Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus
Abstract
:1. Introduction
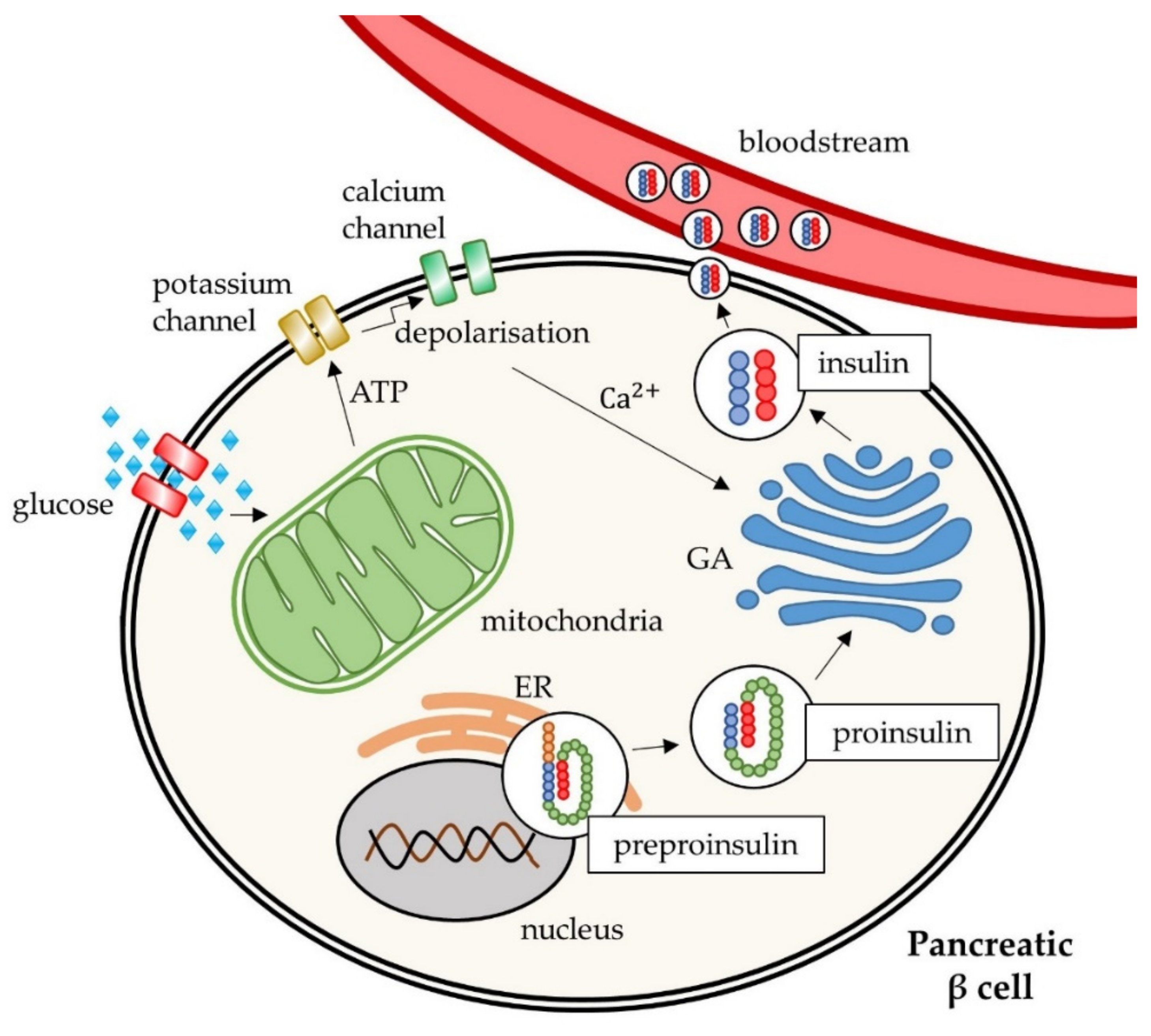
2. Molecular Mechanisms of Type 2 Diabetes Mellitus
3. Treatment of Type 2 Diabetes Mellitus
3.1. Pharmaceutical Drugs for the Treatment of Type 2 Diabetes Mellitus
3.1.1. Insulin Sensitisers
Biguanides
Thiazolidinediones
3.1.2. Insulin Secretagogues
Sulphonylureas
Meglitinides
3.1.3. Alpha-Glucosidase Inhibitors
3.1.4. Incretin-Based Therapies
Glucagon-like Peptide-1 Receptor Agonists
Dipeptidyl Peptidase-4 Inhibitors
3.1.5. SGLT2 Inhibitors
3.2. Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus
3.2.1. Polyphenols
Resveratrol
Curcumin
Tannins
Lignans
3.2.2. Flavonoids
Anthocyanins
Epigallocatechin Gallate
Quercetin
Naringin
Rutin
Kaempferol
3.2.3. Plant Fruits, Vegetables and Other Products
Garlic
Green Tea
Blackcurrant
Rowanberry
Bilberry
Strawberry
Cornelian Cherry
Olive Oil
Sesame Oil
Carrot
3.3. Combination Therapy
3.3.1. Biguanides with Natural Products
3.3.2. Thiazolidinediones with Natural Products
3.3.3. Sulphonylureas with Natural Products
3.3.4. Meglitinides with Natural Products
3.3.5. Alpha-Glucosidase Inhibitors with Natural Products
3.3.6. Incretin-Based Therapies with Natural Products
3.3.7. SGLT2 Inhibitors with Natural Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milibari, A.A.; Matuure, E.Y.; Gadah, E.M. Prevalence, Determinants and Prevention of Type 2 Diabetes Mellitus (T2DM) in Arabic Countries: A Systematic Review Study. Health Sci. J. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [Green Version]
- Sesti, G.; Federici, M.; Lauro, D.; Sbraccia, P.; Lauro, R. Molecular Mechanism of Insulin Resistance in Type 2 Diabetes Mellitus: Role of the Insulin Receptor Variant Forms. Diabetes Metab. Res. Rev. 2001, 17, 363–373. [Google Scholar] [CrossRef]
- Deshmukh, C.; Jain, A.; Nahata, B. Diabetes Mellitus: A Review. Int. J. Pure Appl. Biosci. 2015, 3, 224–230. [Google Scholar]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Corb Aron, R.A.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Sugden, M.; Holness, M. Pathophysiology of Diabetic Dyslipidemia:Implications for Atherogenesis and Treatment. Clin. Lipidol. 2011, 6, 401–411. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primer 2015, 1, 15019. [Google Scholar] [CrossRef]
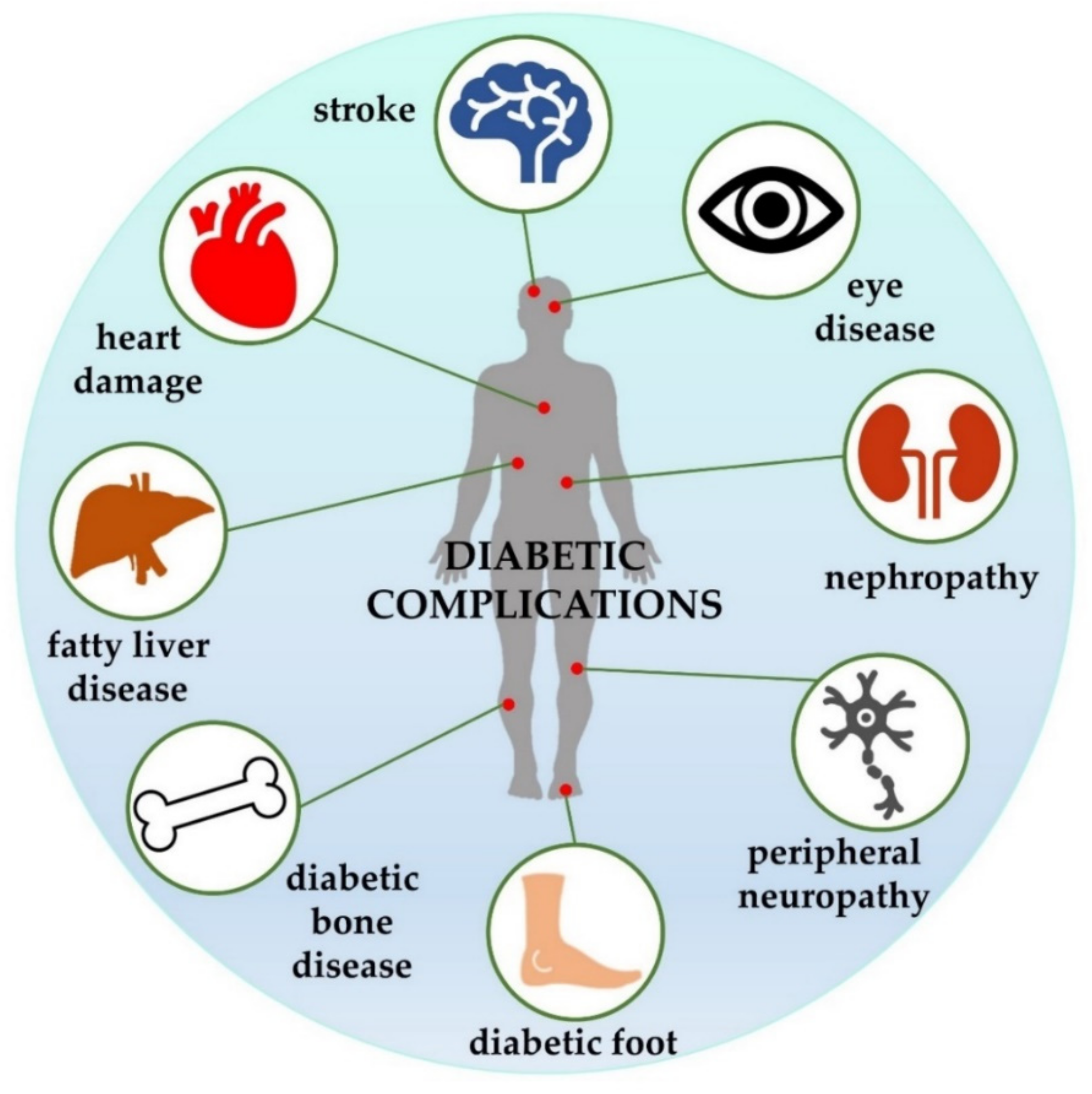
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Baynest, H.W. Classification, Pathophysiology, Diagnosis and Management of Diabetes Mellitus. J. Diabetes Metab. 2015, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Piero, M.N. Diabetes Mellitus–a Devastating Metabolic Disorder. Asian J. Biomed. Pharm. Sci. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Ghodsi, M.; Larijani, B.; Keshtkar, A.A.; Nasli-Esfahani, E.; Alatab, S.; Mohajeri-Tehrani, M.R. Mechanisms Involved in Altered Bone Metabolism in Diabetes: A Narrative Review. J. Diabetes Metab. Disord. 2016, 15, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.; Liu, J. The Effects of Type 1 vs. Type 2 Diabetes on Bone Metabolism. World J. Surg. Surg. Res. 2020, 3, 5. [Google Scholar]
- Govindarajan, G.; Gill, H.; Rovetto, M.; Sowers, J. What Is Insulin Resistance? Heart Metab. 2006, 30, 30–34. [Google Scholar]
- Abdul-Ghani, M.A.; Matsuda, M.; Jani, R.; Jenkinson, C.P.; Coletta, D.K.; Kaku, K.; DeFronzo, R.A. The Relationship between Fasting Hyperglycemia and Insulin Secretion in Subjects with Normal or Impaired Glucose Tolerance. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E401–E406. [Google Scholar] [CrossRef]
- Kaku, K. Pathophysiology of Type 2 Diabetes and Its Treatment Policy. Jpn. Med. Assoc. J. 2010, 53, 41–46. [Google Scholar]
- Cerf, M.E. Beta Cell Physiological Dynamics and Dysfunctional Transitions in Response to Islet Inflammation in Obesity and Diabetes. Metabolites 2020, 10, 452. [Google Scholar] [CrossRef]
- Yu, R.; Hui, H.; Melmed, S. Insulin Secretion and Action. In Endocrinology: Basic and Clinical Principles; Melmed, S., Conn, P.M., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 311–319. ISBN 978-1-59259-829-8. [Google Scholar]
- Gheibi, S.; Ghasemi, A. Insulin Secretion: The Nitric Oxide Controversy. EXCLI J. 2020, 19, 1227–1245. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, B.; Larsen, O.; Gabe, M.B.N.; Christiansen, C.B.; Rosenkilde, M.M.; Drucker, D.J.; Holst, J.J. Insulin Secretion Depends on Intra-Islet Glucagon Signaling. Cell Rep. 2018, 25, 1127–1134.e2. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, N.; Vaziri, N.D.; Dafoe, D.C.; Ichii, H. The Role of Oxidative Stress in Pancreatic β Cell Dysfunction in Diabetes. Int. J. Mol. Sci. 2021, 22, 1509. [Google Scholar] [CrossRef]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting Hepatic Glucose Metabolism in the Treatment of Type 2 Diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, B.J. Insulin Resistance: From Benign to Type 2 Diabetes Mellitus. Rev. Cardiovasc. Med. 2003, 4 (Suppl. S6), S3–S10. [Google Scholar] [PubMed]
- Scheen, A.J. Pathophysiology of Type 2 Diabetes. Acta Clin. Belg. 2003, 58, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodelo, C.; Roura-Guiberna, A.; Olivares-Reyes, J.A. Molecular Mechanisms of Insulin Resistance: An Update. Gac. Med. Mex. 2017, 153, 214–228. [Google Scholar]
- Choi, K.; Kim, Y.-B. Molecular Mechanism of Insulin Resistance in Obesity and Type 2 Diabetes. Korean J. Intern. Med. 2010, 25, 119–129. [Google Scholar] [CrossRef]
- American Diabetes Association Standards of Medical Care in Diabetes—2014. Diabetes Care 2014, 37, S14–S80. [CrossRef] [Green Version]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; del Cañizo-Gómez, F.J. Update on the Treatment of Type 2 Diabetes Mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Ali, A.; Ah Dar, M.; Ayaz, A. Diagnostic Approaches to Diabetes Mellitus and the Role of Vitamins. J. Nutr. Food Sci. 2017, 7. [Google Scholar] [CrossRef]
- Sales, C.H.; de Fatima Campos Pedrosa, L. Magnesium and Diabetes Mellitus: Their Relation. Clin. Nutr. Edinb. Scotl. 2006, 25, 554–562. [Google Scholar] [CrossRef]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of Chromium Supplementation on Glucose Metabolism and Lipids: A Systematic Review of Randomized Controlled Trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef] [Green Version]
- Domingo, J.L.; Gómez, M. Vanadium Compounds for the Treatment of Human Diabetes Mellitus: A Scientific Curiosity? A Review of Thirty Years of Research. Food Chem. Toxicol. 2016, 95, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Suksomboon, N.; Poolsup, N.; Sinprasert, S. Effects of Vitamin E Supplementation on Glycaemic Control in Type 2 Diabetes: Systematic Review of Randomized Controlled Trials. J. Clin. Pharm. Ther. 2011, 36, 53–63. [Google Scholar] [CrossRef]
- Yücel, B.; Topal, E.; Kösoğlu, M. Bee Products as Functional Food. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Waisundara, V., Shiomi, N., Eds.; InTech: London, UK, 2017; pp. 16–33. ISBN 978-953-51-2920-2. [Google Scholar]
