GMP-Compliant Radiosynthesis of [18F]GP1, a Novel PET Tracer for the Detection of Thrombi
Abstract
:1. Introduction
2. Results and Discussion
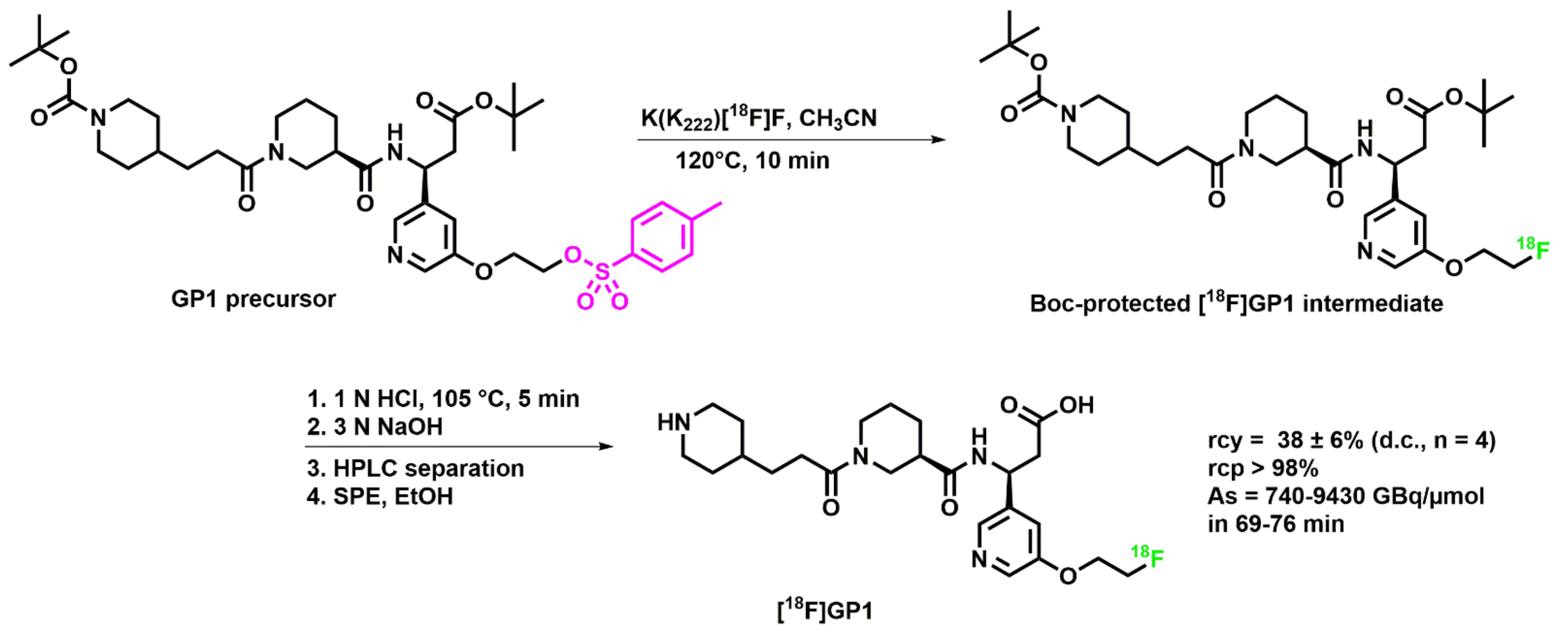
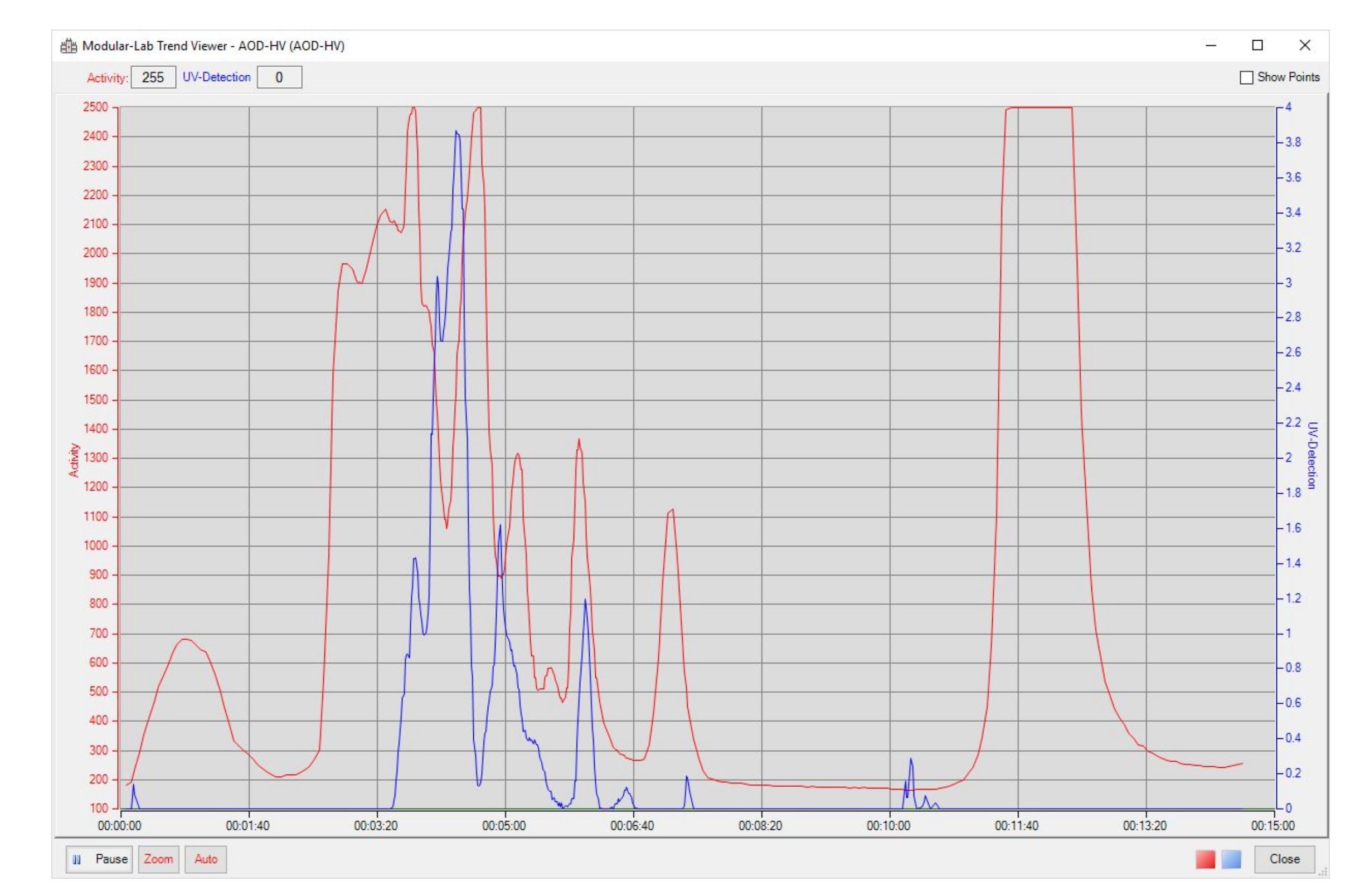
2.1. Optimization of the [18F]GP1 Synthesis
2.2. Clinical Production of [18F]GP1
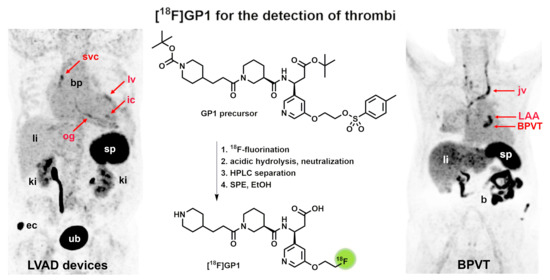
