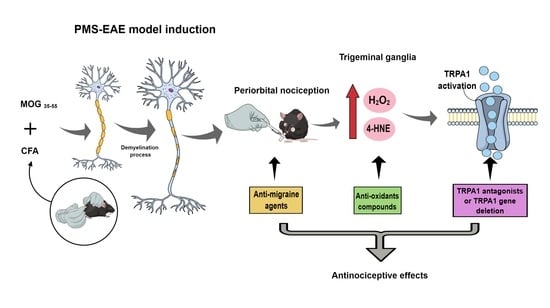
Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation
Abstract
:1. Introduction
2. Results
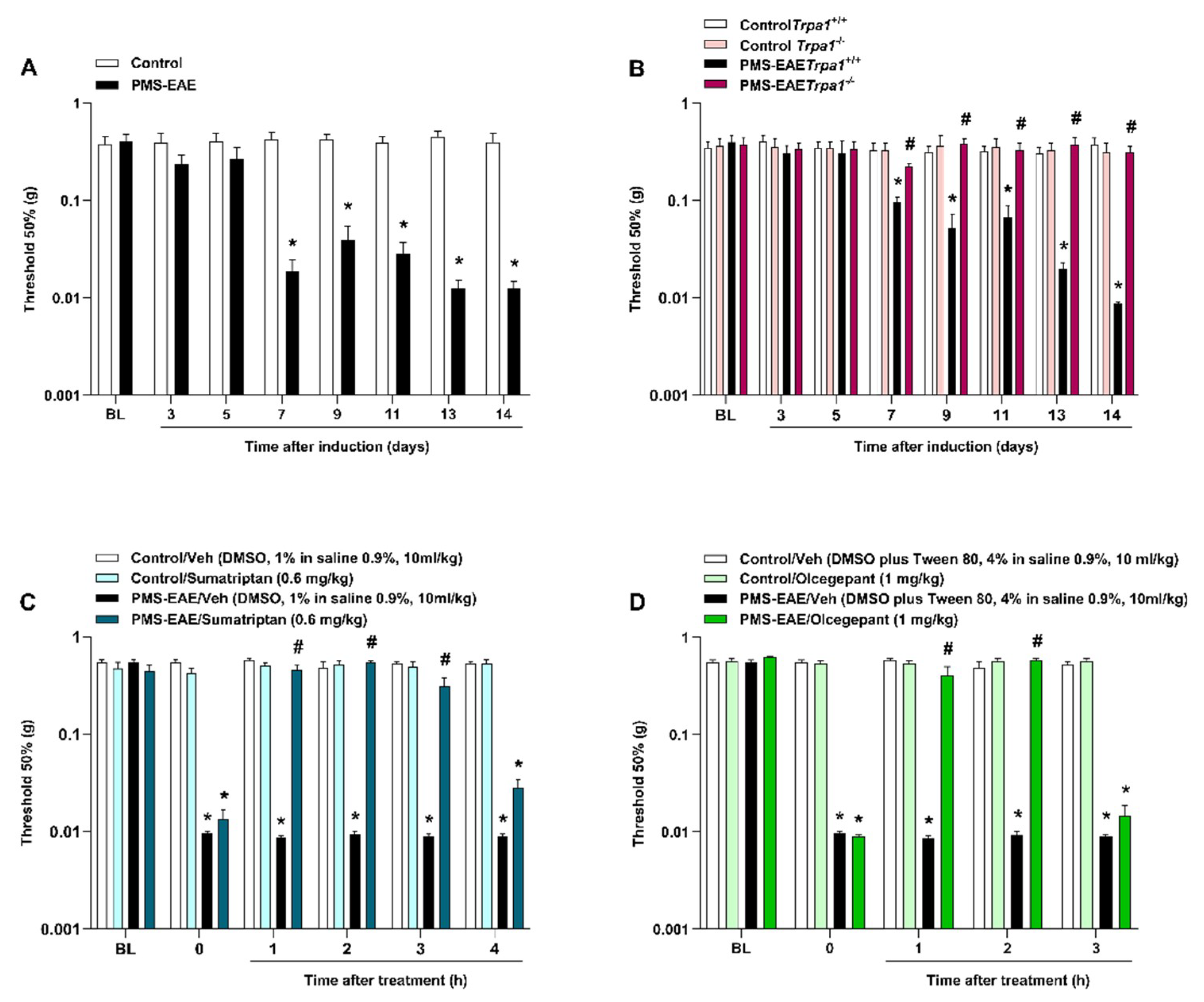
2.1. PMS-EAE Caused PMA Development, but Trpa1−/− Mice Induced to PMS-EAE Did Not Develop PMA and Treatment with Antimigraine Agents Reduced Periorbital Nociception
2.2. TRPA1 Antagonists Attenuated PMA in PMS-EAE-Induced Mice
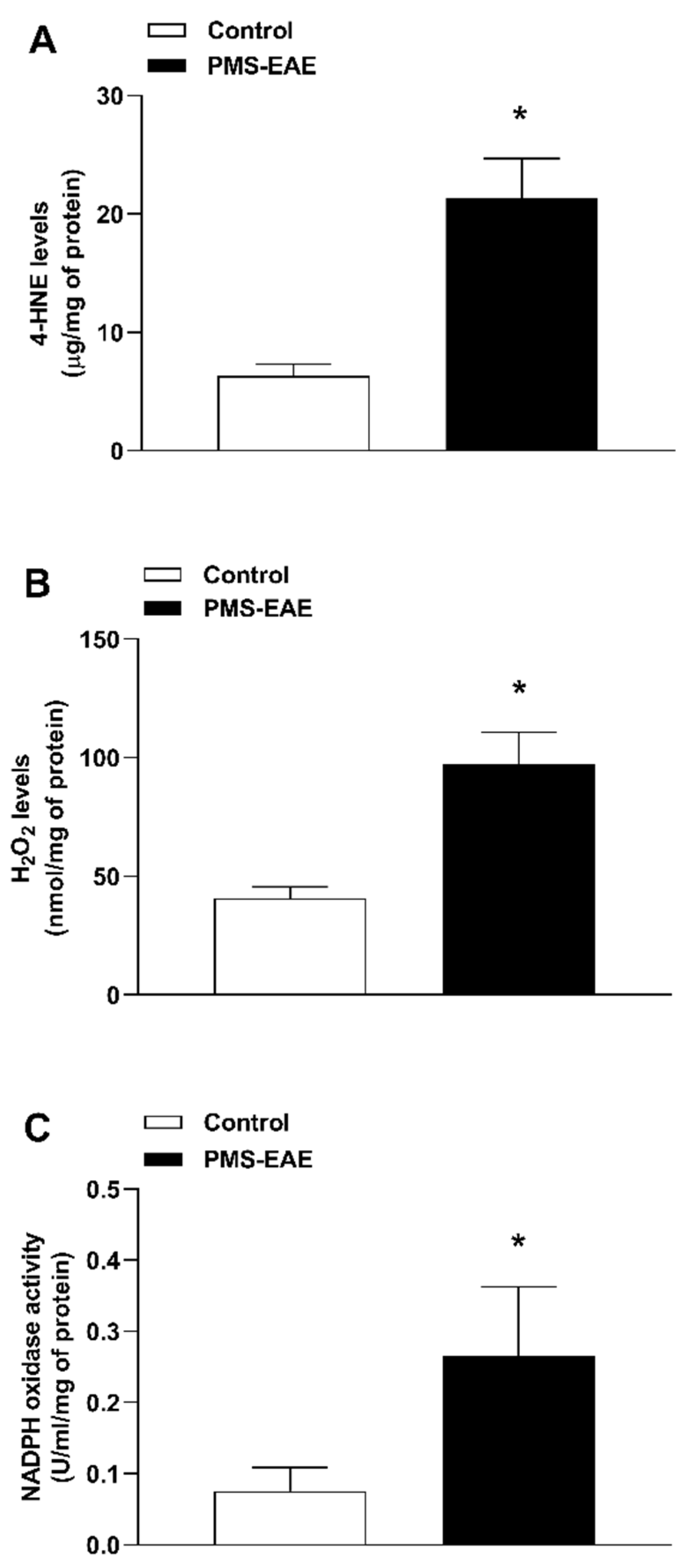
2.3. TRPA1 Endogenous Agonist Levels and NADPH Oxidase Activity Were Increased in PMS-EAE Induced Mice
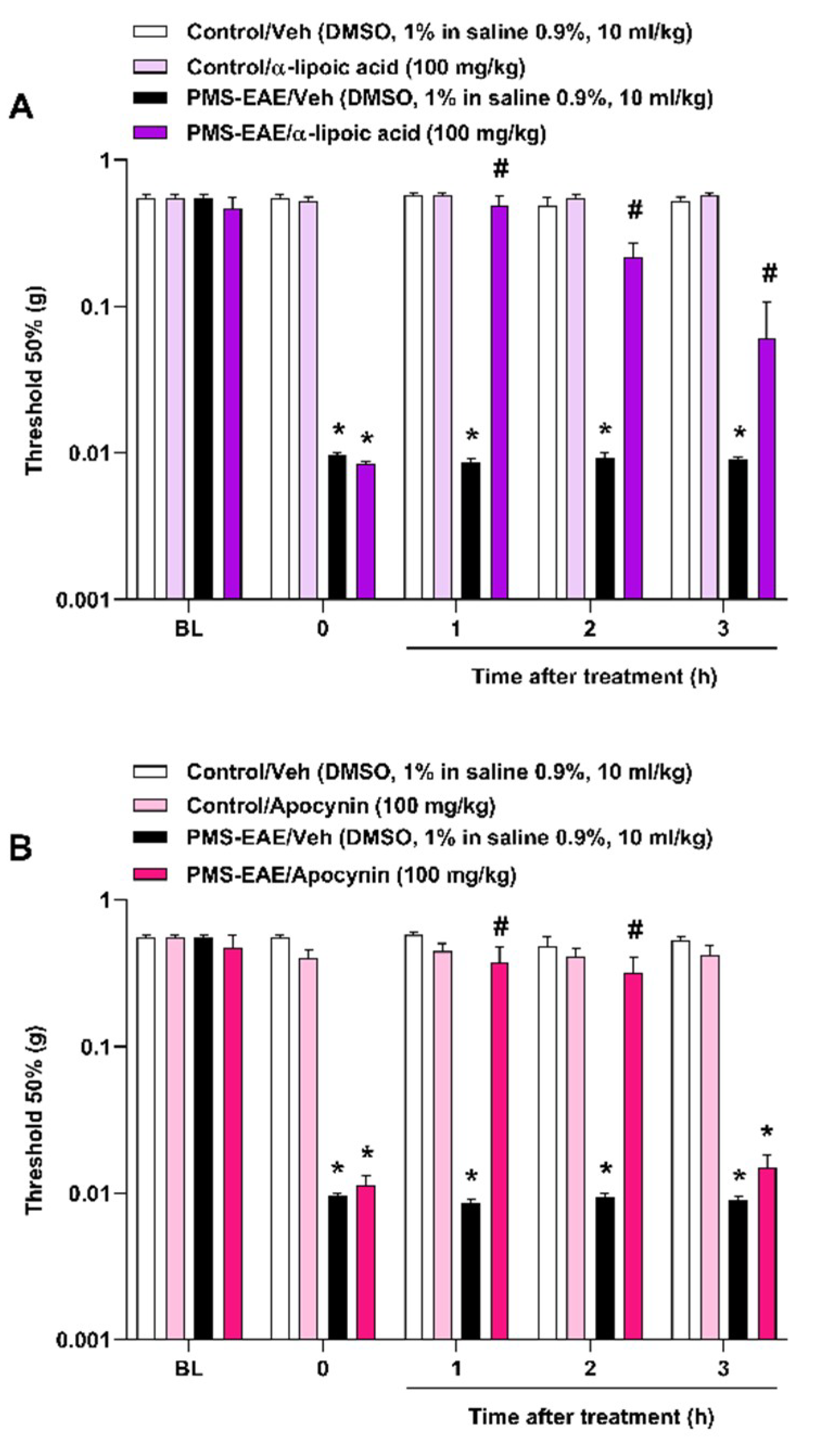
2.4. The Intragastric Treatment of α-Lipoic Acid and Apocynin Attenuated PMA in the PMS-EAE in Induced Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. PMS-Experimental Autoimmune Encephalomyelitis (PMS-EAE) Model Induction
4.4. Assessment of Clinical Signs of PMS-EAE Model
4.5. Periorbital Mechanical Threshold Evaluation
4.6. Treatment Protocols
4.7. Assessment of Oxidative Stress Parameters
4.7.1. TRPA1 Agonist (4-HNE and H2O2) Levels Measurement
4.7.2. Assessment of the Activity of NADPH Oxidase
4.8. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassini, R.; Materazzi, S.; Benemei, S.; Geppetti, P. The TRPA1 Channel in Inflammatory and Neuropathic Pain and Migraine. In Reviews of Physiology, Biochemistry and Pharmacology; Springer-Verlag: Berlin, Germany; New York, NY, USA, 2014; Volume 167, pp. 1–43. [Google Scholar]
- Morgan, C.R.; Bird, E.V.; Robinson, P.P.; Boissonade, F.M. TRPA1 expression in human lingual nerve neuromas in patients with and without symptoms of dysaesthesia. Neurosci. Lett. 2009, 465, 189–193. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Benemei, S.; Patacchini, R.; Trevisani, M.; Geppetti, P. TRP channels. Curr. Opin. Pharmacol. 2015, 22, 18–23. [Google Scholar] [CrossRef]
- Vincent, F.; AJ Duncton, M. TRPV4 Agonists and Antagonists. Curr. Top. Med. Chem. 2011, 11, 2216–2226. [Google Scholar] [CrossRef]
- Chen, Y.; Kanju, P.; Fang, Q.; Lee, S.H.; Parekh, P.K.; Lee, W.; Moore, C.; Brenner, D.; Gereau, R.W.; Wang, F.; et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014, 155, 2662–2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, D.A.; Gentry, C.; Moss, S.; Bevan, S. Transient Receptor Potential A1 Is a Sensory Receptor for Multiple Products of Oxidative Stress. J. Neurosci. 2008, 28, 2485–2494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marone, I.M.; De Logu, F.; Nassini, R.; De Carvalho Goncalves, M.; Benemei, S.; Ferreira, J.; Jain, P.; Puma, S.L.; Bunnett, N.W.; Geppetti, P.; et al. TRPA1/NOX in the soma of trigeminal ganglion neurons mediates migraine-related pain of glyceryl trinitrate in mice. Brain 2018, 141, 2312–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisan, G.; Benemei, S.; Materazzi, S.; De Logu, F.; De Siena, G.; Fusi, C.; Rossato, M.F.; Coppi, E.; Marone, M.; Ferreira, J.; et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes / macrophages and oxidative stress. Brain 2016, 139, 1361–1377. [Google Scholar] [CrossRef] [Green Version]