- McRorie, J.W. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 2: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Pepa, G.; Vetrani, C.; Vitale, M.; Riccardi, G. Wholegrain Intake and Risk of Type 2 Diabetes: Evidence from Epidemiological and Intervention Studies. Nutrients 2018, 10, 1288. [Google Scholar] [CrossRef] [Green Version]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Prnova, M.S.; Svik, K.; Slovak, L.; Kisska, P.; Kovacik, A.; Brindza, J. Consumption of Bee Bread Influences Glycaemia and Development of Diabetes in Obese Spontaneous Diabetic Rats. Biologia 2020, 75, 705–711. [Google Scholar] [CrossRef]
- Martiniakova, M.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Omelka, R. Bee Bread Can Alleviate Lipid Abnormalities and Impaired Bone Morphology in Obese Zucker Diabetic Rats. Molecules 2021, 26, 2616. [Google Scholar] [CrossRef]
- Kadirvelu, A.; Gurtu, S. Potential Benefits of Honey in Type 2 Diabetes Mellitus: A Review. Int. J. Collab. Res. Intern. Med. Public Health 2013, 5, 199–216. [Google Scholar]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 4757893. [Google Scholar] [CrossRef] [Green Version]
- Deepthi, B.; Sowjanya, K.; Lidiya, B.; Bhargavi, R.; Babu, P. A Modern Review of Diabetes Mellitus: An Annihilatory Metabolic Disorder. J. Silico Vitro Pharmacol. 2017, 3. [Google Scholar] [CrossRef]
- Thrasher, J. Pharmacologic Management of Type 2 Diabetes Mellitus: Available Therapies. Am. J. Med. 2017, 130, S4–S17. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; Islam, M.A.; Kamal, M.A.; Gan, S.H. Updates on Managing Type 2 Diabetes Mellitus with Natural Products: Towards Antidiabetic Drug Development. Curr. Med. Chem. 2018, 25, 5395–5431. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M. The Role of Sulphonylureas in the Management of Type 2 Diabetes Mellitus. Drugs 2004, 64, 1339–1358. [Google Scholar] [CrossRef]
- Lazzaroni, E.; Ben Nasr, M.; Loretelli, C.; Pastore, I.; Plebani, L.; Lunati, M.E.; Vallone, L.; Bolla, A.M.; Rossi, A.; Montefusco, L.; et al. Anti-Diabetic Drugs and Weight Loss in Patients with Type 2 Diabetes. Pharmacol. Res. 2021, 171, 105782. [Google Scholar] [CrossRef]
- Raptis, S.A.; Dimitriadis, G.D. Oral Hypoglycemic Agents: Insulin Secretagogues, Alpha-Glucosidase Inhibitors and Insulin Sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S265–S287. [Google Scholar] [CrossRef]
- Drucker, D.J.; Sherman, S.I.; Gorelick, F.S.; Bergenstal, R.M.; Sherwin, R.S.; Buse, J.B. Incretin-Based Therapies for the Treatment of Type 2 Diabetes: Evaluation of the Risks and Benefits. Diabetes Care 2010, 33, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy: A Consensus Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009, 32, 193–203. [Google Scholar] [CrossRef] [Green Version]
- van Baar, M.J.B.; van Ruiten, C.C.; Muskiet, M.H.A.; van Bloemendaal, L.; IJzerman, R.G.; van Raalte, D.H. SGLT2 Inhibitors in Combination Therapy: From Mechanisms to Clinical Considerations in Type 2 Diabetes Management. Diabetes Care 2018, 41, 1543–1556. [Google Scholar] [CrossRef] [Green Version]
- Zangeneh, F.; Kudva, Y.C.; Basu, A. Insulin Sensitizers. Mayo Clin. Proc. 2003, 78, 471–479. [Google Scholar] [CrossRef] [Green Version]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddique, M.A.H.; Begum, A.; Begum, S.; Khan, M.H.; Saiedullah, M.; Haque, A.; Rahman, M.A.; Ali, L. Comparison of Antioxidative Effects of Biguanides and Sulfonylureas Monotherapy on Total Antioxidant Status in Newly-Diagnosed Patients with Type 2 Diabetes Mellitus. Diabetes Case Rep. 2016, 1, 5. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C. Metformin: Its Botanical Background. Pract. Diabetes Int. 2004, 21, 115–117. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and Molecular Mechanisms of Metformin: An Overview. Clin. Sci. (Lond.) 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortizo, A.M.; Sedlinsky, C.; McCarthy, A.D.; Blanco, A.; Schurman, L. Osteogenic Actions of the Anti-Diabetic Drug Metformin on Osteoblasts in Culture. Eur. J. Pharmacol. 2006, 536, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, C.M.; Calori, G.M.; Giannoudis, P.V. Diabetes and Fracture Healing: The Skeletal Effects of Diabetic Drugs. Expert Opin. Drug Saf. 2012, 11, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Paquot, N. Metformin Revisited: A Critical Review of the Benefit-Risk Balance in at-Risk Patients with Type 2 Diabetes. Diabetes Metab. 2013, 39, 179–190. [Google Scholar] [CrossRef]
- Rena, G.; Lang, C.C. Repurposing Metformin for Cardiovascular Disease. Circulation 2018, 137, 422–424. [Google Scholar] [CrossRef] [Green Version]
- MacCallum, L.; Senior, P.A. Safe Use of Metformin in Adults With Type 2 Diabetes and Chronic Kidney Disease: Lower Dosages and Sick-Day Education Are Essential. Can. J. Diabetes 2019, 43, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Diamant, M.; Heine, R.J. Thiazolidinediones in Type 2 Diabetes Mellitus: Current Clinical Evidence. Drugs 2003, 63, 1373–1405. [Google Scholar] [CrossRef]
- Tack, C.; Smits, P. Thiazolidinedione Derivatives in Type 2 Diabetes Mellitus. Neth. J. Med. 2006, 64, 166–174. [Google Scholar]
- Rizos, C.V.; Kei, A.; Elisaf, M.S. The Current Role of Thiazolidinediones in Diabetes Management. Arch. Toxicol. 2016, 90, 1861–1881. [Google Scholar] [CrossRef]
- Davidson, M.A.; Mattison, D.R.; Azoulay, L.; Krewski, D. Thiazolidinedione Drugs in the Treatment of Type 2 Diabetes Mellitus: Past, Present and Future. Crit. Rev. Toxicol. 2018, 48, 52–108. [Google Scholar] [CrossRef] [PubMed]
- El-Atat, F.A.; Nicasio, J.; Clark, L.T.; McFarlane, S.I. An Overview of the Beneficial Cardiovascular Effects of Thiazolidinediones. Therapy 2005, 2, 113–119. [Google Scholar] [CrossRef]
- Pittas, A.G.; Greenberg, A.S. Thiazolidinediones in the Treatment of Type 2 Diabetes. Expert Opin. Pharmacother. 2002, 3, 529–540. [Google Scholar] [CrossRef]
- Sokkar, S.; El-Sharnouby, J.A.; Helmy, A.; El-Bendary, A.; Ahmad, L.S.; Okasha, K. Role Of Peroxisome Proliferator- Activated Receptor Gamma2 (Ppar-ΓG2) Gene Polymorphism In Type 2 Diabetes Mellitus. Eur. J. Gen. Med. 2009, 6, 78–86. [Google Scholar]
- Bösenberg, L.H.; van Zyl, D.G. The Mechanism of Action of Oral Antidiabetic Drugs: A Review of Recent Literature. J. Endocrinol. Metab. Diabetes S. Afr. 2008, 13, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, M.; Scobie, I. The Pathogenesis of Type 2 Diabetes Mellitus. Pract. Diabetes Int. 2002, 19, 255–257. [Google Scholar] [CrossRef]
- Leahy, J.L.; Bonner-Weir, S.; Weir, G.C. Beta-Cell Dysfunction Induced by Chronic Hyperglycemia. Current Ideas on Mechanism of Impaired Glucose-Induced Insulin Secretion. Diabetes Care 1992, 15, 442–455. [Google Scholar] [CrossRef]
- Korytkowski, M.T. Sulfonylurea Treatment of Type 2 Diabetes Mellitus: Focus on Glimepiride. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 606–620. [Google Scholar] [CrossRef]
- Yousef, F.; Mansour, O.; Herbali, J. Sulfonylurea Review. Int. J. Pharm. Pharm. Res. 2018, 11, 54–65. [Google Scholar]
- Aquilante, C.L. Sulfonylurea Pharmacogenomics in Type 2 Diabetes: The Influence of Drug Target and Diabetes Risk Polymorphisms. Expert Rev. Cardiovasc. Ther. 2010, 8, 359–372. [Google Scholar] [CrossRef]
