Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report
Abstract
:1. Introduction
2. Case
2.1. Umbilical Cord-Derived Wharton’s Jelly
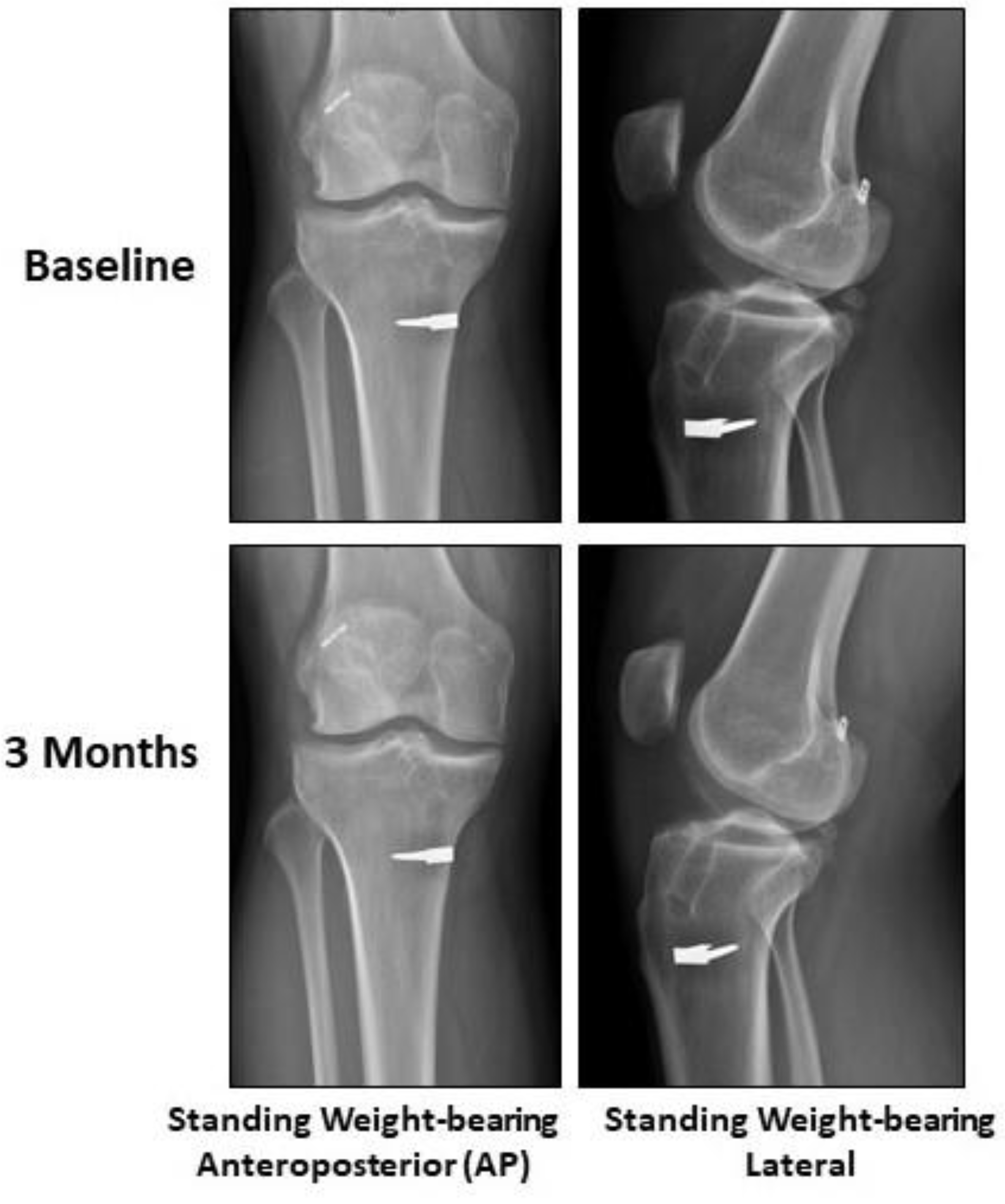
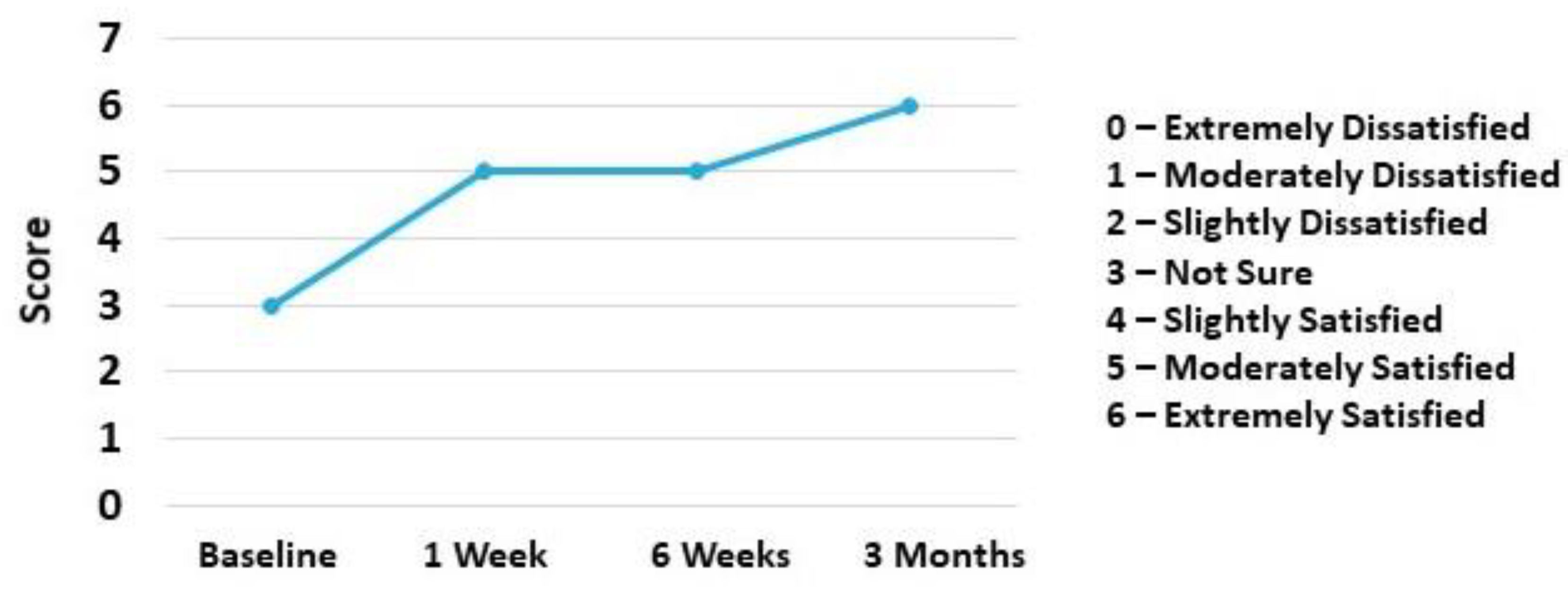
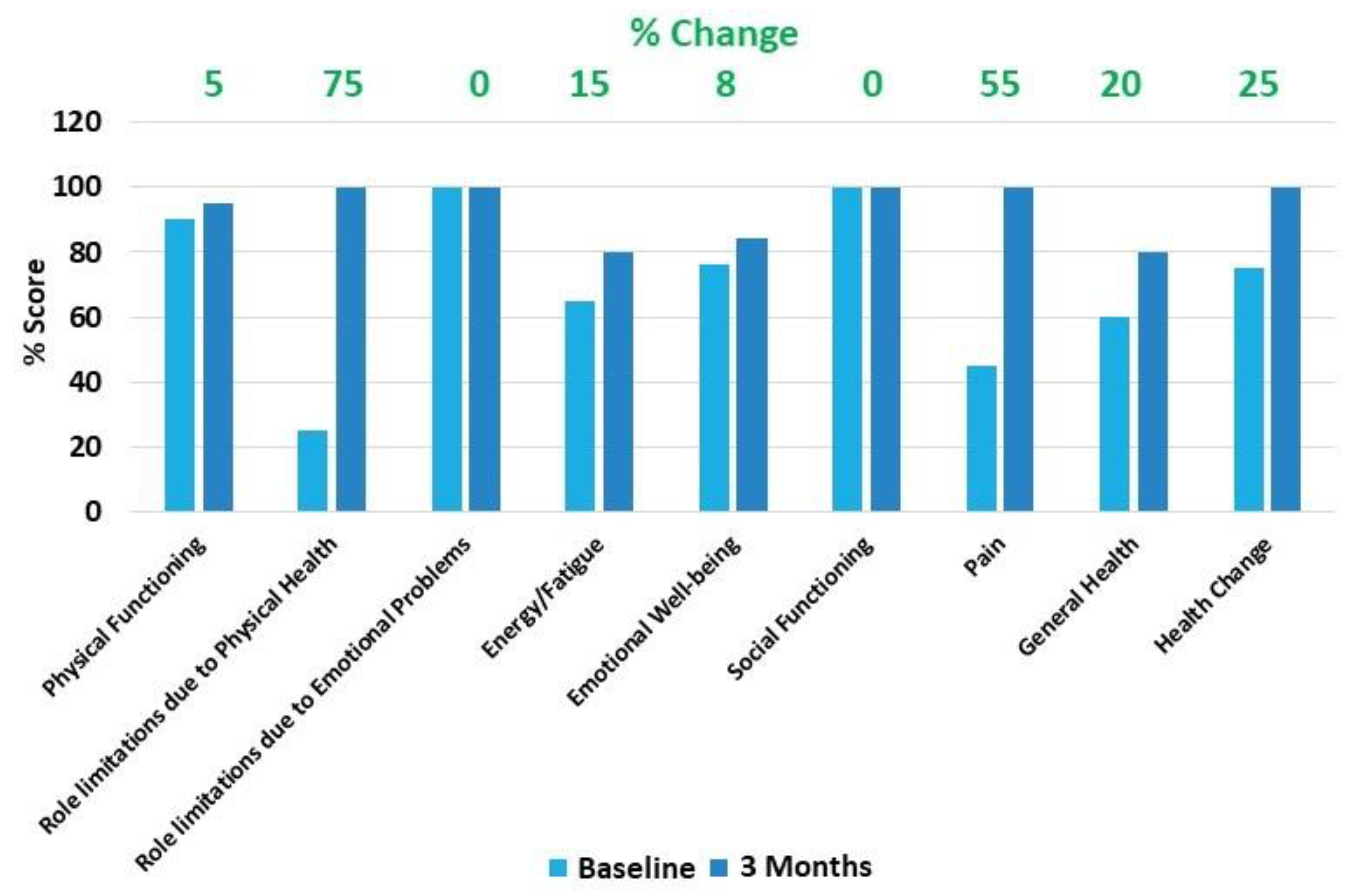
2.2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cisternas, M.G.; Murphy, L.; Sacks, J.J.; Solomon, D.H.; Pasta, D.J.; Helmick, C.G. Alternative Methods for Defining Osteoarthritis and the Impact on Estimating Prevalence in a US Population-Based Survey. Arthritis Care Res. 2016, 68, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losina, E.; Thornhill, T.S.; Rome, B.N.; Wright, J.; Katz, J.N. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by brought in population size and the obesity epidemic. J. Bone Joint Surg. Am. 2012, 94, 201–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; El-Amin, S.F., 3rd; Levy, H.J.; Sze-Tu, R.; Ibim, S.E.; Maffulli, N. Umbilical cord-derived Wharton’s jelly for regenerative medicine applications. J. Orthop. Surg. Res. 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, H.S.; Kim, H.A. Chondrocyte Apoptosis in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Hopman, W.M.; Harrison, M.B.; Coo, H.; Friedberg, E.; Buchanan, M.; VenDenKerkhof, E.G. Associations