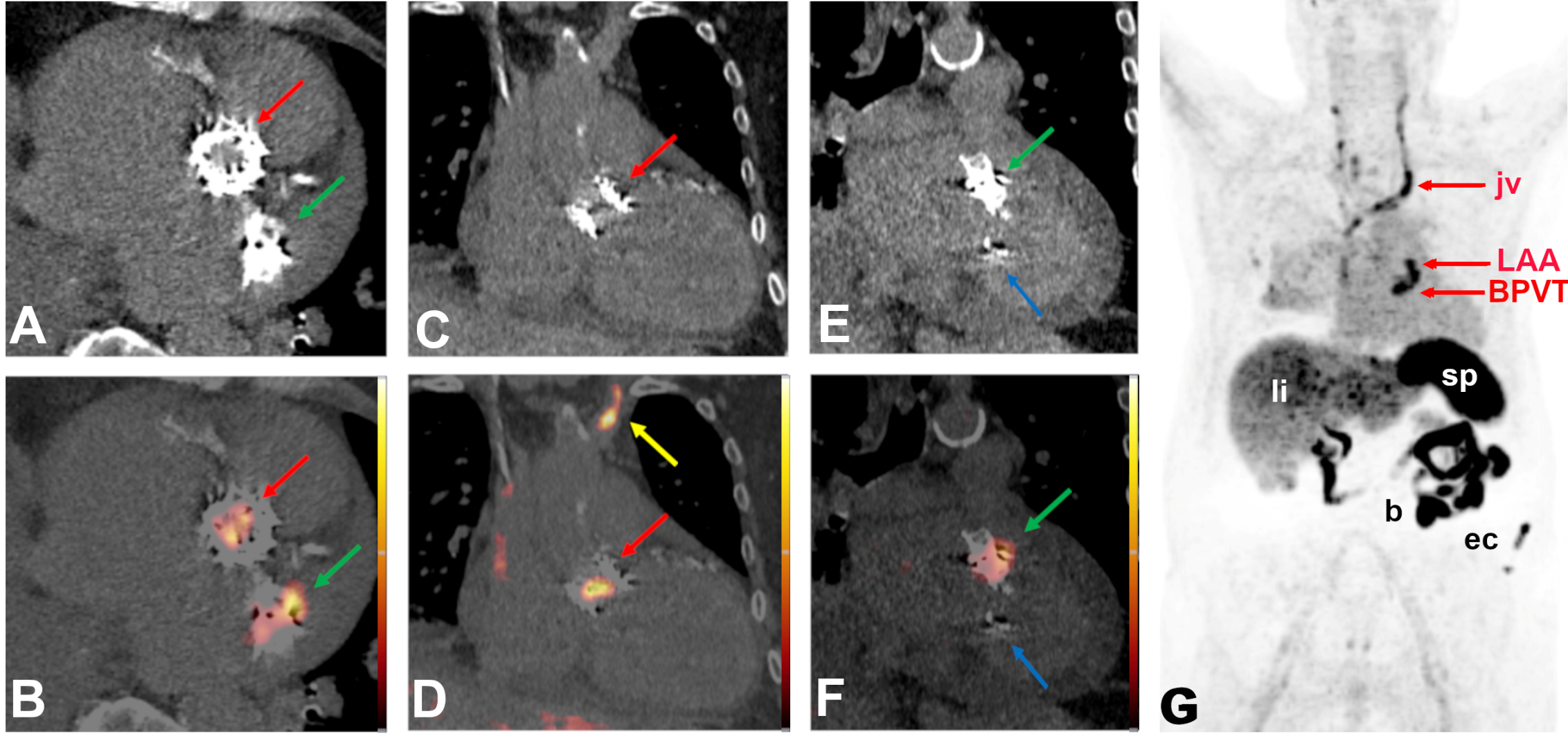
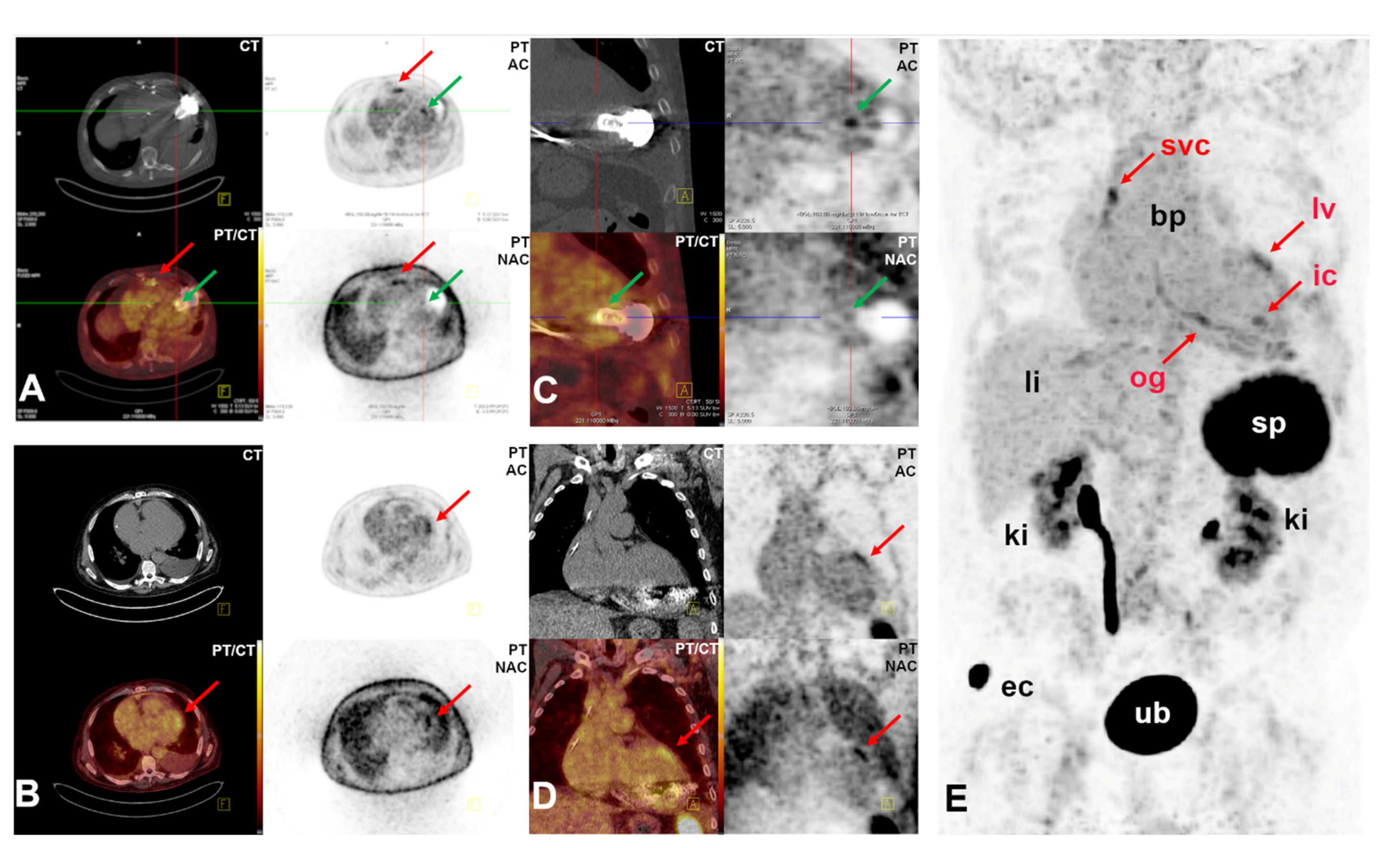
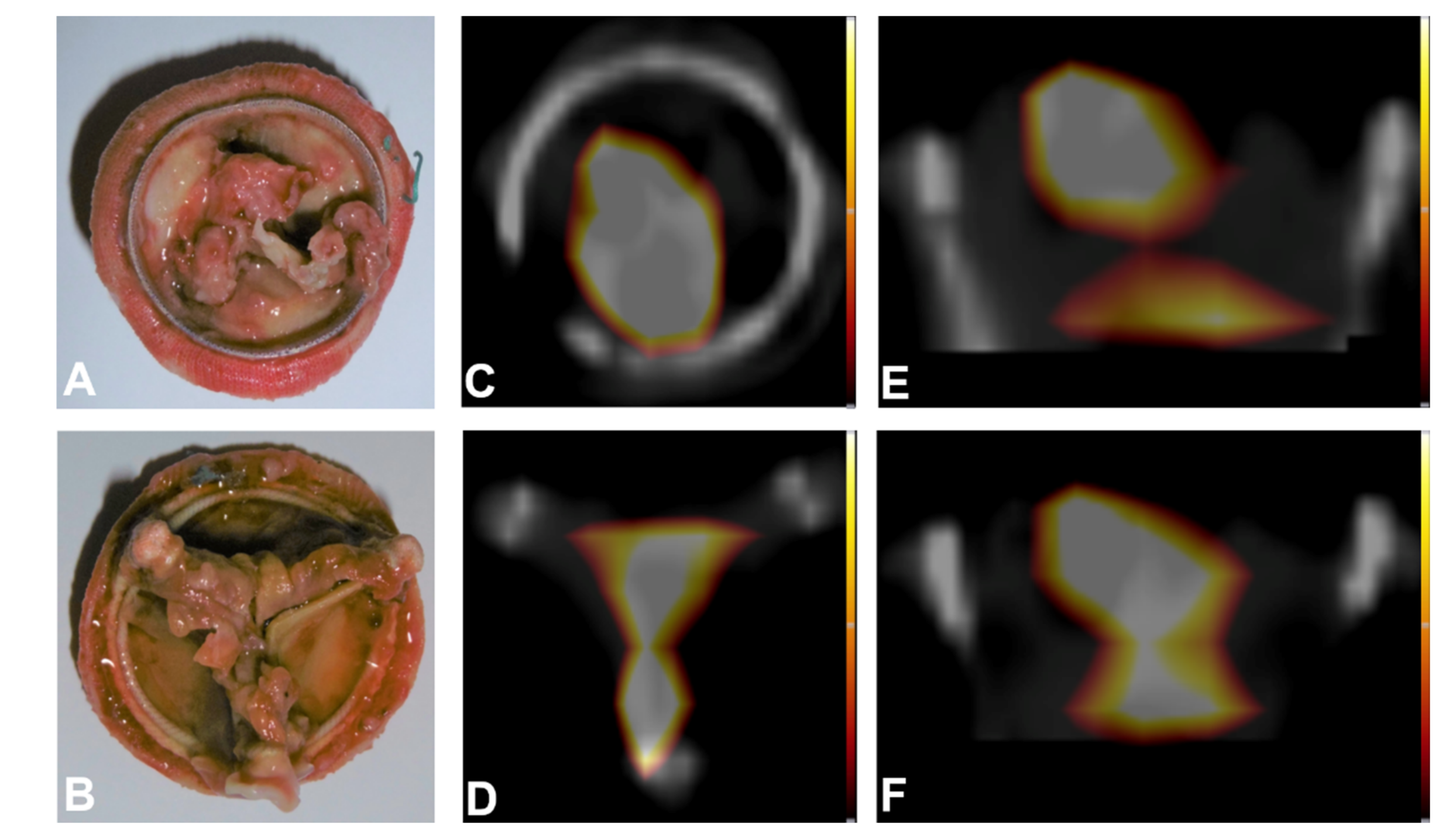
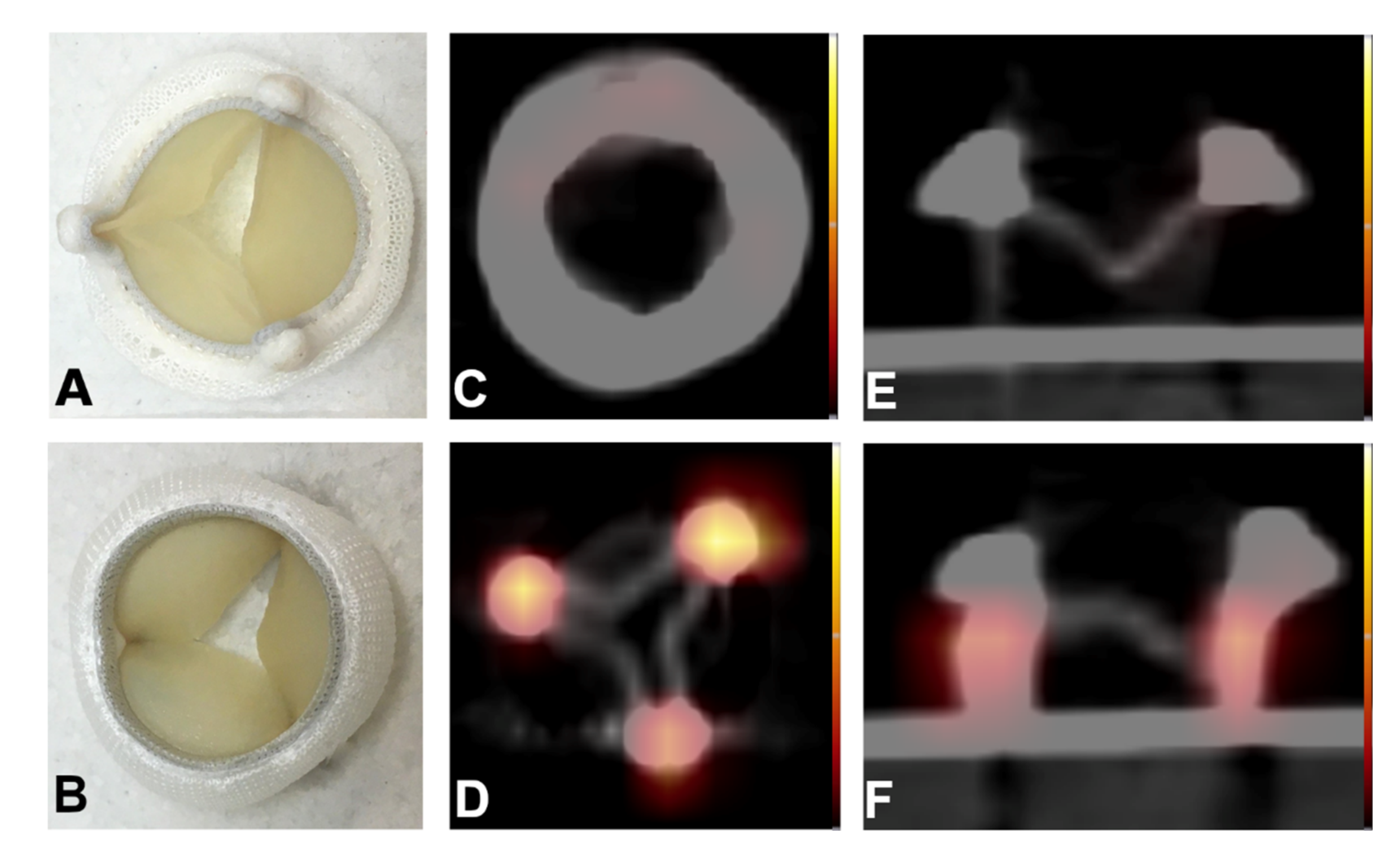
2.3. Proof-of-Concept Examples for Clinical Applications
2.4. In Vitro Experiments
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Chromatographic Methods
3.3. Radiochemistry
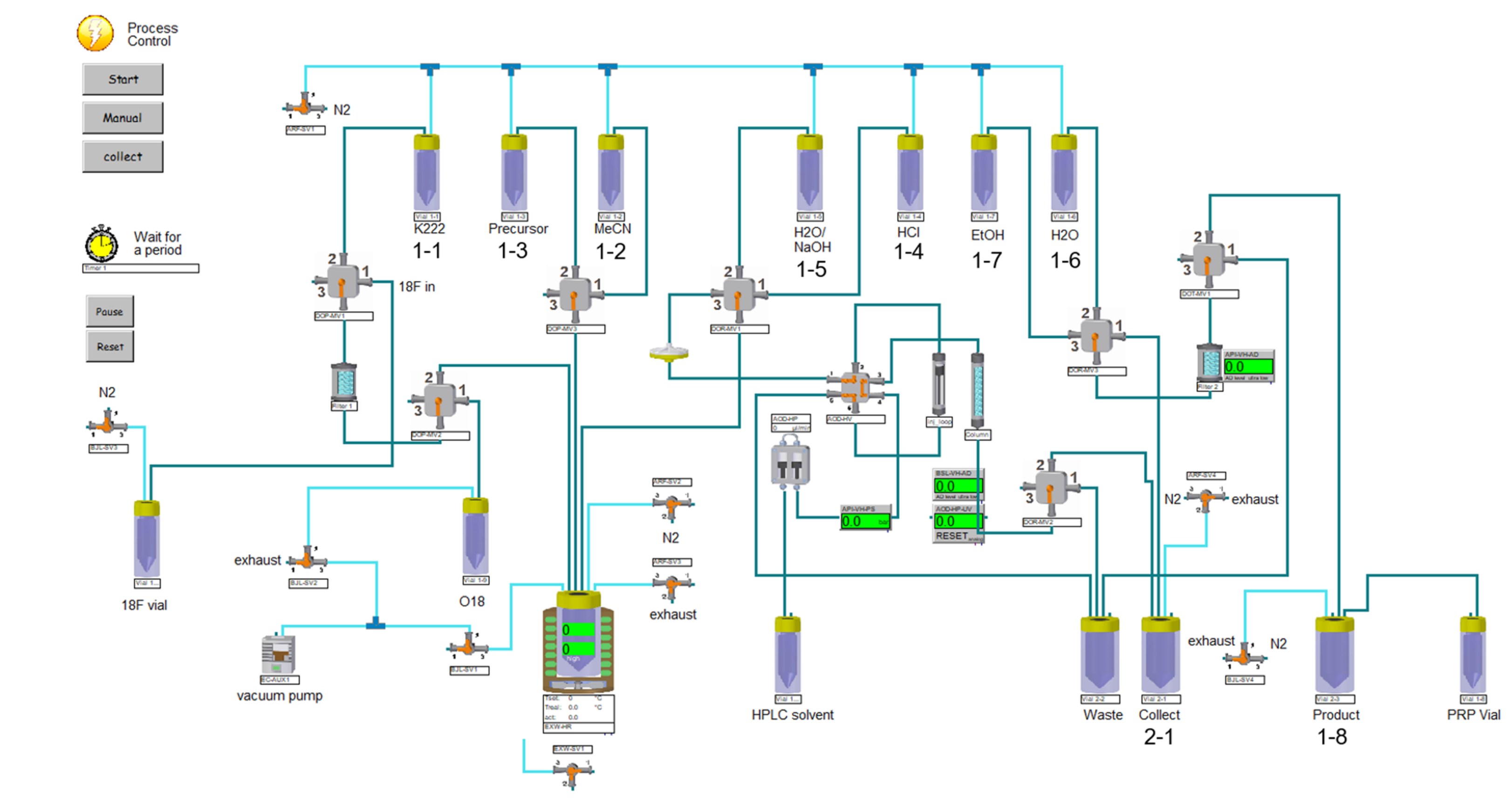