- Shorr, R.I.; Ray, W.A.; Daugherty, J.R.; Griffin, M.R. Incidence and Risk Factors for Serious Hypoglycemia in Older Persons Using Insulin or Sulfonylureas. Arch. Intern. Med. 1997, 157, 1681–1686. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, A.; Rydberg, T.; Sterner, G.; Melander, A. Pharmacokinetics of Glibenclamide and Its Metabolites in Diabetic Patients with Impaired Renal Function. Eur. J. Clin. Pharmacol. 1998, 53, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.S.H. Do Sulfonylurea Drugs Increase the Risk of Cardiac Events? Can. Med. Assoc. J. 2006, 174, 185–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackuliak, P.; Kužma, M.; Payer, J. Effect of Antidiabetic Treatment on Bone. Physiol. Res. 2019, 68, S107–S120. [Google Scholar] [CrossRef]
- Fuhlendorff, J.; Rorsman, P.; Kofod, H.; Brand, C.L.; Rolin, B.; MacKay, P.; Shymko, R.; Carr, R.D. Stimulation of Insulin Release by Repaglinide and Glibenclamide Involves Both Common and Distinct Processes. Diabetes 1998, 47, 345–351. [Google Scholar] [CrossRef]
- Makino, C. Controlled-Release Preparation Containing Meglitinide for Treatment of Type-II Diabetes Mellitus. Ann. Pharmacol. Pharm. 2017, 2, 3. [Google Scholar]
- Landgraf, R. Meglitinide Analogues in the Treatment of Type 2 Diabetes Mellitus. Drugs Aging 2000, 17, 411–425. [Google Scholar] [CrossRef]
- Bailey, T. Options for Combination Therapy in Type 2 Diabetes: Comparison of the ADA/EASD Position Statement and AACE/ACE Algorithm. Am. J. Med. 2013, 126, S10–S20. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative Stress and Inflammatory Markers in Prediabetes and Diabetes. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Guardado-Mendoza, R.; Prioletta, A.; Jiménez-Ceja, L.M.; Sosale, A.; Folli, F. The Role of Nateglinide and Repaglinide, Derivatives of Meglitinide, in the Treatment of Type 2 Diabetes Mellitus. Arch. Med. Sci. 2013, 9, 936–943. [Google Scholar] [CrossRef]
- Obale, B.; Banerjee, M. Safety and Efficacy of Meglitinides in Combination with GLP-1 Analogues—A Case Series. J. Endocrinol. Thyroid Res. 2017, 1, 555559. [Google Scholar] [CrossRef]
- Mohsin, S.; Baniyas, M.M.; AlDarmaki, R.S.; Tekes, K.; Kalász, H.; Adeghate, E.A. An Update on Therapies for the Treatment of Diabetes-Induced Osteoporosis. Expert Opin. Biol. Ther. 2019, 19, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Chougale, A.D.; Ghadyale, V.A.; Panaskar, S.N.; Arvindekar, A.U. Alpha Glucosidase Inhibition by Stem Extract of Tinospora Cordifolia. J. Enzyme Inhib. Med. Chem. 2009, 24, 998–1001. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Biosynthesis of α-Glucosidase Inhibitors by a Newly Isolated Bacterium, Paenibacillus Sp. TKU042 and Its Effect on Reducing Plasma Glucose in a Mouse Model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanefeld, M.; Schaper, F. The Role of Alpha-Glucosidase Inhibitors (Acarbose). In Pharmacotherapy of Diabetes: New Developments: Improving Life and Prognosis for Diabetic Patients; Mogensen, C.E., Ed.; Springer: Boston, MA, USA, 2007; pp. 143–152. ISBN 978-0-387-69737-6. [Google Scholar]
- Meneilly, G.S.; Ryan, E.A.; Radziuk, J.; Lau, D.C.; Yale, J.F.; Morais, J.; Chiasson, J.L.; Rabasa-Lhoret, R.; Maheux, P.; Tessier, D.; et al. Effect of Acarbose on Insulin Sensitivity in Elderly Patients with Diabetes. Diabetes Care 2000, 23, 1162–1167. [Google Scholar] [CrossRef] [Green Version]
- van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; van Weel, C. Alpha-Glucosidase Inhibitors for Patients with Type 2 Diabetes: Results from a Cochrane Systematic Review and Meta-Analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulkhair, W.M.; Abdel-all, W.S.; Bahy, R.H. GENETIC IMPROVEMENT OF ANTIDIABETIC ALPHA-GLUCOSIDASE INHIBITOR PRODUCING STREPTOMYCES SP. Int. J. Pharm. Pharm. Sci. 2018, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.E.; Drucker, D.J. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [Green Version]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their Physiology and Application in the Treatment of Diabetes Mellitus. Diabetes Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef]
- Zappas, M.P.; Gentes, M.; Walton-Moss, B. Use of Incretin Therapy in the Treatment of Type 2 Diabetes Mellitus. J. Nurse Pract. 2017, 13, 418–424. [Google Scholar] [CrossRef]
- Robertson, C. Incretin-Related Therapies in Type 2 Diabetes: A Practical Overview | Diabetes Spectrum. Diabetes Spectr. 2011, 24, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Kendall, D.M.; Cuddihy, R.M.; Bergenstal, R.M. Clinical Application of Incretin-Based Therapy: Therapeutic Potential, Patient Selection and Clinical Use. Am. J. Med. 2009, 122, S37–S50. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. MANAGEMENT OF ENDOCRINE DISEASE: Are All GLP-1 Agonists Equal in the Treatment of Type 2 Diabetes? Eur. J. Endocrinol. 2019, 181, R211–R234. [Google Scholar] [CrossRef]
- Ahrén, B. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes. Diabetes Manag. 2013, 3, 401–413. [Google Scholar] [CrossRef]
- Berra, C.C.; Resi, V.; Mirani, M.; Folini, L.; Rossi, A.; Solerte, S.B.; Fiorina, P. Clinical Efficacy and Predictors of Response to Dulaglutide in Type-2 Diabetes. Pharmacol. Res. 2020, 159, 104996. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Reid, T.S. Practical Use of Glucagon-Like Peptide-1 Receptor Agonist Therapy in Primary Care. Clin. Diabetes 2013, 31, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Scheen, A.J. DPP-4 Inhibitors in the Management of Type 2 Diabetes: A Critical Review of Head-to-Head Trials. Diabetes Metab. 2012, 38, 89–101. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Zhou, Z. Emerging Roles of Dipeptidyl Peptidase 4 Inhibitors: Anti-Inflammatory and Immunomodulatory Effect and Its Application in Diabetes Mellitus. Can. J. Diabetes 2014, 38, 473–479. [Google Scholar] [CrossRef]
- Singh, A.-K.; Yadav, D.; Sharma, N.; Jin, J.-O. Dipeptidyl Peptidase (DPP)-IV Inhibitors with Antioxidant Potential Isolated from Natural Sources: A Novel Approach for the Management of Diabetes. Pharmaceuticals 2021, 14, 586. [Google Scholar] [CrossRef]
- Ahrén, B. Dipeptidyl Peptidase-4 Inhibitors: Clinical Data and Clinical Implications. Diabetes Care 2007, 30, 1344–1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, R.; Bridgeman, M.B. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors In the Management of Diabetes. Pharm. Ther. 2010, 35, 509–513. [Google Scholar]
- Kazakos, K. Incretin Effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 2011, 93, S32–S36. [Google Scholar] [CrossRef]
- Packer, M. Is the Popularity of Dipeptidyl-Peptidase-4 Inhibitors Justified? Insights From Mechanistic Studies and Clinical Trials. Am. J. Med. 2018, 131, e287–e289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nespoux, J.; Vallon, V. SGLT2 Inhibition and Kidney Protection. Clin. Sci. (Lond.) 2018, 132, 1329–1339. [Google Scholar] [CrossRef]
- Moradi-Marjaneh, R.; Paseban, M.; Sahebkar, A. Natural Products with SGLT2 Inhibitory Activity: Possibilities of Application for the Treatment of Diabetes. Phytother. Res. 2019, 33, 2518–2530. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Al Harrasi, A.; Hussain, H.; Hamaed, A.; Supuran, C.T. The Management of Diabetes Mellitus-Imperative Role of Natural Products against Dipeptidyl Peptidase-4, α-Glucosidase and Sodium-Dependent Glucose Co-Transporter 2 (SGLT2). Bioorganic Chem. 2019, 86, 305–315. [Google Scholar] [CrossRef]