- Edelmayer, R.M.; Le, L.N.; Yan, J.; Wei, X.; Nassini, R.; Materazzi, S.; Preti, D.; Appendino, G.; Geppetti, P.; Dodick, D.W.; et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012, 153, 1949–1958. [Google Scholar] [CrossRef] [Green Version]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [Green Version]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The “headache tree” via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135, 376–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eberhardt, M.; Dux, M.; Namer, B.; Miljkovic, J.; Cordasic, N.; Will, C.; Kichko, T.I.; De La Roche, J.; Fischer, M.; Suárez, S.A.; et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat. Commun. 2014, 5, 4381. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, T.; Dublin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One 2009, 4, e7596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S.; Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 2008, 27, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Grant, A.D. Vascular Actions of Calcitonin Gene-Related Peptide and Adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef] [Green Version]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies—Successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Hargreaves, R.; Olesen, J. Calcitonin Gene-Related Peptide Modulators—The History and Renaissance of a New Migraine Drug Class. Headache 2019, 59, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Villalón, C.M.; Maassenvandenbrink, A. Basic mechanisms of migraine and its acute treatment. Pharmacol. Ther. 2012, 136, 319–333. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Hargreaves, R.J. Mechanisms of action of serotonin 5-HT1B/D agonists: Insights into migraine pathophysiology using rizatriptan. Neurology 2000, 55, S8–S14. [Google Scholar] [PubMed]
- Hassler, S.N.; Ahmad, F.B.; Burgos-Vega, C.C.; Boitano, S.; Vagner, J.; Price, T.J.; Dussor, G. Protease activated receptor 2 (PAR2) activation causes migraine-like pain behaviors in mice. Cephalalgia 2019, 39, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Vega, C.C.; Ahn, D.D.-U.; Bischoff, C.; Wang, W.; Horne, D.; Wang, J.; Gavva, N.; Dussor, G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2016, 36, 185–193. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Landini, L.; Janal, M.N.; Puma, S.L.; Cesaris, F. De Migraine-provoking substances evoke periorbital allodynia in mice. J. Headache Pain 2019, 1, 1–9. [Google Scholar]
- Koldbro, A.; Kumar, D.; Olesen, J.; Jørn, L.; Jansen-olesen, I. Pharmacological Reports Effect of TRPA1 activator allyl isothiocyanate ( AITC ) on rat dural and pial arteries. Pharmacol. Rep. 2019, 71, 565–572. [Google Scholar] [CrossRef]
- Roberto, G.; Raschi, E.; Piccinni, C.; Conti, V.; Vignatelli, L.; D’Alessandro, R.; De Ponti, F.; Poluzzi, E. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: Systematic review of observational studies. Cephalalgia 2015, 35, 118–131. [Google Scholar] [CrossRef]
