Extracorporeal Photopheresis in Children with Chronic Graft-Versus-Host Disease
Abstract
1. Introduction
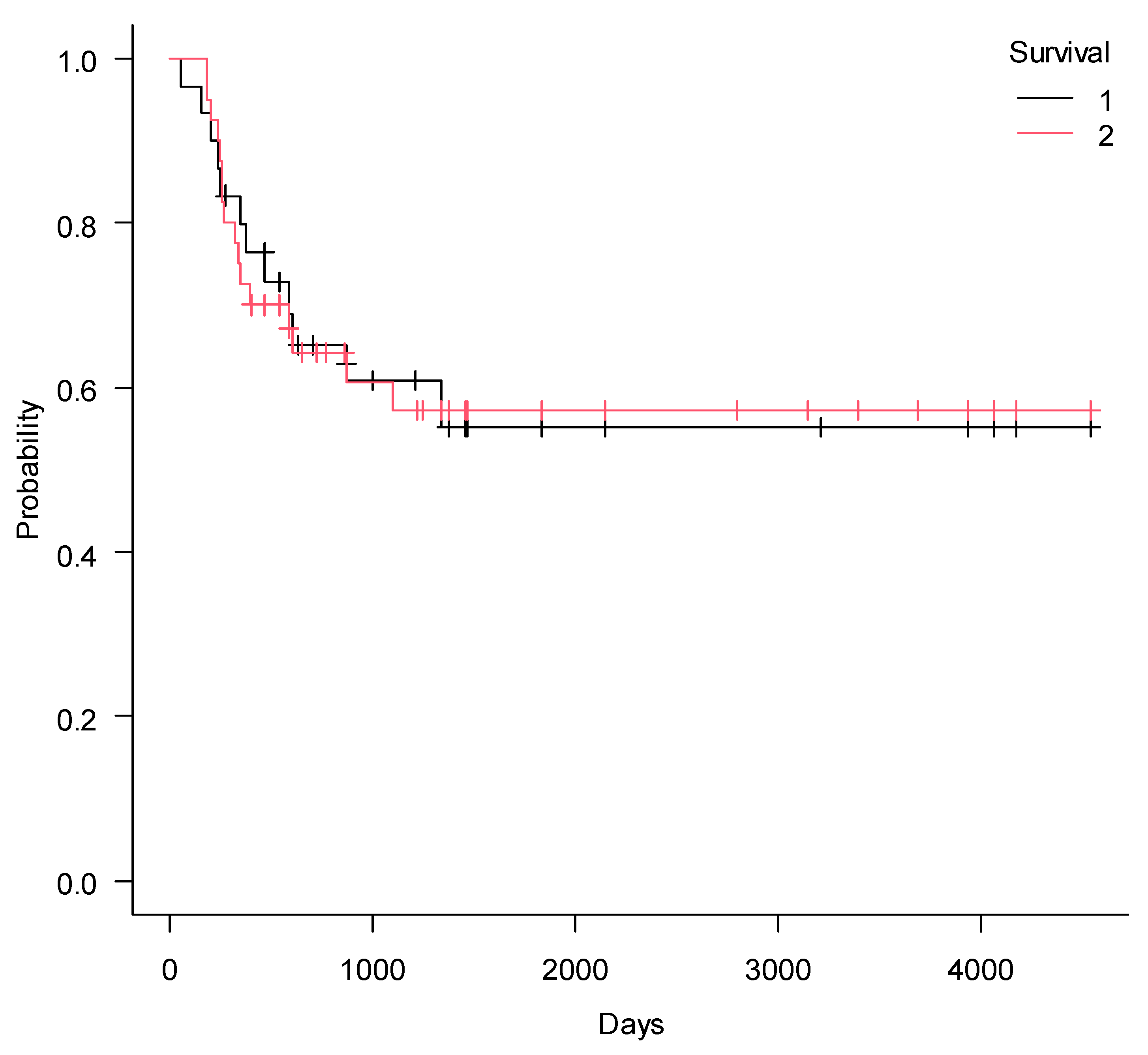
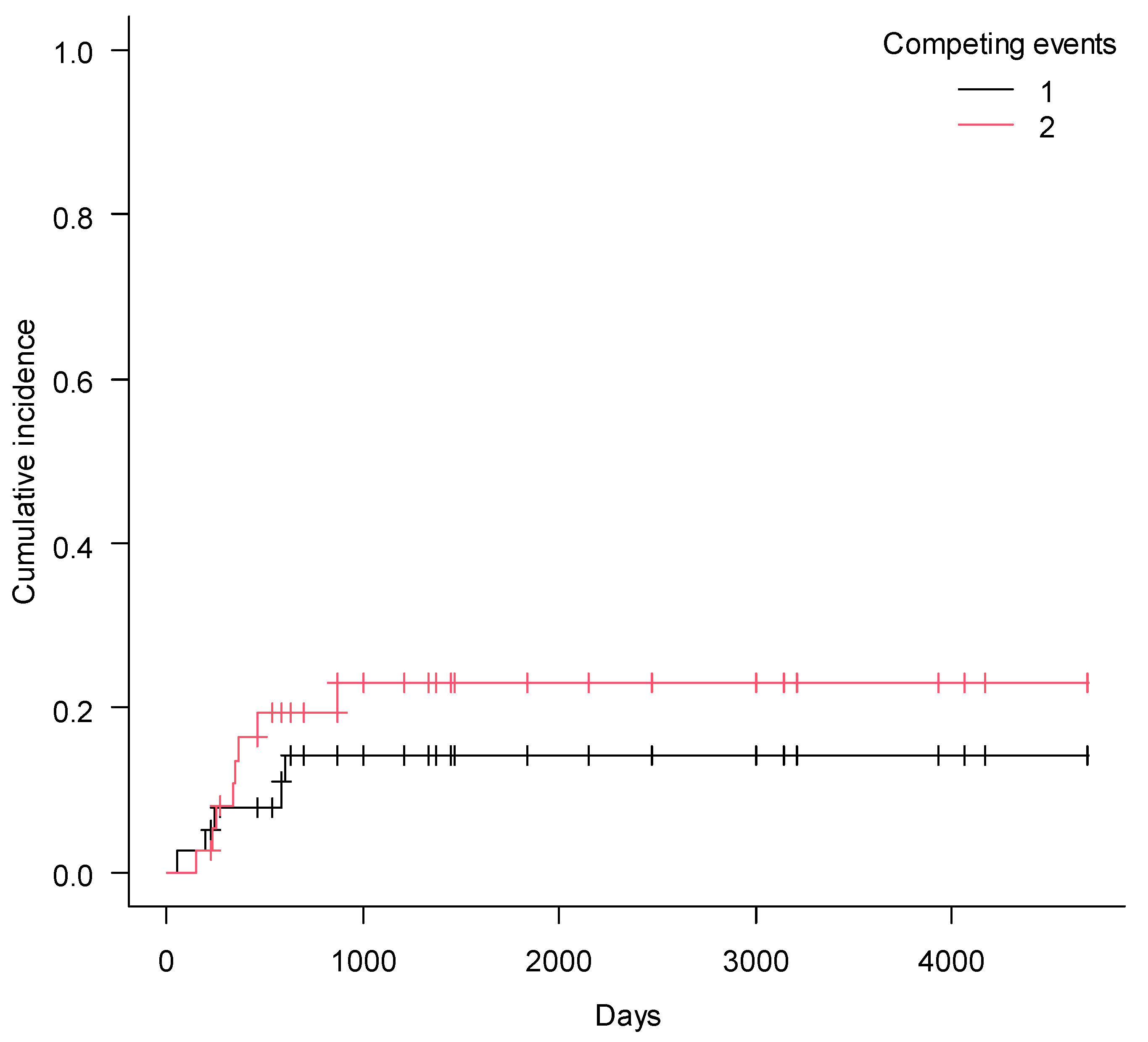
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inagaki, J.; Moritake, H.; Nishikawa, T.; Hyakuna, N.; Okada, M.; Suenobu, S.-I.; Nagai, K.; Honda, Y.; Shimomura, M.; Fukano, R.; et al. Long-Term Morbidity and Mortality in Children with Chronic Graft-versus-Host Disease Classified by National Institutes of Health Consensus Criteria after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2015, 21, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; O’Donnell, P.V.; Fuchs, E.J. Post-Transplantation Cyclophosphamide for Tolerance Induction in HLA-Haploidentical Bone Marrow Transplantation. Semin. Oncol. 2012, 39, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Pirogova, O.V.; Moiseev, I.S.; Alyansky, A.L.; Babenko, E.V.; Darskaya, E.I.; Slesarchuk, O.A.; Bondarenko, S.N.; Afanasyev, B.V. Risk-adapted graft-versus host disease prophylaxis with post-transplantation cyclophosphamide in related, unrelated and haploidentical stem cell transplantations. Cell. Ther. Transplant. 2016, 5, 54–56. [Google Scholar] [CrossRef][Green Version]
- Modi, B.; Hernandez-Henderson, M.; Yang, D.; Klein, J.; Dadwal, S.; Kopp, E.; Huelsman, K.; Mokhtari, S.; Ali, H.; Al Malki, M.M.; et al. Ruxolitinib as Salvage Therapy for Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.; Perales, M.-A.; Schroeder, M.A.; Ali, H.; Shah, N.N.; Chen, Y.-B.; Fazal, S.; Dawkins, F.W.; Arbushites, M.C.; Tian, C.; et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): A multicenter, open-label phase 2 trial. Blood 2020, 135, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.; Levine, J.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Kolb, H.-J. Graft versus host disease: From experiments to clinical insight. Cell. Ther. Transplant. 2010, 2, 3. [Google Scholar]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; Brink, M.R.V.D.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Heshmati, F. Updating ECP action mechanisms. Transfus. Apher. Sci. 2014, 50, 330–339. [Google Scholar] [CrossRef]