- Tentolouris, A.; Vlachakis, P.; Tzeravini, E.; Eleftheriadou, I.; Tentolouris, N. SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int. J. Environ. Res. Public. Health 2019, 16, 2965. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A Paradigm of Proactive Medicine. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nisar, B.; Sultan, A.; Rubab, S.L. Comparison of Medicinally Important Natural Products versus Synthetic Drugs-A Short Commentary. Nat. Prod. Chem. Res. 2018, 6. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Grossman, L.D.; Roscoe, R.; Shack, A.R. Complementary and Alternative Medicine for Diabetes. Can. J. Diabetes 2018, 42 (Suppl. S1), S154–S161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An Update on Natural Compounds in the Remedy of Diabetes Mellitus: A Systematic Review. J. Tradit. Complement. Med. 2018, 8, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.M.; Guarino, M.P.; Barroso, S.; Gil, M.M. Phytopharmacological Strategies in the Management of Type 2 Diabetes Mellitus. Foods 2020, 9, 271. [Google Scholar] [CrossRef] [Green Version]
- Tariq, R.; Khan, K.; Ali, R.; Naeem Wain, Z. Natural Remedies for Diabetes Mellitus. Int. Curr. Pharm. J. 2016, 5, 97. [Google Scholar] [CrossRef]
- Kumari, M.S.; Lakshmi, K.N.; Jyothi, A.S.; Prasanthi, T. Natural herbs vs. allopathic drugs: To treat diabetes. Am. J. Pharm. Sci. 2016, 3, 415–422. [Google Scholar]
- Shaikh, D.A.; Patil, R. Natural Diabetes Treatment: An Overview. Int. J. Sci. Res. 2019, 8, 5. [Google Scholar]
- Buchholz, T.; Melzig, M.F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Greń, A.; Massányi, P. Antidiabetic and Antioxidant Potential of Plant Extracts; SUA: Nitra, Slovakia, 2016. [Google Scholar]
- Zhu, X.; Wu, C.; Qiu, S.; Yuan, X.; Li, L. Effects of Resveratrol on Glucose Control and Insulin Sensitivity in Subjects with Type 2 Diabetes: Systematic Review and Meta-Analysis. Nutr. Metab. 2017, 14. [Google Scholar] [CrossRef]
- Nanjan, M.J.; Betz, J. Resveratrol for the Management of Diabetes and Its Downstream Pathologies. Eur. Endocrinol. 2014, 10, 31–35. [Google Scholar] [CrossRef]
- Szkudelski, T.; Szkudelska, K. Resveratrol and Diabetes: From Animal to Human Studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and Their Effects on Diabetes Management: A Review. Med. J. Islam. Repub. Iran 2017, 31, 134. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.; Monteiro-Alfredo, T.; Silva, S.; Matafome, P. Curcumin Derivatives for Type 2 Diabetes Management and Prevention of Complications. Arch. Pharm. Res. 2020, 43, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural Products for the Treatment of Type 2 Diabetes Mellitus: Pharmacology and Mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Aba, P.; Asuzu, I. Mechanisms of Actions of Some Bioactive Anti-Diabetic Principles from Phytochemicals of Medicinal Plants: A Review. Indian J. Nat. Prod. Resour. 2018, 9, 85–96. [Google Scholar]
- Kumari, M.; Jain, S. Tannin: An Antinutrient with Positive Effect to Manage Diabetes. Res. J. Recent Sci. 2012, 1, 1–8. [Google Scholar]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and Vascular Complications of Diabetes: An Update. Phytomed. Int. J. Phytother. Phytopharm. 2019, 56, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, A.M.; Brown, B.D.; Cunnane, S.C.; Domitrovich, S.G.; Adams, E.R.; Bobowiec, C.E. Daily Flaxseed Consumption Improves Glycemic Control in Obese Men and Women with Pre-Diabetes: A Randomized Study. Nutr. Res. 2013, 33, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fofana, B.; Roy, M.; Ghose, K.; Yao, X.-H.; Nixon, M.-S.; Nair, S.; Nyomba, G.B.L. Flaxseed Lignan Secoisolariciresinol Diglucoside Improves Insulin Sensitivity through Upregulation of GLUT4 Expression in Diet-Induced Obese Mice. J. Funct. Foods 2015, 18, 1–9. [Google Scholar] [CrossRef]
- Prasad, K. Secoisolariciresinol Diglucoside from Flaxseed Delays the Development of Type 2 Diabetes in Zucker Rat. J. Lab. Clin. Med. 2001, 138, 32–39. [Google Scholar] [CrossRef]
- Draganescu, D.; Andritoiu, C.; Hritcu, D.; Dodi, G.; Popa, M.I. Flaxseed Lignans and Polyphenols Enhanced Activity in Streptozotocin-Induced Diabetic Rats. Biology 2021, 10, 43. [Google Scholar] [CrossRef]
- Guo, H.; Xia, M. Chapter 12—Anthocyanins and Diabetes Regulation. In Polyphenols: Mechanisms of Action in Human Health and Disease (Second Edition); Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 135–145. ISBN 978-0-12-813006-3. [Google Scholar]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin Gallate Supplementation Alleviates Diabetes in Rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Landeka Jurčević, I.; Đikić, D.; Rogić, D.; Odeh, D.; Balta, V.; Perak Junaković, E.; Terzić, S.; Jutrić, D. Effect of Propolis on Diet-Induced Hyperlipidemia and Atherogenic Indices in Mice. Antioxidants 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortsäter, H.; Grankvist, N.; Wolfram, S.; Kuehn, N.; Sjöholm, Å. Diet Supplementation with Green Tea Extract Epigallocatechin Gallate Prevents Progression to Glucose Intolerance in Db/Db Mice. Nutr. Metab. 2012, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and in Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, e9340637. [Google Scholar] [CrossRef] [PubMed]
- Bahattab, O.; Shaikhomar, O. Physiological Effect of Quercetin as a Natural Flavonoid to Be Used as Hypoglycemic Agent in Diabetes Mellitus Type II Rats. Saudi J. Biomed. Res. 2021, 6, 10–17. [Google Scholar]
- Malakul, W.; Pengnet, S. Effect of Naringin on Insulin Resistance and Oxidative Stress in Fructose Fed Rats. Naresuan Univ. J. Sci. Technol. 2018, 26, 10–18. [Google Scholar]
- Alam, M.A.; Kauter, K.; Brown, L. Naringin Improves Diet-Induced Cardiovascular Dysfunction and Obesity in High Carbohydrate, High Fat Diet-Fed Rats. Nutrients 2013, 5, 637–650. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Liang, W.; Zhang, D.; Kang, J.; Meng, X.; Yang, J.; Yang, L.; Xue, N.; Gao, Q.; Han, S.; Gou, X. Protective Effects of Rutin on Liver Injury in Type 2 Diabetic Db/Db Mice. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 107, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Alkhalidy, H.; Moore, W.; Wang, A.; Luo, J.; McMillan, R.P.; Wang, Y.; Zhen, W.; Hulver, M.W.; Liu, D. Kaempferol Ameliorates Hyperglycemia through Suppressing Hepatic Gluconeogenesis and Enhancing Hepatic Insulin Sensitivity in Diet-Induced Obese Mice. J. Nutr. Biochem. 2018, 58, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol Alleviates Insulin Resistance via Hepatic IKK/NF-ΚB Signal in Type 2 Diabetic Rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Khan, R. Effects of Garlic on Blood Glucose Levels and HbA1c in Patients with Type 2 Diabetes Mellitus. J. Med. Plant Res. 2011, 5, 2922–2928. [Google Scholar]
- Emami, S.; Rouhani, M.; Azadbakht, L. The Effect of Garlic Intake on Glycemic Control in Humans: A Systematic Review and Meta-Analysis. Prog. Nutr. 2017, 19, 10–18. [Google Scholar] [CrossRef]
- Hou, L.; Liu, Y.; Zhang, Y. Garlic Intake Lowers Fasting Blood Glucose: Meta-Analysis of Randomized Controlled Trials. Asia Pac. J. Clin. Nutr. 2015, 24, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gupta, A.K.; Walia, A. Effect of Green Tea on Diabetes Mellitus. ACTA Sci. Nutr. Health 2019, 3, 27–31. [Google Scholar]