- Dodick, D.W.; Martin, V. Triptans and CNS side-effects: Pharmacokinetic and metabolic mechanisms. Cephalalgia 2004, 24, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kaniecki, R.G. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother. 2009;9:1379-1391: Comments. Headache 2010, 50, 333. [Google Scholar] [CrossRef]
- Borkum, J.M. CGRP and Brain Functioning: Cautions for Migraine Treatment. Headache 2019, 59, 1339–1357. [Google Scholar] [CrossRef]
- O’Connor, A.B.; Schwid, S.R.; Herrmann, D.N.; Markman, J.D.; Dworkin, R.H. Pain associated with multiple sclerosis: Systematic review and proposed classification. Pain 2008, 137, 96–111. [Google Scholar] [CrossRef]
- Thompson, A.J.; Toosy, A.T.; Ciccarelli, O. Pharmacological management of symptoms in multiple sclerosis: Current approaches and future directions. Lancet. Neurol. 2010, 9, 1182–1199. [Google Scholar] [CrossRef]
- Khan, N.; Smith, M.T. Multiple sclerosis-induced neuropathic pain: Pharmacological management and pathophysiological insights from rodent EAE models. Inflammopharmacology 2014, 22, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solaro, C.; Trabucco, E.; Messmer Uccelli, M. Pain and multiple sclerosis: Pathophysiology and treatment topical collection on demyelinating disorders. Curr. Neurol. Neurosci. Rep. 2013, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Adamian, L.; Fiocchi, J.; Hoyt, D.; Ernst, C.; Kaye, A.D.; Viswanath, O. Advances in the Understanding and Management of Chronic Pain in Multiple Sclerosis: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 1–11. [Google Scholar] [CrossRef]
- Elmazny, A.; Hamdy, S.M.; Abdel-Naseer, M.; Shalaby, N.M.; Shehata, H.S.; Kishk, N.A.; Nada, M.A.; Mourad, H.S.; Hegazy, M.I.; Abdelalim, A.; et al. Interferon-beta-induced headache in patients with multiple sclerosis: Frequency and characterization. J. Pain Res. 2020, 13, 537–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Mantia, L.; Mantia, L. Headache and multiple sclerosis: Clinical and therapeutic correlations. Neurol. Sci. 2009, 30, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.; Pardo, G.; Rabadi, M. Headache and Its Management in Patients With Multiple Sclerosis. Curr. Treat. Opt. Neurol. 2018, 20, 10. [Google Scholar] [CrossRef]
- Thorburn, K.; Paylor, J.; Webber, C.; Winship, I.; Kerr, B. Facial hypersensitivity and trigeminal pathology in mice with experimental autoimmune encephalomyelitis. Pain 2016, 157, 627–642. [Google Scholar] [CrossRef]
- Duffy, S.S.; Perera, C.J.; Makker, P.G.S.; Lees, J.G.; Carrive, P.; Moalem-Taylor, G. Peripheral and central neuroinflammatory changes and pain behaviors in an animal model of multiple sclerosis. Front. Immunol. 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Lyons, D.N.; Kniffin, T.C.; Zhang, L.P.; Danaher, R.J.; Miller, C.S.; Bocanegra, J.L.; Carlson, C.R.; Westlund, K.N. Trigeminal Inflammatory Compression (TIC) injury induces chronic facial pain and susceptibility to anxiety-related behaviors. Neuroscience 2015, 295, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Krzyzanowska, A.; Pittolo, S.; Cabrerizo, M.; Sánchez-López, J.; Krishnasamy, S.; Venero, C.; Avendaño, C. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J. Neurosci. Methods 2011, 201, 46–54. [Google Scholar] [CrossRef]