- Besnier, D.P.; Chabannes, D.; Mahe, B.; Mussini, J.M. Treatment of graft-versus-host disease by extracorpo-real photochemotherapy: A pilot study. Transplantation 1997, 64, 49–54. [Google Scholar] [CrossRef]
- Knobler, R.; Arenberger, P.; Arun, A.; Assaf, C.; Bagot, M.; Berlin, G.; Bohbot, A.; Calzavara-Pinton, P.; Child, F.; Cho, A.; et al. European dermatology forum—Updated guidelines on the use of extracorporeal photopheresis 2020—Part 1. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2693–2716. [Google Scholar] [CrossRef]
- Flowers, M.E.D.; Apperley, J.F.; van Besien, K.; Elmaagacli, A.; Grigg, A.; Reddy, V.; Bacigalupo, A.; Kolb, H.-J.; Bouzas, L.; Michallet, M.; et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood 2008, 112, 2667–2674. [Google Scholar] [CrossRef]
- Jagasia, M.; Scheid, C.; Socié, G.; Ayuk, F.A.; Tischer, J.; Donato, M.L.; Bátai, Á.; Chen, H.; Chen, S.-C.; Chin, T.; et al. Randomized controlled study of ECP with methoxsalen as first-line treatment of patients with moderate to severe cGVHD. Blood Adv. 2019, 3, 2218–2229. [Google Scholar] [CrossRef]
- McKenna, K.E.; Whittaker, S.; Rhodes, L.; Taylor, P.; Lloyd, J.; Ibbotson, S.; Russell-Jones, R. Evidence-based practice of photopheresis 1987–2001: A report of a workshop of the British Photodermatology Group and the U.K. Skin Lymphoma Group. Br. J. Dermatol. 2005, 154, 7–20. [Google Scholar] [CrossRef]
- Sarantopoulos, S.; Cardones, A.R.; Sullivan, K.M. How I treat refractory chronic graft-versus-host disease. Blood 2019, 133, 1191–1200. [Google Scholar] [CrossRef]
- Jagasia, M.H.; Greinix, H.T.; Arora, M.; Williams, K.M.; Wolff, D.; Cowen, E.W.; Palmer, J.; Weisdorf, D.; Treister, N.S.; Cheng, G.-S.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 389–401.e1. [Google Scholar] [CrossRef]