3.3.1. Synthesis module
3.3.2. Production Procedures
3.3.3. [18F]Fluoride Production
3.3.4. [18F]GP1 Production
- Transfer of the aqueous [18F]fluoride to “18F vial”.
- Trapping of [18F]fluoride on the QMA ion exchange cartridge.
- Elution from the QMA cartridge with K222/K2CO3 solution into the reactor.
- Evaporation of the solvent in the reactor at 125 °C under nitrogen flow and vacuum.
- Addition of acetonitrile from vial 1-3. Evaporation at 125 °C under nitrogen flow and vacuum.
- Cooling of the reactor to 80 °C. Addition of the precursor in anhydrous acetonitrile from vial 1-2.
- Heating the reaction mixture in the reactor at 120 °C with closed exhaust for 10 min.
- Cooling the reactor to 80 °C, addition of HCl from vial 1-5.
- Heating at 105 °C for 5 min with closed exhaust.
- Cooling the reactor to 80 °C. Addition of WFI/NaOH from vial 1-4.
- Transfer of the reaction mixture through the vented Cathivex®-filter to be loaded onto the HPLC loop.
- Injection of the loop content onto the HPLC column and elution with 86% 10 mM glycine buffer pH 10.0/14% ethanol at 5 mL/min.
- Manual collection of the product fraction in vial 2-1 pre-loaded with 70 mL WFI.
- Transfer of the resulting mixture from vial 2-1 through the solid phase extraction cartridge (tC18 Plus short, Sep-Pak).
- Rinsing of the SPE with 5 mL WFI from vial 1-6.
- Elution of the product off the SPE using 1.5 mL ethanol from vial 1-7. Collecting the product in vial 1-8, pre-loaded with 16.5 mL sodium ascorbate solution.
- Using nitrogen pressure, passing the product solution from product vial 1-8 through a 0.22 μm sterile membrane filter into the sterile, filter-vented final product vial.
- Samples for quality control, sterility testing and reserve sample were withdrawn in a laminar-airflow (LAF) cabinet with grade A environment inside.