- Avona, A.; Burgos-Vega, C.; Burton, M.D.; Akopian, A.N.; Price, T.J.; Dussor, G. Dural Calcitonin Gene-Related Peptide Produces Female-Specific Responses in Rodent Migraine Models. J. Neurosci. 2019, 39, 4323–4331. [Google Scholar] [CrossRef] [Green Version]
- Dalenogare, D.P.; Theisen, M.C.; Peres, D.S.; Fialho, M.F.P.; Lückemeyer, D.D.; de David Antoniazzi, C.T.; Kudsi, S.Q.; de Amorim Ferreira, M.; dos Santos Ritter, C.; Ferreira, J.; et al. TRPA1 activation mediates nociception behaviors in a mouse model of relapsing-remitting experimental autoimmune encephalomyelitis. Exp. Neurol. 2020, 328, 113241. [Google Scholar] [CrossRef]
- Ritter, C.; Dalenogare, D.P.; de Almeida, A.S.; Pereira, V.L.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.; Kudsi, S.Q.; Ferreira, J.; et al. Nociception in a Progressive Multiple Sclerosis Model in Mice Is Dependent on Spinal TRPA1 Channel Activation. Mol. Neurobiol. 2020, 57, 2420–2435. [Google Scholar] [CrossRef] [PubMed]
- Foley, P.L.; Vesterinen, H.M.; Laird, B.J.; Sena, E.S.; Colvin, L.A.; Chandran, S.; MacLeod, M.R.; Fallon, M.T. Prevalence and natural history of pain in adults with multiple sclerosis: Systematic review and meta-analysis. Pain 2013, 154, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Ouchchane, L.; Guy, N.; Bayle, D.J.; Dallel, R.; Clavelou, P. Migraine headaches and pain with neuropathic characteristics: Comorbid conditions in patients with multiple sclerosis. Pain 2013, 154, 2691–2699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Mantia, L.; Prone, V. Headache in multiple sclerosis and autoimmune disorders. Neurol. Sci. 2015, 36 (Suppl. 1), 75–78. [Google Scholar] [CrossRef]
- Diener, H.C.; Holle, D.; Solbach, K.; Gaul, C. Medication-overuse headache: Risk factors, pathophysiology and management. Nat. Rev. Neurol. 2016, 12, 575–583. [Google Scholar] [CrossRef]
- Vuralli, D.; Wattiez, A.-S.; Russo, A.F.; Bolay, H. Behavioral and cognitive animal models in headache research. J. Headache Pain 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Lionetto, L.; Negro, A.; Casolla, B.; Simmaco, M.; Martelletti, P. Sumatriptan succinate: Pharmacokinetics of different formulations in clinical practice. Expert Opin. Pharmacother. 2012, 13, 2369–2380. [Google Scholar] [CrossRef]
- Scott, A. Sumatriptan clinical pharmacokinetics. Clin. Pharmacokinet. 1994, 27, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kopruszinski, C.M.; Navratilova, E.; Swiokla, J.; Dodick, D.W.; Chessell, I.P.; Porreca, F. A novel, injury-free rodent model of vulnerability for assessment of acute and preventive therapies reveals temporal contributions of CGRP-receptor activation in migraine-like pain. Cephalalgia 2021, 41, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.-C.; Charles, A.; Goadsby, P.J.; Holle, D. New therapeutic approaches for the prevention and treatment of migraine. Lancet. Neurol. 2015, 14, 1010–1022. [Google Scholar] [CrossRef]