- Waller, E.K.; Miklos, D.; Cutler, C.; Arora, M.; Jagasia, M.H.; Pusic, I.; Flowers, M.E.; Logan, A.C.; Nakamura, R.; Chang, S.; et al. Ibrutinib for Chronic Graft-versus-Host Disease After Failure of Prior Therapy: 1-Year Update of a Phase 1b/2 Study. Biol. Blood Marrow Transplant. 2019, 25, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Maas-Bauer, K.; Kiote-Schmidt, C.; Bertz, H.; Apostolova, P.; Wäsch, R.; Ihorst, G.; Finke, J.; Zeiser, R. Ruxolitinib–ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021, 56, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Greinix, H.T.; Worel, N.; Just, U.; Knobler, R. Extracorporeal photopheresis in acute and chronic graft-versus-host disease. Transfus. Apher. Sci. 2014, 50, 349–357. [Google Scholar] [CrossRef]
- Jacobsohn, D.A.; Arora, M.; John, P.K.; Hassebroek, A.; Flowers, M.E.; Cutler, C.S.; Ur-bano-Ispizua, A.; Bolwell, B.J.; Antin, J.H.; Boyiadzis, M.; et al. Risk factors associated with increased nonrelapse mortality and with poor overall sur-vival in children with chronic graft-versus-host disease. Blood 2011, 118, 4472–4479. [Google Scholar] [CrossRef]
- Perotti, C.; Del Fante, C.; Tinelli, C.; Viarengo, G.; Scudeller, L.; Zecca, M.; Locatelli, F.; Salvaneschi, L. Extracorporeal photochemotherapy in graft-versus-host disease: A longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion 2010, 50, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, G.; Labopin, M.; Beohou, E.; Dalissier, A.; Dalle, J.H.; Cornish, J.; Zecca, M.; Samarasinghe, S.; Gibson, B.; Locatelli, F.; et al. Impact of Conditioning Regimen on Outcomes for Children with Acute Myeloid Leukemia Undergoing Transplantation in First Complete Remission. An Analysis on Behalf of the Pediatric Disease Working Party of the European Group for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 467–474. [Google Scholar] [CrossRef]
