on Microbes | References |
|---|---|---|---|---|
| ANTIDEPRESSANTs—SSRIs | ||||
| Sertraline (third generation of selective serotonin reuptake inhibitors) | C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. dubliniensis, C. krusei, A. fumigatus, A. flavus, A. terreus, C. neofromans, S. cerevisiae, A. baumanii, H. influenzae, C. jejuni, H. pylori, S. aureus, P. aeruginosa, S. epidermidis, E. faecalis, C. difficile, B. fragilis, Prevotella spp | 9–775 µM (3–237 µg/mL) | Fungicidal; inhibits hyphal elongation and phospholipase activity, reduces secreted aspartyl proteinases (SAP) production, inhibits fungal viability, has antifungal and anti-biofilm effects, displays a synergistic effect with fluconazole, causes mitochondrial depolarization and cell membrane damage, induces autophagy | [50,51,56,57,58,59,60,61,62,63] |
| Fluoxetine (third generation of selective serotonin reuptake inhibitors) | C. albicans, E. coli P. aeruginosa, S. aureus, Coxsackievirus, E. coli, A. baumanii | 130 µM–13 mM (40–4000 µg/mL) | Inhibits cell growth; promotes mitochondrial depolarization and membrane damage; decreases metabolic activity of mature biofilms; displays synergistic interaction with azoles such as fluconazole, downregulates SAP genes expression and extracellular phospholipase activity, inhibits bacterial growth and synergizes with antibiotics, reduces the synthesis of viral RNA and proteins. | [52,56,58,64,65,66,67] |
| Paroxetine (third generation of selective serotonin reuptake inhibitors) | C. albicans, E. coli, A. baumanii | 110–282 µM (40–101 µg/mL) | Inhibits cell growth; promotes mitochondrial depolarization and membrane damage in yeast, inhibits bacterial growth. | [56,58,67,68,69] |
| ANTIDEPRESSANTs—MAOIs | ||||
| Clorgyline (MonoAmine Oxidase Inhibitor) | C. albicans, C. glabrata | 5.1 µM (1.4 µg/mL) | Broad-spectrum inhibitor of several fungal efflux pumps, displays a synergistic interaction with fluconazole | [56,70] |
| Phenelzine (MonoAmine Oxidase Inhibitor) | Salmonella | 30–100 µM (4–14 µg/mL) | Inhibits TYR oxidoreductase | [71] |
| ANTIDEPRESSANTS—TCAs | ||||
| Doxepin (increases the levels of norepinephrine, along with blocking histamine, acetylcholine, and serotonin) | C. albicans, C. glabrata, C. parapsilosis, C. krusei, C. utilis | 716 µM (200 μg/mL) | Inhibits hyphae and biofilm formation, kills cells in a mature yeast biofilm | [72] |
| Imipramine (increases the levels of serotonin and norepinephrine and blocks some serotonin, adrenergic, histamine, and cholinergic receptors) | C. albicans, C. glabrata C. parapsilosis, C. krusei, C. utilis | 142 µM (40 μg/mL) | Inhibits hyphae and biofilm formation, kills cells in a mature yeast biofilm | [72] |
| Nortryptiline (blocks the reuptake of norepinephrine, binds to alpha-adrenergic, histaminergic, and cholinergic receptors) | C. albicans, C. glabrata, C. parapsilosis, C. krusei, C. utilis | 190 µM (50 μg/mL) | Inhibits hyphae and biofilm formation, kills cells in a mature biofilm, induces cell lysis, and displays synergistic activity with amphotericin B | [72,73] |
| Amitriptyline (Inhibits the reuptake of serotonin and norepinephrine) | Staphylococcus spp., Bacillus spp., V. cholerae, Micrococcus spp, L. sporogenes, Citrobacter spp. | 36–722 µM (10–200 μg/mL) | Inhibits microbial growth | [53] |
| Amoxapine (Reduces the uptake of serotonin and noradrenaline) | Salmonella, Y. pestis | 1–100 µM (0.3–30 µg/mL) | Inhibits GUS-mediated hydrolysis of d-glucuronides, reduces cytotoxicity in murine macrophages | [54,71] |
| ANTIPSYCHOTIC—NEUROLEPTIC | ||||
| Aripiprazole (partial agonist of serotonin and dopamine receptors) | C. albicans | 11–111 µM (5–50 µg/mL) | At low doses, it inhibits biofilm formation, as well as yeast-to-hyphal transition and flocculation; at high doses, disrupts lipid rafts, induces membrane damage | [74] |
| Bromperidol (antagonist of the dopamine receptor) | Mycobacterium smegmatis, M. tubercolosis, C. albicans, C. glabrata, A. terreus | 119–142 µM (50–60 µg/mL) | Acts synergistically with spectinomycin on Mycobacterium, is bactericidal, interacts positively with azoles | [55,75] |
| ANTIPSYCHOTIC—BENZODIAZEPINE | ||||
| Diazepam (increases the effect of the neurotransmitter GABA) | C. albicans | 108 µM–14 mM (31.25–4000 μg/mL) | Inhibits growth, hyphae formation, and biofilm growth | [76] |
| Lorazepam (enhancer of the effect of the inhibitory neurotransmitter gamma-aminobutyric acid on GABA receptors) | C. albicans | 96 µM–12 mM (31.25–4000 μg/mL) | Inhibits growth, hyphae formation, and biofilm growth | [76] |
| Midazolam (promotes the action of GABA) | C. albicans, S. aureus, E. faecalis, E. coli, P. aeruginosa, A. baumanii | 95 µM–12 mM (31.25–4000 μg/mL) | Inhibits growth, hyphae formation, and biofilm growth, inhibits bacterial growth | [76,77,78] |
| ANTIPSYCHOTIC—ATYPICAL | ||||
| Zotepine (High affinity to dopamine receptors, affects serotonin receptors, its active metabolite, norzotepine, serves as a potent norepinephrine reuptake inhibitor) | C. albicans | 0.1–40 µM (0.03–12 µg/mL) | Inhibits biofilm development | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caldara, M.; Marmiroli, N. Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals 2021, 14, 915. https://doi.org/10.3390/ph14090915
Caldara M, Marmiroli N. Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals. 2021; 14(9):915. https://doi.org/10.3390/ph14090915
Chicago/Turabian StyleCaldara, Marina, and Nelson Marmiroli. 2021. "Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications" Pharmaceuticals 14, no. 9: 915. https://doi.org/10.3390/ph14090915
APA StyleCaldara, M., & Marmiroli, N. (2021). Antimicrobial Properties of Antidepressants and Antipsychotics—Possibilities and Implications. Pharmaceuticals, 14(9), 915. https://doi.org/10.3390/ph14090915