- Xu, F.; Sun, W. Network Meta-Analysis of Calcitonin Gene-Related Peptide Receptor Antagonists for the Acute Treatment of Migraine. Front. Pharmacol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negro, A.; Martelletti, P. Gepants for the treatment of migraine. Expert Opin. Investig. Drugs 2019, 28, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Iovino, M.; Feifel, U.; Yong, C.; Wolters, J.; Wallenstein, G. Safety, tolerability and pharmacokinetics of BIBN 4096 BS, the first selective small molecule calcitonin gene-related peptide receptor antagonist, following single intravenous administration in healthy volunteers. Cephalalgia 2004, 24, 645–656. [Google Scholar] [CrossRef]
- Burgos-Vega, C.C.; Quigley, L.D.; Trevisan, G.; Yan, F.; Asiedu, M.; Jacobs, B.; Motina, M.; Safdar, N.; Yousuf, H.; Avona, A.; et al. Non-invasive dural stimulation in mice: A novel preclinical model of migraine. Cephalalgia 2019, 39, 123–134. [Google Scholar] [CrossRef]
- Nassini, R.; Fusi, C.; Materazzi, S.; Coppi, E.; Tuccinardi, T.; Marone, I.M.; De Logu, F.; Preti, D.; Tonello, R.; Chiarugi, A.; et al. The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. Br. J. Pharmacol. 2015, 172, 3397. [Google Scholar] [CrossRef]
- Hearn, L.; Derry, S.; Moore, R. Single dose dipyrone (metamizole) for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2016, 4, CD011421. [Google Scholar] [CrossRef]
- Brune, K. Next generation of everyday analgesics. Am. J. Ther. 2002, 9, 215–223. [Google Scholar] [CrossRef]
- Ramacciotti, A.S.; Soares, B.G.O.; Atallah, A.N. Dipyrone for acute primary headaches. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef] [Green Version]
- Vallano, A.; Malouf, J.; Payrulet, P.; Baños, J. Analgesic use and pain in the hospital settings. Eur. J. Clin. Pharmacol. 2007, 63, 619–626. [Google Scholar] [CrossRef]
- Levy, M.; Zylber-Katz, E.; Rosenkranz, B. Clinical Pharmacokinetics of Dipyrone and its Metabolites. Clin. Pharmacokinet. 1995, 28, 216–234. [Google Scholar] [CrossRef]
- Volz, M.; Kellner, H. Kinetics and metabolism of pyrazolones (propyphenazone, aminopyrine and dipyrone). Br. J. Clin. Pharmacol. 1980, 10, 299S–308S. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, D.; Grubb, B.D.; Wang, M. ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J. Headache Pain 2019, 20, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, A.S.; Rigo, F.K.; De Prá, S.D.-T.T.; Milioli, A.M.; Pereira, G.C.; Lückemeyer, D.D.; Antoniazzi, C.T.D.D.; Kudsi, S.Q.; de Araújo, D.S.M.; de Oliveira, S.M.; et al. Role of transient receptor potential ankyrin 1 (TRPA1) on nociception caused by a murine model of breast carcinoma. Pharmacol. Res. 2020, 152, 104576. [Google Scholar] [CrossRef]