- Paina, O.V.; Kozhokar, P.V.; Borovkova, A.S.; Frolova, A.S.; Ekushov, K.A.; Bykova, T.A.; Rakhmanova, Z.Z.; Galas, M.A.; Khabirova, A.G.; Markova, I.V.; et al. Ten year expierence of allogeneic haploaidentical hematopoietic stem cell transplantation with non-manipulated grafts in children and adolescents with high risk acute leukemia. Cell. Ther. Transplant. 2018, 7, 20–27. [Google Scholar] [CrossRef][Green Version]
- Beynarovich, A.V.; Babenko, E.V.; Moiseev, I.S.; Paina, O.V.; Pirogova, O.V.; Rudakova, T.A.; Gindina, T.L.; Darskaya, E.I.; Morozova, E.V.; Bondarenko, S.N.; et al. Haploidentical stem cell transplantation in adults for the treatement of hematological diseases: Results of a single center (CIC 725). Cell. Ther. Transplant. 2019, 8, 26–35. [Google Scholar] [CrossRef]
- Apisarnthanarax, N.; Donato, M.L.; Korbling, M.; Couriel, D.R.; Gajewski, J.L.; Giralt, S.; Khouri, I.F.; Hosing, C.; E Champlin, R.; Duvic, M.; et al. Extracorporeal photopheresis therapy in the management of steroid-refractory or steroid-dependent cutaneous chronic graft-versus-host disease after allogeneic stem cell transplantation: Feasibility and results. Bone Marrow Transplant. 2003, 31, 459–465. [Google Scholar] [CrossRef]
- Flinn, A.M.; Macheka, S.; Slatter, M.; Ewins, A.; Gibson, B.; Lawson, S.; Tailby, A.; Lucchini, G.; New, H.; James, B.; et al. A survey of extracorporeal photopheresis treatment in pediatric patients in the United Kingdom. eJHaem 2020, 1, 293–296. [Google Scholar] [CrossRef]
- Buense, R.; Duarte, I.A.G.; Bouer, M. Localized scleroderma: Assessment of the therapeutic response to phototherapy. An. Bras. Dermatol. 2012, 87, 63–69. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, R.A.; Schwartz, J.; Schneiderman, J. Extracorporeal photopheresis in pediatric patients: Practical and technical considerations. J. Clin. Apher. 2017, 32, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Gaczkowski, A.; Scharffetter-Kochanek, K.; Borberg, H. Small-scale extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma: A report of 3 cases. Transfus. Apher. Sci. 2005, 32, 197–203. [Google Scholar] [CrossRef]