between chronic disease, age and physical and mental health status. Chronic Dis. Can. 2009, 29, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassel, S.; Muschter, D. Recent advances in the treatment of osteoarthritis. F1000Res 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.m.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Bedard, N.A.; DeMik, D.E.; Glass, N.A.; Burnett, R.A.; Bozic, K.J.; Callaghan, J.J. Impact of Clinical Practice Guidelines on Use of Intra-Articular Hyaluronic Acid and Corticosteroid Injections for Knee Osteoarthritis. J. Bone Joint Surg. Am. 2018, 100, 827–834. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Wallace, S.; Luu, H.H.; Shi, L.L.; Lee, M.J.; Chen, A.F. Do We Need to Wait 3 Months After Corticosteroid Injections to Reduce the Risk of Infection After Total Knee Arthroplasty? J. Am. Acad. Orthop. Surg. 2021, 29, e714–e721. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Cady, C.; Fauser, A.M.; Rodriguez, H.C.; Mistovich, R.J.; Potty, A.G.R.; Maffulli, N. Cell-free Stem Cell-Derived Extract Formulation for Regenerative Medicine Applications. Int. J. Mol. Sci. 2020, 21, 9364. [Google Scholar] [CrossRef] [PubMed]
- Fabre, H.; Ducret, M.; Degoul, O.; Rodriguez, J.; Perrier-Groult, E.; Aubert-Foucher, E.; Pasdeloup, M.; Auxenfans, C.; McGuckin, C.; Forraz, N.; et al. Characterization of Different Sources of Human MSCs Expanded in Serum-Free Conditions with Quantification of Chondrogenic Induction in 3D. Stem Cells Int. 2019, 2019, 2186728. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.J.; Prinsen, C.A.; Christensen, R.; Bartels, E.M.; Terwee, C.B.; Roos, E.M. Knee Injury and Osteoarthritis Outcome Score (KOOS): Systematic review and meta-analysis of measurement properties. Osteoarthr. Cartilage 2016, 24, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolognese, J.A.; Schnitzer, T.J.; Ehrich, E.W. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthr. Cartil. 2003, 11, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Autologous Protein Solution Injection for the Treatment of Knee Osteoarthritis: 3-Year Results. Am. J. Sports Med. 2020, 48, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Lee, C.E.; Levy, H.J.; El-Amin, S.F., 3rd. Umbilical cord-derived Wharton’s jelly for treatment of knee osteoarthritis: Study protocol for a non-randomized, open-label, multi-center trial. J. Orthop. Surg. Res. 2021, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Carson, E.W.; Bascharon, R.A.; Delfino, K.; Levy, H.J.; El-Amin, S.F., 