- Sághy, É.; Sipos, É.; Ács, P.; Bölcskei, K.; Pohóczky, K.; Kemény, Á.; Sándor, Z.; Szőke, É.; Sétáló, G.; Komoly, S.; et al. TRPA1 deficiency is protective in cuprizone-induced demyelination???A new target against oligodendrocyte apoptosis. Glia 2016, 64, 2166–2180. [Google Scholar] [CrossRef] [PubMed]
- Bölcskei, K.; Kriszta, G.; Sághy, É.; Payrits, M.; Sipos, É.; Vranesics, A.; Berente, Z.; Ábrahám, H.; Ács, P.; Komoly, S.; et al. Behavioural alterations and morphological changes are attenuated by the lack of TRPA1 receptors in the cuprizone-induced demyelination model in mice. J. Neuroimmunol. 2018, 320, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [Green Version]
- Brône, B.; Peeters, P.; Marrannes, R.; Mercken, M.; Nuydens, R.; Meert, T.; Gijsen, H. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol. Appl. Pharmacol. 2008, 231, 150–156. [Google Scholar] [CrossRef]
- Andrè, E.; Campi, B.; Materazzi, S.; Trevisani, M.; Amadesi, S.; Massi, D.; Creminon, C.; Vaksman, N.; Nassini, R.; Civelli, M.; et al. Cigarette smoke–induced neurogenic inflammation is mediated by α,β-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 2008, 118, 2574–2582. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessac, B.F.; Sivula, M.; Hehn, C.A.; Escalera, J.; Cohn, L.; Jordt, S.E. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 2008, 118, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Hinman, A.; Chuang, H.-H.; Bautista, D.M.; Julius, D. TRP channel activation by reversible covalent modification. Proc. Natl. Acad. Sci. USA 2006, 103, 19564–19568. [Google Scholar] [CrossRef] [Green Version]
- Wetzels, S.; Vanmierlo, T.; Scheijen, J.; van Horssen, J.; Amor, S.; Somers, V.; Schalkwijk, C.; Hendriks, J.; Wouters, K. Methylglyoxal-Derived Advanced Glycation Endproducts Accumulate in Multiple Sclerosis Lesions. Front. Immunol. 2019, 10, 855. [Google Scholar] [CrossRef]
- Tully, M.; Tang, J.; Zheng, L.; Acosta, G.; Tian, R.; Hayward, L.; Race, N.; Mattson, D.; Shi, R.; Brilot, F.; et al. Systemic Acrolein Elevations in Mice With Experimental Autoimmune Encephalomyelitis and Patients With Multiple Sclerosis. Front. Neurol. 2018, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Shi, R. New insights in the pathogenesis of multiple sclerosis-role of acrolein in neuronal and myelin damage. Int. J. Mol. Sci. 2013, 14, 20037–20047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tully, M.; Zheng, L.; Shi, R. Acrolein detection: Potential theranostic utility in multiple sclerosis and spinal cord injury. Expert Rev. Neurother. 2014, 14, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.; Sun, W.; Zheng, L.; Brookes, S.; Tully, M.; Shi, R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune encephalomyelitis mouse. Neuroscience 2011, 173, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Thorlund, K.; Toor, K.; Wu, P.; Chan, K.; Druyts, E.; Ramos, E.; Bhambri, R.; Donnet, A.; Stark, R.; Goadsby, P.J. Comparative tolerability of treatments for acute migraine: A network meta-analysis. Cephalalgia 2017, 37, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.A.; Gentry, C.; Light, E.; Vastani, N.; Vallortigara, J.; Bierhaus, A.; Fleming, T.; Bevan, S. Methylglyoxal Evokes Pain by Stimulating TRPA1. PLoS ONE 2013, 8, e77986. [Google Scholar] [CrossRef]