- Messina, C.; Locatelli, F.; Lanino, E.; Uderzo, C.; Zacchello, G.; Cesaro, S.; Pillon, M.; Perotti, C.; Del Fante, C.; Faraci, M.; et al. Extracorporeal photochemotherapy for paediatric patients with graft-versus-host disease after haematopoietic stem cell transplantation. Br. J. Haematol. 2003, 122, 118–127. [Google Scholar] [CrossRef]
- Heshmati, F Mechanisms of action of extracorporeal photochemotherapy. Transfus. Apher. Sci. 2003, 29, 61–70. [CrossRef]
- Lee, S.J.; Wolff, D.; Kitko, C.; Koreth, J.; Inamoto, Y.; Jagasia, M.; Pidala, J.; Olivieri, A.; Martin, P.J.; Przepiorka, D.; et al. Measuring Therapeutic Response in Chronic Graft-versus-Host Disease. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IV. The 2014 Response Criteria Working Group Report. Biol. Blood Marrow Transplant. 2015, 21, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
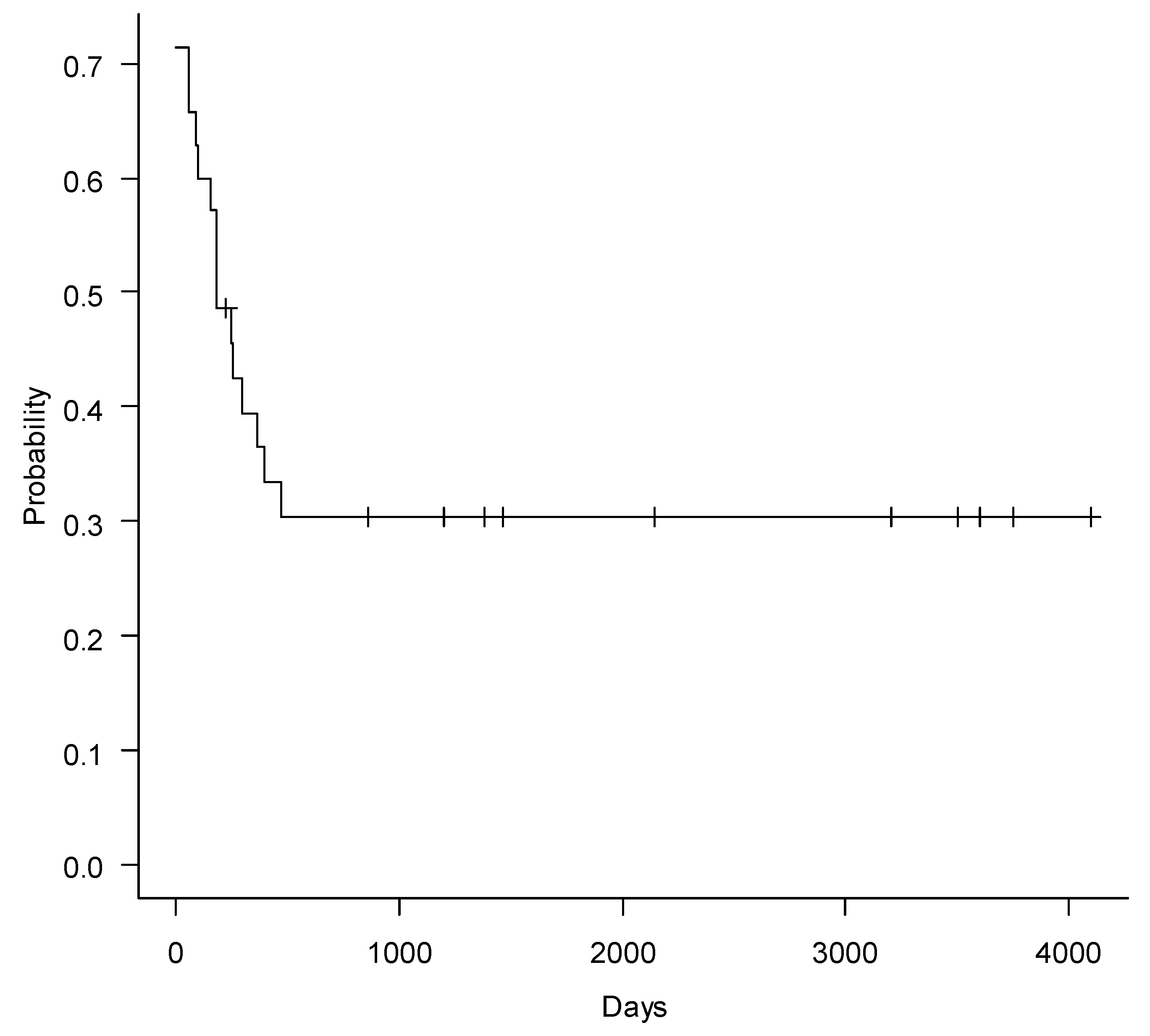
| № | Age | Diagnosis | cGVHD Severity | Affected Organs | Steroids Prior ECP, mg/kg/day | Number of ECP Procedures | Response to ECP | Steroids Post ECP, mg/kg/day | Concomitant IST | Requirement for IST Escalation after ECP | Status (Cause) | Follow-Up, Days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | MDS | severe | skin, mucous membranes | 0.5 | 8 | CR | 0.1 | no | no | alive | 1466 |
| 2 | 5 | AML | moderate | skin | 0.4 | 5 | CR | 0 | CsA | no | alive | 4541 |
| 3 | 16 | ALL | moderate | skin, lungs | 0 | 12 | PR | 0 | CsA | no | alive | 4064 |
| 4 | 10 | ALL | severe | skin | 1 | 7 | progression | 1 | Tx | yes | dead (ALL) | 180 |
| 5 | 10 | ALL | moderate | skin | 0.5 | 4 | progression | 2 | ruxolitinib | yes | alive | 721 |
| 6 | 18 | ALL | severe | skin, mucous membranes | 0.3 | 4 | PR | 0.3 | ibrutinib | no | alive | 463 |
| 7 | 10 | BAL | moderate | skin, mucous membranes, lungs | 0.5 | 8 | PR | 0.2 | no | no | dead (ALL) | 1097 |