- Liu, K.; Zhou, R.; Wang, B.; Chen, K.; Shi, L.-Y.; Zhu, J.-D.; Mi, M.-T. Effect of Green Tea on Glucose Control and Insulin Sensitivity: A Meta-Analysis of 17 Randomized Controlled Trials. Am. J. Clin. Nutr. 2013, 98, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks Containing Anthocyanin-Rich Blackcurrant Extract Decrease Postprandial Blood Glucose, Insulin and Incretin Concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, Y.; Ozeki, A.; Tani, T.; Tsuda, T. Blackcurrant Extract Ameliorates Hyperglycemia in Type 2 Diabetic Mice in Association with Increased Basal Secretion of Glucagon-Like Peptide-1 and Activation of AMP-Activated Protein Kinase. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry Components Inhibit α-Glucosidase in Vitro: Synergies between Acarbose and Polyphenols from Black Currant and Rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Güder, A.; Gür, M.; Engin, M.S. Antidiabetic and Antioxidant Properties of Bilberry (Vaccinium myrtillus Linn.) Fruit and Their Chemical Composition. J. Agric. Sci. Technol. 2015, 17, 401–414. [Google Scholar]
- Sidorova, Y.; Shipelin, V.; Mazo, V.; Zorin, S.N.; Petrov, N.; Kochetkova, A.A. Comparative Studies of Antidiabetic Activity of Bilberry Leaf Extract in Wistar Rats with STZ-Induced Diabetes and Zucker Diabetic Fatty Rats. Int. Food Res. J. 2018, 25, 1288–1294. [Google Scholar]
- Huang, Y.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Maximizing the Health Effects of Strawberry Anthocyanins: Understanding the Influence of the Consumption Timing Variable. Food Funct. 2016, 7, 4745–4752. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A Dose-Response Evaluation of Freeze-Dried Strawberries Independent of Fiber Content on Metabolic Indices in Abdominally Obese Individuals with Insulin Resistance in a Randomized, Single-Blinded, Diet-Controlled Crossover Trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Svik, K.; Prnova, M.S.; Slovak, L.; Kovacik, A.; Lory, V.; Zorad, S.; et al. Cornelian Cherry Fruit Improves Glycaemia and Manifestations of Diabetes in Obese Zucker Diabetic Fatty Rats. Res. Vet. Sci. 2019, 126, 118–123. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive Oil Nutraceuticals in the Prevention and Management of Diabetes: From Molecules to Lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, B.; Saravanan, R.; Pugalendi, K.V. Influence of Sesame Oil on Blood Glucose, Lipid Peroxidation, and Antioxidant Status in Streptozotocin Diabetic Rats. J. Med. Food 2005, 8, 377–381. [Google Scholar] [CrossRef]
- Kumar, S.; Shachi, K.; Prasad, N.; Dubey, N.K.; Dubey, U. Anti-Diabetic, Haematinic and Anti-Cholesterolmic Effects of Carrot (Daucus Carota Linn.) Juice Metabolites to Cure Alloxan Monohydrate Induced Type-1 Diabetes in Albino Rats. J. Diabetes Metab. Disord. Control 2020, 7, 37–40. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Tijjani, H.; Zangoma, M.H.; Mohammed, Z.S.; Obidola, S.M.; Egbuna, C.; Abdulai, S.I. Polyphenols: Classifications, Biosynthesis and Bioactivities. In Functional Foods and Nutraceuticals: Bioactive Components, Formulations and Innovations; Egbuna, C., Dable Tupas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 389–414. ISBN 978-3-030-42319-3. [Google Scholar]
- Abbas, M.; Saeed, F.; Anjum, F.; Afzaal, M.; Tufail, T.; Bashir, M.; Ishtiaq, A.; Hussain, S.; Suleria, H. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20. [Google Scholar] [CrossRef] [Green Version]
- Oyenihi, O.R.; Oyenihi, A.B.; Adeyanju, A.A.; Oguntibeju, O.O. Antidiabetic Effects of Resveratrol: The Way Forward in Its Clinical Utility. J. Diabetes Res. 2016, 2016, 9737483. [Google Scholar] [CrossRef]
- Bo, S.; Gambino, R.; Ponzo, V.; Cioffi, I.; Goitre, I.; Evangelista, A.; Ciccone, G.; Cassader, M.; Procopio, M. Effects of Resveratrol on Bone Health in Type 2 Diabetic Patients. A Double-Blind Randomized-Controlled Trial. Nutr. Diabetes 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Fu, M.; Gao, S.-H.; Liu, J.-L. Curcumin and Diabetes: A Systematic Review. Evid.-Based Complement. Altern. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef]
- He, J.; Yang, X.; Liu, F.; Li, D.; Zheng, B.; Abdullah, A.O.; Liu, Y. The Impact of Curcumin on Bone Osteogenic Promotion of MC3T3 Cells under High Glucose Conditions and Enhanced Bone Formation in Diabetic Mice. Coatings 2020, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Al-Salih, R.M. Clinical Experimental Evidence: Synergistic Effect of Gallic Acid and Tannic Acid as Antidiabetic and Antioxidant Agents. Thi-Qar Med. J. 2010, 4, 109–119. [Google Scholar]
- Asgar, M.A. Anti-Diabetic Potential of Phenolic Compounds: A Review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Kumari, M.; Jain, S. Screening of Potential Sources of Tannin and Its Therapeutic Application. Int. J. Nutr. Food Sci. 2015, 4, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Kunyanga, C.N.; Imungi, J.K.; Okoth, M.; Momanyi, C.; Biesalski, H.K.; Vadivel, V. Antioxidant and Antidiabetic Properties of Condensed Tannins in Acetonic Extract of Selected Raw and Processed Indigenous Food Ingredients from Kenya. J. Food Sci. 2011, 76, C560–C567. [Google Scholar] [CrossRef]
- Félicien, M.K.; Wendo, F.; Séverin, M.; Kadima, J. Comparative Hypoglycemic Activity of Flavonoids and Tannins Fractions of Stachytarpheta Indica (L.) Vahl Leaves Extracts in Guinea-Pigs and Rabbits. Int. J. Pharm. Pharm. Res. 2016, 5, 48–57. [Google Scholar]
- Morada, N.; Metillo, E.; Uy, M.; Oclarit, J. Toxicity and Hypoglycemic Effect of Tannin-Containing Extract from the Mangrove Tree Sonneratia Alba Sm. Bull. Environ. Pharmacol. Life Sci. 2016, 5, 58–64. [Google Scholar]
- Velayutham, R.; Sankaradoss, N.; Ahamed, K.F.H.N. Protective Effect of Tannins from Ficus Racemosa in Hypercholesterolemia and Diabetes Induced Vascular Tissue Damage in Rats. Asian Pac. J. Trop. Med. 2012, 5, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wedick, N.M.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Gut Microbiota Metabolites of Dietary Lignans and Risk of Type 2 Diabetes: A Prospective Investigation in Two Cohorts of U.S. Women. Diabetes Care 2014, 37, 1287–1295. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a Flaxseed-Derived Lignan Supplement in Type 2 Diabetic Patients: A Randomized, Double-Blind, Cross-Over Trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef] [PubMed]