3rd. Safety and efficacy of umbilical cord-derived Wharton’s Jelly compared to hyaluronic acid and saline for knee osteoarthritis: Study protocol for a randomized, controlled, single-blind, multi-center trial. J. Orthop. Surg. Res. 2021, 16, 352. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Rodriguez, H.C.; Potty, A.G.; Levy, H.J.; El-Amin III, S.F. Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report. Pharmaceuticals 2021, 14, 883. https://doi.org/10.3390/ph14090883
Gupta A, Rodriguez HC, Potty AG, Levy HJ, El-Amin III SF. Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report. Pharmaceuticals. 2021; 14(9):883. https://doi.org/10.3390/ph14090883
Chicago/Turabian StyleGupta, Ashim, Hugo C. Rodriguez, Anish G. Potty, Howard J. Levy, and Saadiq F. El-Amin III. 2021. "Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report" Pharmaceuticals 14, no. 9: 883. https://doi.org/10.3390/ph14090883
APA StyleGupta, A., Rodriguez, H. C., Potty, A. G., Levy, H. J., & El-Amin III, S. F. (2021). Treatment of Knee Osteoarthritis with Intraarticular Umbilical Cord-Derived Wharton’s Jelly: A Case Report. Pharmaceuticals, 14(9), 883. https://doi.org/10.3390/ph14090883