| 8 | 13 | ALL | moderate | joints, gut | 0.6 | 6 | PR | 0.25 | CsA | no | alive | 774 |
| 9 | 5 | AML | moderate | mucous membranes | 0.5 | PR | 0.5 | no | no | alive | 256 | |
| 10 | 13 | BAL | severe | liver | 0.3 | 10 | PR | 0 | sirolimus | no | dead (BAL) | 396 |
| 11 | 8 | CML | severe | mucous membranes | 1 | 24 | PR | 0.25 | imatinib | no | alive | 3929 |
| 12 | 12 | AML | severe | skin, liver, lungs | 0.7 | 20 | CR | 0.1 | imatinib | yes | alive | 1834 |
| 13 | 1 | ALL | severe | skin, lungs | 0.25 | 2 | PR | 0.1 | CsA | no | alive | 3394 |
| 14 | 13 | ALL | moderate | skin, liver | 0.4 | 2 | PR | 0.4 | ruxolitinib | no | dead (ALL) | 352 |
| 15 | 3 | Hurler | mild | skin, mucous membranes | 0.5 | 6 | PR | 0.2 | ruxolitinib | yes | alive | 864 |
| 16 | 5 | AML | moderate | skin, gut | 0.5 | 26 | PR | 0.3 | imatinib | no | alive | 1217 |
| 17 | 16 | AML | severe | skin, mucous membranes, liver, lungs | 0.4 | 33 | unchanged | 0.75 | rituximab | yes | dead (cGVHD) | 604 |
| 18 | 18 | AML | severe | mucous membranes, liver | 0.6 | 12 | PR | 0.25 | Tx | no | alive | 3683 |
| 19 | 13 | ALL | mild | skin, mucous membranes | 1 | 5 | PR | 0.5 | sirolimus | yes | dead (cGVHD) | 201 |
| 20 | 16 | AML | mild | skin, liver | 0.8 | 28 | PR | 0 | Tx | no | alive | 1379 |
| 21 | 4 | Fanconi | severe | skin, gut | 0.5 | 6 | PR | 0.25 | Tx | no | alive | 3140 |
| 22 | 11 | ALL | severe | skin | 0.4 | 24 | CR | 0.1 | ruxolitinib | no | alive | 1334 |
| 23 | 14 | Fanconi | severe | skin, joints, lungs | 0.6 | 12 | PR | 0.4 | ruxolitinib | no | dead (Fanconi) | 259 |
| 24 | 1 | AML | severe | skin, mucous membranes, liver | 0.25 | 10 | PR | 0.1 | ruxolitinib | no | alive | 1453 |
| 25 | 8 | ALL | moderate | skin, gut, liver | 0.7 | 18 | PR | 0.25 | Tx, mmf | no | dead (ALL) | 182 |
| 26 | 15 | AML | severe | skin, mucous membranes, lungs | 0.4 | 2 | progression | 0.5 | Tx | yes | dead (cGVHD) | 249 |
| 27 | 7 | ALL | moderate | mucous membranes | 0.6 | 37 | PR | 0.8 | sirolimus | yes | alive | 648 |