- Bhathena, S.J.; Velasquez, M.T. Beneficial Role of Dietary Phytoestrogens in Obesity and Diabetes. Am. J. Clin. Nutr. 2002, 76, 1191–1201. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The Possible Role of Flavonoids in the Prevention of Diabetic Complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef] [Green Version]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Cásedas, G.; Gómez, C.; Moliner, C.; Valero, M.S.; López, V. The Role of Anthocyanins as Antidiabetic Agents: From Molecular Mechanisms to in Vivo and Human Studies. J. Physiol. Biochem. 2021, 77, 109–131. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Gajski, G.; Garaj-Vrhovac, V.; Jazvinšćak Jembrek, M.; Kosalec, I. Assessment of DNA Damage and Lipid Peroxidation in Diabetic Mice: Effects of Propolis and Epigallocatechin Gallate (EGCG). Mutat. Res. 2013, 757, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-Y.; Kim, S.-P.; Song, D.-K. Effects of (-)-Epigallocatechin-3-Gallate on Pancreatic Beta-Cell Damage in Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2006, 541, 115–121. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.-S. Anti-Diabetic Effect of Cotreatment with Quercetin and Resveratrol in Streptozotocin-Induced Diabetic Rats. Biomol. Ther. 2018, 26, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Hassan, J.; Khalid, A.; Falah, H. Sheri Effect of Quercetin Supplement on Some Bone Mineralization Biomarkers in Diabetic Type 2 Patients. Adv. Pharmacol. Pharm. 2018, 6, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Qi, Q.-L.; Wang, M.-T.; Li, Q.-Y. Therapeutic Potential of Naringin: An Overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Liu, S.; Dong, J.; Bian, Q. A Dual Regulatory Effect of Naringenin on Bone Homeostasis in Two Diabetic Mice Models. Tradit. Med. Mod. Med. 2020, 3, 101–108. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and Antioxidant Effect of Rutin, a Polyphenolic Flavonoid, in Streptozotocin-Induced Diabetic Wistar Rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Sattanathan, K.; Dhanapal, C.K.; Umarani, R.; Manavalan, R. Beneficial Health Effects of Rutin Supplementation in Patients with Diabetes Mellitus. J. Appl. Pharm. Sci. 2011, 1, 227–231. [Google Scholar]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes. Clin. Nutr. 2020, 35, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D. Flavonol Kaempferol Improves Chronic Hyperglycemia-Impaired Pancreatic Beta-Cell Viability and Insulin Secretory Function. Eur. J. Pharmacol. 2011, 670, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Tzeng, T.-F.; Liou, S.-S.; Chang, Y.-S.; Liu, I.-M. Kaempferol Regulates the Lipid-Profile in High-Fat Diet-Fed Rats through an Increase in Hepatic PPARα Levels. Planta Med. 2011, 77, 1876–1882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, J. The Effects of Green Tea on Obesity and Type 2 Diabetes. Diabetes Metab. J. 2013, 37, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, T.; Leary, L.; Brooks, W.B. The Effect of an Extract of Green and Black Tea on Glucose Control in Adults with Type 2 Diabetes Mellitus: Double-Blind Randomized Study. Metabolism. 2007, 56, 1340–1344. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Liao, Y.-L.; Lin, S.-C.; Tsai, T.-H.; Huang, C.-J.; Chou, P. Does Supplementation with Green Tea Extract Improve Insulin Resistance in Obese Type 2 Diabetics? A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Altern. Med. Rev. J. Clin. Ther. 2011, 16, 157–163. [Google Scholar] [CrossRef]
- Mirzaei, K.; Hossein-Nezhad, A.; Karimi, M.; Hosseinzadeh Attar, M.J.; Jafari, N.; Najmafshar, A.; Larijani, B. Effect of Green Tea Extract on Bone Turnover Markers in Type 2 Diabetic Patients; A Double- Blind, Placebo-Controlled Clinical Trial Study. Daru 2009, 17, 38–44. [Google Scholar]
- Cao, L.; Park, Y.; Lee, S.; Kim, D.-O. Extraction, Identification, and Health Benefits of Anthocyanins in Blackcurrants (Ribes nigrum L.). Appl. Sci. 2021, 11, 1863. [Google Scholar] [CrossRef]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical Profiling of Fruit Powders of Twenty Sorbus L. Cultivars. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry Extracts Are Not Created Equal: The Role of Non Anthocyanin Fraction. Discovering the “Dark Side of the Force” in a Preliminary Study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar] [PubMed]
- Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z.; et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila Melanogaster High-Sugar Diet Model. Antioxid. Basel Switz. 2020, 9, 1067. [Google Scholar] [CrossRef] [PubMed]
- Abdulazeez, S.S.; Ponnusamy, P. Report: Antioxidant and Hypoglycemic Activity of Strawberry Fruit Extracts against Alloxan Induced Diabetes in Rats. Pak. J. Pharm. Sci. 2016, 29, 255–260. [Google Scholar]
- Xu, Y.; Xie, L.; Xie, J.; Liu, Y.; Chen, W. Pelargonidin-3-O-Rutinoside as a Novel α-Glucosidase Inhibitor for Improving Postprandial Hyperglycemia. Chem. Commun. 2018, 55, 39–42. [Google Scholar] [CrossRef]
- Shamsi, F.; Asgari, S.; Rafieian-Kopaei, M.; Kazemi, S.; Adelnia, A. Effects of Cornus mas L. on Blood Glucose, Insulin and Histopathology of Pancreas in Alloxan-Induced Diabetic Rats. J. Isfahan Med. Sch. 2011, 29, 929–938. [Google Scholar]
- Rafieian-kopaei, M.; Asgary, S.; Adelnia, A.; Khazaei, M.; Kazemi, S.; Shamsi, F. The Effects of Cornelian Cherry on Atherosclerosis and Atherogenic Factors in Hypercholesterolemic Rabbits. J. Med. Plants Res. 2010, 5, 2670–2676. [Google Scholar]
- Gholamrezayi, A.; Yaghubi, E.; Ghafouri, A. A Review of Probable Effects of Cornelian Cherry Fruit. J. Biochem. Technol. 2019, 2, 71–74. [Google Scholar]
- Narimani-Rad, M.; Lotfi, A.; Mesgari Abbasi, M.; Abdollahi, B. The Effect of Cornelian Cherry (Cornus mas L.) Extract on Serum Ghrelin and Corticosterone Levels in Rat Model. J. Pharm. Biomed. Sci. 2013, 3, 7–9. [Google Scholar]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis-Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Omelka, R.; Blahova, J.; Kovacova, V.; Babikova, M.; Mondockova, V.; Kalafova, A.; Capcarova, M.; Martiniakova, M. Cornelian Cherry Pulp Has Beneficial Impact on Dyslipidemia and Reduced Bone Quality in Zucker Diabetic Fatty Rats. Animals 2020, 10, 2435. [Google Scholar] [CrossRef]
- Martiniakova, M.; Blahova, J.; Kovacova, V.; Mondockova, V.; Babosova, R.; Kalafova, A.; Capcarova, M.; Omelka, R. Effects of Bee Bread, Cornelian Cherries Treatment on the Femoral Bone Structure Using Zucker Diabetic Fatty Rats as an Animal Model. Veterinární Medicína 2021, 66, 8. [Google Scholar] [CrossRef]
- Rajput, T.; Yousaf, M.; Naveed, A.; Khan, S.; Azeem, Z. Hypolipidemic Effect of Extra Virgin Olive Oil in Diabetic Rats. J. Rawalpindi Med. Coll. 2012, 16, 70–72. [Google Scholar]
- Pérez-Martínez, P.; García-Ríos, A.; Delgado-Lista, J.; Pérez-Jiménez, F.; López-Miranda, J. Mediterranean Diet Rich in Olive Oil and Obesity, Metabolic Syndrome and Diabetes Mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A Mediterranean Diet Enriched with Olive Oil Is Associated with Higher Serum Total Osteocalcin Levels in Elderly Men at High Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological Agents and Natural Compounds: Available Treatments for Osteoporosis. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2020, 71, 307–320. [Google Scholar] [CrossRef]
- Mitra, A. Study on the Benefits of Sesame Oil Over Coconut Oil in Patients of Insulin Resistance Syndrome, Notably Type 2 Diabetes and Dyslipidaemia. J. Hum. Ecol. 2007, 22, 61–66. [Google Scholar] [CrossRef]
- Sankar, D.; Ali, A.; Sambandam, G.; Rao, R. Sesame Oil Exhibits Synergistic Effect with Anti-Diabetic Medication in Patients with Type 2 Diabetes Mellitus. Clin. Nutr. Edinb. Scotl. 2011, 30, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Khaki, A.A.; Khaki, A.; Ahmadi-Ashtiani, H.R.; Rastegar, H.; Rezazadeh, S.; Babazadeh, D.; Zahedi, A.; Ghanbari, Z. Treatment Effects of Ginger Rhizome & Extract of Carrot Seed on Diabetic Nephropathy in Rat. J. Med. Plants 2010, 9, 75–80. [Google Scholar]