- Park, J.; Zheng, L.; Acosta, G.; Vega-Alvarez, S.; Chen, Z.; Muratori, B.; Cao, P.; Shi, R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J. Neurochem. 2015, 135, 987–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, D. Migraine pain, meningeal inflammation, and mast cells. Curr. Pain Headache Rep. 2009, 13, 237–240. [Google Scholar] [CrossRef]
- Möhrke, J.; Kropp, P.; Zettl, U.K. Headaches in Multiple Sclerosis Patients Might Imply an Inflammatorial Process. PLoS ONE 2013, 8, 69570. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Araya, I.; JM, T.; AR, B.; JG, C. Contribution of intraganglionic CGRP to migraine-like responses in male and female rats. Cephalalgia 2020, 40, 689–700. [Google Scholar] [CrossRef]
- Afroz, S.; Arakaki, R.; Iwasa, T.; Oshima, M.; Hosoki, M.; Inoue, M.; Baba, O.; Okayama, Y.; Matsuka, Y. CGRP Induces Differential Regulation of Cytokines from Satellite Glial Cells in Trigeminal Ganglia and Orofacial Nociception. Int. J. Mol. Sci. 2019, 20, 711. [Google Scholar] [CrossRef] [Green Version]
- Loy, B.D.; Fling, B.W.; Horak, F.B.; Bourdette, D.N.; Spain, R.I. Effects of lipoic acid on walking performance, gait, and balance in secondary progressive multiple sclerosis. Complement. Ther. Med. 2018, 41, 169–174. [Google Scholar] [CrossRef]
- Waslo, C.; Bourdette, D.; Gray, N.; Wright, K.; Spain, R. Lipoic Acid and Other Antioxidants as Therapies for Multiple Sclerosis. Curr. Treat. Opt. Neurol. 2019, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; De Prá, S.D.T.; de David Antoniazzi, C.T.; Kudsi, S.Q.; Ferro, P.R.; Landini, L.; Rigo, F.K.; de Bem Silveira, G.; Silveira, P.C.L.; Oliveira, S.M.; et al. Macrophages and Schwann cell TRPA1 mediate chronic allodynia in a mouse model of complex regional pain syndrome type I. Brain. Behav. Immun. 2020, 88, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mcgrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.S.; Woolf, C.J.; Corey, D.P. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olechowski, C.J.; Parmar, A.; Miller, B.; Stephan, J.; Tenorio, G.; Tran, K.; Leighton, J.; Kerr, B.J. A diminished response to formalin stimulation reveals a role for the glutamate transporters in the altered pain sensitivity of mice with experimental autoimmune encephalomyelitis (EAE). Pain 2010, 149, 565–572. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Dutra, R.C.; Bento, A.F.; Leite, D.F.P.; Manjavachi, M.N.; Marcon, R.; Bicca, M.A.; Pesquero, J.B.; Calixto, J.B. The role of kinin B1 and B2 receptors in the persistent pain induced by experimental autoimmune encephalomyelitis (EAE) in mice: Evidence for the involvement of astrocytes. Neurobiol. Dis. 2013, 54, 82–93. [Google Scholar] [CrossRef]