| 28 | 6 | ALL | severe | skin, mucous membranes, lungs | 0 | 35 | unchanged | 0 | rituximab | yes | dead (cGVHD) | 587 |
| 29 | 6 | ALL | severe | skin, mucous membranes, gut, liver | 0 | 40 | PR | 0 | imatinib | yes | alive | 1246 |
| 30 | 3 | BAL | moderate | skin, mucous membranes | 0.4 | 4 | CR | 0 | Tx | no | alive | 4167 |
| 31 | 17 | BAL | severe | mucous membranes | 0.3 | 10 | progression | 0 | imatinib | yes | dead (BAL) | 325 |
| 32 | 13 | ALL | moderate | mucous membranes | 0.5 | 12 | PR | 0.25 | sirolimus | no | alive | 342 |
| 33 | 15 | ALL | moderate | skin | 1 | 34 | CR | 0.1 | no | alive | 400 | |
| 34 | 7 | JMML | moderate | skin | 0.25 | 5 | CR | 0.1 | Tx | no | alive | 2800 |
| 35 | 16 | HL | severe | mucous membranes | 0.4 | 14 | PR | 0.25 | ruxolitinib | yes | alive | 590 |
| 36 | 17 | ALL | severe | mucous membranes | 0.7 | 5 | progression | 0.7 | CsA | yes | dead (ALL) | 252 |
| 37 | 16 | CML | severe | skin, joints | 0.3 | 34 | PR | 0.3 | CsA | yes | alive | 3568 |
| 38 | 17 | HL | severe | skin, mucous membranes, lungs | 0.5 | 46 | unchanged | 0.5 | ruxolitinib | yes | alive | 536 |
| 39 | 9 | JMLL | moderate | skin, mucous membranes | 0.4 | 42 | PR | 0.25 | sirolimus | no | alive | 2146 |
| 40 | 16 | AML | severe | skin, mucous membranes | 0 | 10 | progression | 0 | imatinib, Tx | yes | dead (AML) | 870 |
| 41 | 18 | ALL | severe | skin | 0.4 | 8 | progression | 0 | Tx, mmf | yes | dead (ALL) | 270 |
| 42 | 17 | AML | severe | skin, liver | 0.5 | 8 | progression | 0.75 | no | yes | dead (cGVHD) | 239 |
| Efficiency of ECP in Children with cGVHD | |||||||
|---|---|---|---|---|---|---|---|
| Response | Skin (n = 32) | Mucous Membrane (n = 22) | Lungs (n = 9) | Liver (n = 10) | Gut (n = 5) | Joints (n = 3) | Overall Response (n = 42) |
| Complete response, n (%) | 7 (22%) | 2 (9%) | 1 (11%) | 2 (20%) | N/A | N/A | 7 (17%) |
| Partial response, n (%) | 17 (53%) | 14 (64%) | 1 (11%) | 6 (60%) | 4 (80%) | 2 (67%) | 24 (57%) |