- Pouraboli, I.; Ranjbar, B. The Effect of Daucus Carota Seeds Extract on Lipid Profile, LFT and Kidney Function Indicators in Streptozocin-Induced Diabetic Rats. Int. J. Plant Sci. Ecol. 2015, 3, 84–87. [Google Scholar]
- Nicolle, C.; Cardinault, N.; Aprikian, O.; Busserolles, J.; Grolier, P.; Rock, E.; Demigné, C.; Mazur, A.; Scalbert, A.; Amouroux, P.; et al. Effect of Carrot Intake on Cholesterol Metabolism and on Antioxidant Status in Cholesterol-Fed Rat. Eur. J. Nutr. 2003, 42, 254–261. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, F.A. Alpha-Glucosidase Inhibitors in the Early Treatment of Type 2 Diabetes. Vasc. Health Risk Manag. 2008, 4, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Consoli, A.; Formoso, G. Do Thiazolidinediones Still Have a Role in Treatment of Type 2 Diabetes Mellitus? Diabetes Obes. Metab. 2013, 15, 967–977. [Google Scholar] [CrossRef]
- Dicker, D. DPP-4 Inhibitors: Impact on Glycemic Control and Cardiovascular Risk Factors. Diabetes Care 2011, 34 (Suppl. S2), S276–S278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninčević, V.; Omanović Kolarić, T.; Roguljić, H.; Kizivat, T.; Smolić, M.; Bilić Ćurčić, I. Renal Benefits of SGLT 2 Inhibitors and GLP-1 Receptor Agonists: Evidence Supporting a Paradigm Shift in the Medical Management of Type 2 Diabetes. Int. J. Mol. Sci. 2019, 20, 5831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A. Combination Therapy with GLP-1 Receptor Agonist and SGLT2 Inhibitor. Diabetes Obes. Metab. 2017, 19, 1353–1362. [Google Scholar] [CrossRef]
- Dludla, P.V.; Silvestri, S.; Orlando, P.; Gabuza, K.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Johnson, R.; Muller, C.J.F.; et al. Exploring the Comparative Efficacy of Metformin and Resveratrol in the Management of Diabetes-Associated Complications: A Systematic Review of Preclinical Studies. Nutrients 2020, 12, 739. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Gupta, P.; Lal, V. Effect of Co-Administration of Allium Sativum Extract and Metformin on Blood Glucose of Streptozotocin Induced Diabetic Rats. J. Intercult. Ethnopharmacol. 2013, 2, 81. [Google Scholar] [CrossRef]
- Prasad, N. Influence of Curcumin on Pioglitazone Metabolism and Pk/Pd: Diabetes Mellitus. Conference Proceedings of 3rd World Congress on Diabetes & Metabolism, Hyderabad, India, September 24. J. Diabetes Metab. 2012, 3, 80. [Google Scholar]
- Khayatnouri, M.; Bahari, K.; Safarmashaei, S. Study of the Effect of Gliclazide and Garlic Extract on Blood Sugar Level in STZ-Induced Diabetic Male Mice. Adv. Environ. Biol. 2011, 5, 1751–1755. [Google Scholar]
- Rani, T.; Sujatha, S.; Veeresham, C. Pharmacokinetic and Pharmacodynamic Interaction of Curcumin with Glimepiride in Normal and Diabetic Rats. Pharmacogn. Commun. 2012, 2, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, P.; Babu, R.B.; Narsimha, R.Y. Effect of repaglinide and curcumin combination on oxidative stress and biochemical parameters in stz induced diabetic rats. Eur. J. Biomed. Pharm. Sci. 2017, 4, 384–388. [Google Scholar]
- Gao, J.; Xu, P.; Wang, Y.; Wang, Y.; Hochstetter, D. Combined Effects of Green Tea Extracts, Green Tea Polyphenols or Epigallocatechin Gallate with Acarbose on Inhibition against α-Amylase and α-Glucosidase in Vitro. Molecules 2013, 18, 11614–11623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, N.M.; Millar, P.J.B.; Pathak, V.; Flatt, P.R.; Gault, V.A. Beneficial Metabolic Effects of Dietary Epigallocatechin Gallate Alone and in Combination with Exendin-4 in High Fat Diabetic Mice. Mol. Cell. Endocrinol. 2018, 460, 200–208. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.; Ma, Y.; Shao, Y.; Zhang, J.; Yuan, G.; Guo, X. Resveratrol Attenuates Dapagliflozin-Induced Renal Gluconeogenesis via Activating the PI3K/Akt Pathway and Suppressing the FoxO1 Pathway in Type 2 Diabetes. Food Funct. 2021, 12, 1207–1218. [Google Scholar] [CrossRef]
- Kannappan, S.; Anuradha, C.V. Insulin Sensitizing Actions of Fenugreek Seed Polyphenols, Quercetin & Metformin in a Rat Model. Indian J. Med. Res. 2009, 129, 401–408. [Google Scholar] [PubMed]
- Poonam, T.; Prakash, G.P.; Kumar, L.V. Influence of Allium Sativum Extract on the Hypoglycemic Activity of Glibenclamide: An Approach to Possible Herb-Drug Interaction. Drug Metabol. Drug Interact. 2013, 28, 225–230. [Google Scholar] [CrossRef]
- Williamson, G.; Sheedy, K. Effects of Polyphenols on Insulin Resistance. Nutrients 2020, 12, 135. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Aydin, E.; Gauer, J.S.; Pyner, A.; Williamson, G.; Kerimi, A. Green and Chamomile Teas, but Not Acarbose, Attenuate Glucose and Fructose Transport via Inhibition of GLUT2 and GLUT5. Mol. Nutr. Food Res. 2017, 61, 1700566. [Google Scholar] [CrossRef]
- González-Abuín, N.; Martínez-Micaelo, N.; Margalef, M.; Blay, M.; Arola-Arnal, A.; Muguerza, B.; Ardévol, A.; Pinent, M. A Grape Seed Extract Increases Active Glucagon-like Peptide-1 Levels after an Oral Glucose Load in Rats. Food Funct. 2014, 5, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Amadi, C.N.; Orisakwe, O.E. Herb-Induced Liver Injuries in Developing Nations: An Update. Toxics 2018, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering Gut Microbiota in Treatment of Type 2 Diabetes Mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
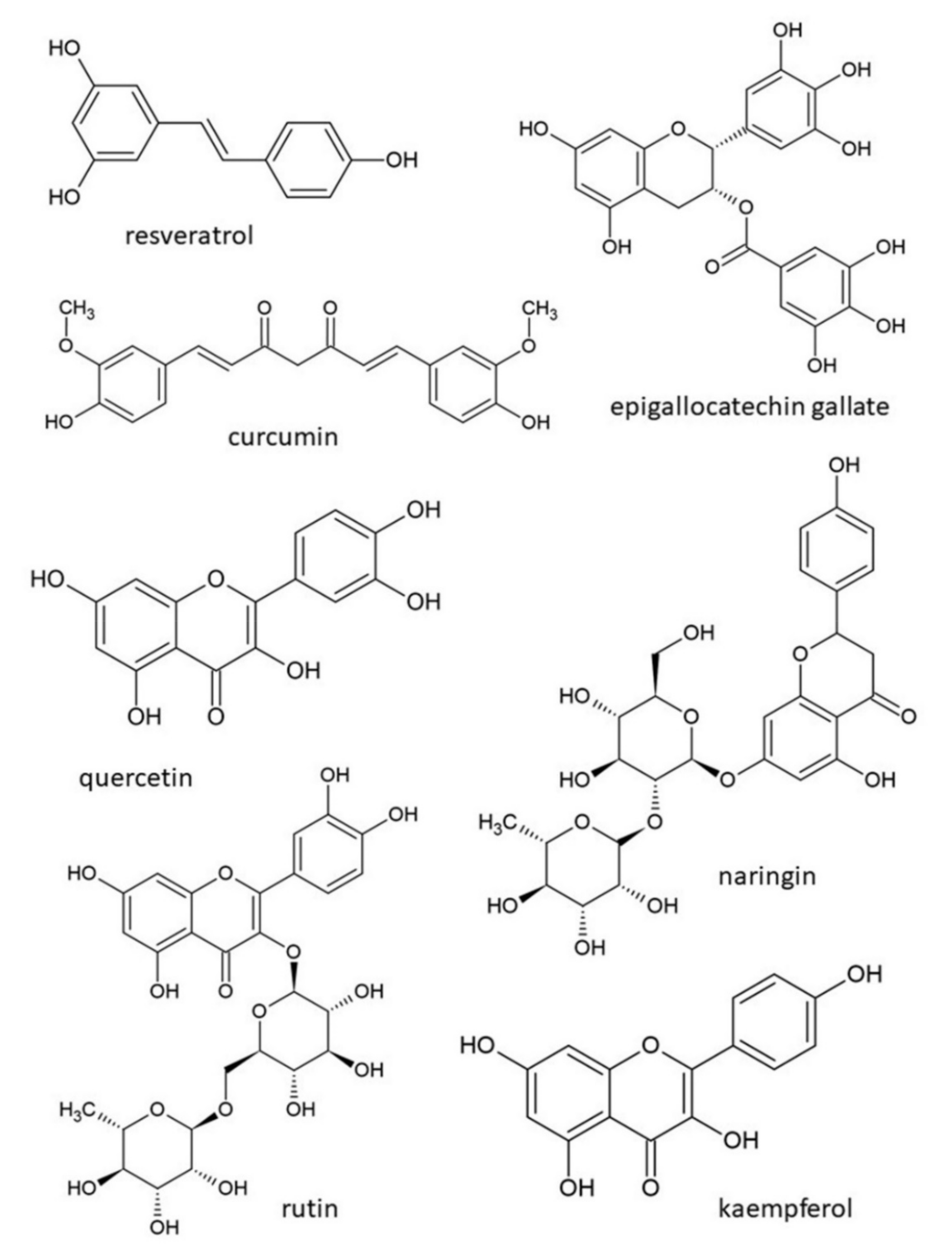
| Natural Products | Models Used | Known Effects and Mechanisms of Action | References |
|---|---|---|---|
| NON-FLAVONOID POLYPHENOLS | |||
| resveratrol | patients with T2DM (9 randomized controlled trials, n = 283) | improves glucose control and insulin sensitivity | [124] |
| diet-induced obese mice and rats, ZDF rats | enhances insulin sensitivity by activation of deacetylases sirtuins 1–7, protects β-cells from oxidative stress, interacts with PPARγ receptor | [124,125,126,127] | |
| in vitro (different cell lines) | improves insulin signalling and inhibits oxidative stress | [125,126] | |
| curcumin | obese patients (randomized, cross-over study, n = 16) | reduces postprandial glycaemia | [128] |
| C57BL/6J HFD-induced obese mice | improves insulin sensitivity | [128] | |
| STZ-induced rats | supports pancreatic cell viability by inhibition of lipid peroxidation, NF-κB activation and reduction of inflammatory cytokine levels | [129] | |
| in vitro (enzyme inhibition assay) | inhibits alpha-amylase and alpha-glucosidase activities | [43] | |
| tannins | in vitro (enzyme inhibition assay) | inhibit alpha-glucosidase activity | [130] |
| in vitro (3T3-L1 adipose cells) | improve insulin sensitivity | [130] | |
| in vivo and in vitro models | have insulin-like effect, stimulate glucose transport, reduce formation and accumulation of AGEs | [131,132] | |
| lignans | patients with pre-diabetes (randomized cross-over trial, n = 25) | decrease blood glucose levels, delay postprandial glucose absorption, reduce inflammation and oxidative stress | [133] |
| diet-induced obese mice | reduce blood glucose and insulin levels, improve oral glucose tolerance, insulin signalling and insulin sensitivity | [134] | |
| STZ-induced rats, ZDF rats | delay onset of diabetes | [135,136] | |
| FLAVONOIDS | |||
| anthocyanins | several cross-over trials on humans | prevent free radical production and lipid peroxidation, increase insulin secretion, improve insulin resistance, inhibit alpha-glucosidase activity | [137] |