- Olechowski, C.J.; Truong, J.J.; Kerr, B.J. Neuropathic pain behaviours in a chronic-relapsing model of experimental autoimmune encephalomyelitis (EAE). Pain 2009, 141, 156–164. [Google Scholar] [CrossRef]
- Olechowski, C.J.; Tenorio, G.; Sauve, Y.; Kerr, B.J. Changes in nociceptive sensitivity and object recognition in experimental autoimmune encephalomyelitis (EAE). Exp. Neurol. 2013, 241, 113–121. [Google Scholar] [CrossRef]
- Dixon, W.J. Efficient Analysis of Experimental Observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Marmura, M.J.; Silberstein, S.D.; Schwedt, T.J. The acute treatment of migraine in adults: The american headache society evidence assessment of migraine pharmacotherapies. Headache 2015, 55, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.J. Acute migraine treatment in adults. Headache 2015, 55, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- da Silva Brum, E.; Becker, G.; Fialho, M.F.P.; Casoti, R.; Trevisan, G.; Oliveira, S.M. TRPA1 involvement in analgesia induced by Tabernaemontana catharinensis ethyl acetate fraction in mice. Phytomedicine 2019, 54, 248–258. [Google Scholar] [CrossRef]
- Leary, S.C.; Hill, B.C.; Lyons, C.N.; Carlson, C.G.; Michaud, D.; Kraft, C.S.; Ko, K.; Glerum, D.M.; Moyes, C.D. Chronic Treatment with Azide in Situ Leads to an Irreversible Loss of Cytochrome c Oxidase Activity via Holoenzyme Dissociation. J. Biol. Chem. 2002, 277, 11321–11328. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalenogare, D.P.; Ritter, C.; Bellinaso, F.R.A.; Kudsi, S.Q.; Pereira, G.C.; Fialho, M.F.P.; Lückemeyer, D.D.; Antoniazzi, C.T.d.D.; Landini, L.; Ferreira, J.; et al. Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation. Pharmaceuticals 2021, 14, 831. https://doi.org/10.3390/ph14080831
Dalenogare DP, Ritter C, Bellinaso FRA, Kudsi SQ, Pereira GC, Fialho MFP, Lückemeyer DD, Antoniazzi CTdD, Landini L, Ferreira J, et al. Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation. Pharmaceuticals. 2021; 14(8):831. https://doi.org/10.3390/ph14080831
Chicago/Turabian StyleDalenogare, Diéssica Padilha, Camila Ritter, Fernando Roberto Antunes Bellinaso, Sabrina Qader Kudsi, Gabriele Cheiran Pereira, Maria Fernanda Pessano Fialho, Débora Denardin Lückemeyer, Caren Tatiane de David Antoniazzi, Lorenzo Landini, Juliano Ferreira, and et al. 2021. "Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation" Pharmaceuticals 14, no. 8: 831. https://doi.org/10.3390/ph14080831
APA StyleDalenogare, D. P., Ritter, C., Bellinaso, F. R. A., Kudsi, S. Q., Pereira, G. C., Fialho, M. F. P., Lückemeyer, D. D., Antoniazzi, C. T. d. D., Landini, L., Ferreira, J., Bochi, G. V., Oliveira, S. M., De Logu, F., Nassini, R., Geppetti, P., & Trevisan, G. (2021). Periorbital Nociception in a Progressive Multiple Sclerosis Mouse Model Is Dependent on TRPA1 Channel Activation. Pharmaceuticals, 14(8), 831. https://doi.org/10.3390/ph14080831