| Unchanged, n (%) | 4 (12.5%) | 3 (13.5%) | 6 (67%) | 1 (10%) | 1 (20%) | 1 (33%) | 3 (7%) |
| Progression, n (%) | 4 (12.5%) | 3 (13.5%) | 1 (11%) | 1 (10%) | N/A | N/A | 8 (19%) |
| Variable | HR | 95%CI | p |
|---|---|---|---|
| Response to ECP | 7.8 | 2.7–23.1 | 0.0002 |
| Sex | 2.3 | 0.6–8.7 | 0.2 |
| Disease risk group (HR vs. SR) | 1.964 | 9–0.4 | 0.4 |
| Time interval from HSCT to ECP | 0.4 | 0.1–1.5 | 0.2 |
| Ruxolitinib− vs. Ruxolitinib+ | 1.7 | 0.5–6.9 | 0.4 |
| ECP monotherapy vs. ECP in combination therapy | 0.6 | 0.2–2.4 | 0.5 |
| cGVHD severity (severe vs. moderate) | 2.656 | 0.8–8.3 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, A.; Estrina, M.; Paina, O.; Bykova, T.; Osipova, A.; Kozhokar, P.; Rakhmanova, Z.; Solodova, I.; Morozova, E.; Alyansky, A.; et al. Extracorporeal Photopheresis in Children with Chronic Graft-Versus-Host Disease. Pharmaceuticals 2021, 14, 808. https://doi.org/10.3390/ph14080808
Kozlov A, Estrina M, Paina O, Bykova T, Osipova A, Kozhokar P, Rakhmanova Z, Solodova I, Morozova E, Alyansky A, et al. Extracorporeal Photopheresis in Children with Chronic Graft-Versus-Host Disease. Pharmaceuticals. 2021; 14(8):808. https://doi.org/10.3390/ph14080808
Chicago/Turabian StyleKozlov, Andrey, Maria Estrina, Olesia Paina, Tatiana Bykova, Anna Osipova, Polina Kozhokar, Zhemal Rakhmanova, Irina Solodova, Elena Morozova, Alexander Alyansky, and et al. 2021. "Extracorporeal Photopheresis in Children with Chronic Graft-Versus-Host Disease" Pharmaceuticals 14, no. 8: 808. https://doi.org/10.3390/ph14080808
APA StyleKozlov, A., Estrina, M., Paina, O., Bykova, T., Osipova, A., Kozhokar, P., Rakhmanova, Z., Solodova, I., Morozova, E., Alyansky, A., Kulagina, I., Gevorgian, A., Dotsenko, A., Moiseev, I., Chukhlovin, A., Kulagin, A., Bondarenko, S., Semenova, E., & Zubarovskaya, L. (2021). Extracorporeal Photopheresis in Children with Chronic Graft-Versus-Host Disease. Pharmaceuticals, 14(8), 808. https://doi.org/10.3390/ph14080808