| epigallocatechin gallate | db/db mice, ZDF rats | affects gluconeogenesis by downregulation of enzyme phosphoenolpyruvate carboxykinase, increases tyrosine phosphorylation of insulin receptors | [138] |
| db/db mice, alloxan-induced diabetic mice | has antioxidant and anti-inflammatory abilities, enhances glucose-stimulated insulin secretion | [129,139,140] | |
| db/db mice | increases number and size of pancreatic islets | [129] | |
| quercetin | in vivo and in vitro models | enhances glucose uptake by a MAPK insulin-dependent mechanism, increases phosphorylation of PI3K/Akt signalling pathways, interacts with PPARγ receptor, inhibits alpha-glucosidase and alpha-amylase activities | [127,141,142] |
| STZ- induced rats | improves β-cells action | [143] | |
| naringin | fructose-fed rats | improves insulin signalling | [144] |
| high-carbohydrate + high fat-fed rats | improves mitochondrial dysfunction in the liver | [145] | |
| high fat-fed rats | reduces blood glucose and cholesterol levels by upregulation of PPARγ | [146] | |
| in vitro (rat skeletal L6 myoblast cell line) | upregulates of 5′ AMPK in skeletal muscle cells | [146] | |
| in vivo and in vitro models | inhibits serum DPP-4 levels | [129] | |
| db/db mice | enhances hepatic glycolysis and glycogen concentration, reduces hepatic gluconeogenesis | [129] | |
| rutin | db/db mice | reduces blood glucose level, modulates insulin secretion, inhibits AGEs formation, positively affects IRS-2/PI3K/Akt/GSK-3β signalling pathway | [147] |
| kaempferol | diet-induced obese mice | prevents hyperglycaemia development, suppresses hepatic gluconeogenesis by reducing pyruvate carboxylase activity | [148] |
| STZ-induced + high-fat diet rats | improves insulin sensitivity by inhibiting pro-inflammatory cytokines, leading to reduced inflammatory responses and hepatic inflammatory lesions | [149] | |
| PLANT FRUITS, VEGETABLES AND OTHER PRODUCTS | |||
| garlic | in vivo and in vitro models | increases insulin secretion and sensitivity | [150,151] |
| obese patients with T2DM (open-label, prospective, comparative trial, n = 60) | reduces postprandial blood glucose level | [152] | |
| green tea | in vivo and in vitro models | increases insulin secretion, lowers blood glucose levels, improves insulin resistance, reduces diabetic complications | [153] |
| in vitro (adipocytes isolated from rats) | reduces concentration of glucose and increases insulin binding | [154] | |
| blackcurrant | patients (randomized, double-blind, cross-over trial, n = 23) | reduces postprandial blood glucose level | [155] |
| diet-induced obese mice, high-fructose diet rats | decreases blood glucose levels, enhances glucose tolerance | [156] | |
| in vivo and in vitro models | delays carbohydrate digestion, inhibits glucose absorption in the intestine | [155] | |
| in vitro (enzyme inhibition assay) | inhibits alpha-glucosidase and alpha-amylase activities, increases the effect of acarbose | [145,157] | |
| rowanberry | in vitro (enzyme inhibition assay) | inhibits alpha-glucosidase and alpha-amylase activities, increases the effect of acarbose | [157] |
| in vitro (enzyme inhibition assay) | inhibits alpha-amylase | [158] | |
| bilberry | in vitro (enzyme inhibition assay) | inhibits alpha-amylase and alpha-glucosidase activities | [159] |
| ZDF rats | decreases hyperglycaemia, positively affects body weight gain | [160] | |
| STZ-induced rats | reduces glucose level and weight loss | [160] | |
| strawberry | obese patients (single-blinded, cross-over trial, n = 14) | lowers postprandial glucose concentrations, improves insulin sensitivity | [161] |
| obese patients with insulin resistance (randomized, single-blinded, diet-controlled crossover trial, n = 21) | decreases postprandial glucose concentrations, improves insulin sensitivity | [162] | |
| cornelian cherry | ZDF rats | reduces blood glucose level | [163] |
| olive oil | in vivo and in vitro models | acts preventively against inflammation and oxidative stress in pancreatic β-cells, improves β-cells capacity, insulin resistance and adipocyte differentiation | [164] |
| sesame oil | STZ-induced rats | reduces blood glucose level | [165] |
| in vitro (3T3-L1 adipose cells) | inhibits alpha-glucosidase activity, possesses insulin-like effect | [43] | |
| carrot | alloxan-induced diabetic rats | has antidiabetic, haematinic and anti-cholesterolemic impacts | [166] |
| Combination Therapy | Models Used | Known Synergistic Effects and Mechanisms of Action | References |
|---|---|---|---|
| BIGUANIDES WITH NATURAL PRODUCTS | |||
| metformin + resveratrol | in vivo and in vitro models | enhances hyperglycaemia, dyslipidaemia, insulin resistance, pro-inflammatory response, and lipid peroxidation | [234] |
| metformin + garlic extract | STZ-induced rats | reduces blood glucose levels | [235] |
| THIAZOLIDINEDIONES WITH NATURAL PRODUCTS | |||
| pioglitazone + curcumin | alloxan-induced diabetic rats | has beneficial impact on the pharmacokinetics and pharmacodynamics | [236] |
| SULPHONYLUREAS WITH NATURAL PRODUCTS | |||
| glibenclamide + sesame oil | patients with T2DM (open label study, n = 60) | has anti-hyperglycaemic, anti-hypercholesterolemic effects, antioxidant activity | [225] |
| glibenclamide + garlic extract | STZ-induced rats | has hypoglycaemic effect, increases body weight | [47] |
| gliclazide + garlic extract | STZ-induced rats | reduces blood glucose level | [237] |
| glimepiride + curcumin | STZ-induced rats | has protective effects against diabetic alterations associated with selected biochemical parameters and total antioxidant status | [238] |
| MEGLITINIDES WITH NATURAL PRODUCTS | |||
| repaglinide + curcumin | STZ-induced rats | reduces ROS and lipid peroxidation, enhances total proteins and serum insulin levels | [239] |
| ALPHA-GLUCOSIDASE INHIBITORS WITH NATURAL PRODUCTS | |||
| acarbose + green tea extract | in vitro (enzyme inhibition assay) | inhibits alpha-amylase and alpha-glucosidase activities | [240] |
| INCRETIN-BASED THERAPIES WITH NATURAL PRODUCTS | |||
| exendin-4 + epigallocatechin gallate | high-fat diabetic mice | improves glycaemic control, insulin release, insulin sensitivity and dyslipidaemia | [241] |
| SGLT2 INHIBITORS WITH NATURAL PRODUCTS | |||
| dapagliflozin + resveratrol | ob/ob mice + HK-2 cells | alleviates dapagliflozin-induced renal glucose production and gluconeogenesis through activating the PI3K/Akt pathway and supressing FoxO1 activation | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. https://doi.org/10.3390/ph14080806
Blahova J, Martiniakova M, Babikova M, Kovacova V, Mondockova V, Omelka R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals. 2021; 14(8):806. https://doi.org/10.3390/ph14080806
Chicago/Turabian StyleBlahova, Jana, Monika Martiniakova, Martina Babikova, Veronika Kovacova, Vladimira Mondockova, and Radoslav Omelka. 2021. "Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus" Pharmaceuticals 14, no. 8: 806. https://doi.org/10.3390/ph14080806
APA StyleBlahova, J., Martiniakova, M., Babikova, M., Kovacova, V., Mondockova, V., & Omelka, R. (2021). Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals, 14(8), 806. https://doi.org/10.3390/ph14080806