3.4. Quality Control
3.5. Qualification and Validation
3.5.1. Validation of the Synthesis Procedure
3.5.2. Validation of Analytical Methods
3.6. [18F]GP1 PET/CT Imaging
3.7. Experimental in Vitro Set Up
3.8. Western Blot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report from the American Heart Association. Circulation 2017, 135, 146–603. [Google Scholar] [CrossRef]
- Springer, T.A.; Zhu, J.; Xiao, T. Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J. Cell. Biol. 2008, 182, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, A.W.; Koglin, N.; Dinkelborg, L.M. Commentary to 18F-GP1, a Novel PET Tracer Designed for High-Sensitivity, Low-Background Detection of Thrombi: Imaging Activated Platelets in Clots-Are We Getting There? Mol. Imaging 2018, 17, 1536012117749052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, S.Y.; Kwon, T.W.; Jin, S.; Kwon, S.U.; Sung, C.; Oh, S.J.; Lee, S.J.; Oh, J.S.; Han, Y.; Cho, Y.-P.; et al. A phase 1, first-in-human study of (18)F-GP1 positron emission tomography for imaging acute arterial thrombosis. EJNMMI Res. 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Oh, I.; Chae, S.Y.; Jin, J.; Oh, S.J.; Lee, S.J.; Koglin, N.; Berndt, M.; Stephens, A.W.; Oh, J.S.; et al. Radiation dosimetry of [18F]GP1 for imaging activated glycoprotein IIb/IIIa receptors with positron emission tomography in patients with acute thromboembolism. Nucl. Med. Biol 2019, 72-73, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, J.S.; Han, Y.; Chae, S.Y.; Jin, S.; Sung, C.; Son, H.J.; Oj, S.J.; Lee, S.J.; Oh, J.S.; et al. Glycoprotein IIb/IIIa receptor imaging with 18F-GP1 positron emission tomography for acute venous thromboembolism: An open-label, non-randomized, first-in-human phase 1 study. J. Nucl. Med. 2019, 60, 244–249. [Google Scholar] [CrossRef] [Green Version]
- Lohrke, J.; Siebeneicher, H.; Berger, M.; Reinhardt, M.; Berndt, M.; Mueller, A.; Zerna, M.; Koglin, N.; Oden, F.; Bauser, M.; et al. 18F-GP1, a Novel PET Tracer Designed for High-Sensitivity, Low-Background Detection of Thrombi. J. Nucl. Med. 2017, 58, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prosthetic Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.T.; Holmes, D.R.; Mack, M.J.; Delgado, V.; Makkar, R.; Blanke, P.; Leipsic, J.A.; Leon, M.B.; Bax, J.J. Leaflet immobility and thrombosis in transcatheter aortic valve replacement. Eur. Heart. J. 2020, 41, 3184–3197. [Google Scholar] [CrossRef]
- Puri, R.; Auffret, V.; Rodes-Cabau, J. Bioprosthetic Valve Thrombosis. J. Am. Coll. Cardiol. 2017, 69, 2193–2211. [Google Scholar] [CrossRef]
- Rosseel, L.; De Backer, O.; Sondergaard, L. Clinical Valve Thrombosis and Subclinical Leaflet Thrombosis Following Transcatheter Aortic Valve Replacement: Is There a Need for a Patient-Tailored Antithrombotic Therapy? Front. Cardiovasc. Med. 2019, 6, 44. [Google Scholar] [CrossRef]
- Blanke, P.; Leipsic, J.A.; Popma, J.J.; Yakubov, S.J.; Deeb, G.M.; Gada, H.; Mumtaz, M.; Ramlawi, B.; Kleiman, N.S.; Sorajja, P.; et al. Bioprosthetic Aortic Valve Leaflet Thickening in the Evolut Low Risk Sub-Study. J. Am. Coll. Cardiol. 2020, 75, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Blanke, P.; Leipsic, J.A.; Thourani, V.; Chakravarty, T.; Brown, D.; Trento, A.; Guyton, R.; Babaliaros, V.; Williams, M.; et al. Subclinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Valves: PARTNER 3 Cardiac Computed Tomography Substudy. J. Am. Coll. Cardiol. 2020, 75, 3003–3015. [Google Scholar] [CrossRef]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Julaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Del Trigo, M.; Munoz-Garcia, A.J.; Latib, A.; Wijeysundera, H.C.; Nombela-Franco, L.; Gutierrez, E.; Cheema, A.N.; Serra, V.; Amat-Santos, I.J.; Kefer, J.; et al. Impact of anticoagulation therapy on valve haemodynamic deterioration following transcatheter aortic valve replacement. Heart 2018, 104, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Hugenberg, V.; Burchert, W.; Preuss, R.; Koglin, N.; Berndt, M.; Stephens, A.; Feldmann, C.; Kassner, A.; Milting, H. Detection of Thrombi inside LVADs using F-18-GP1 PET/CT—Preliminary Results. EJNMMI 2019, 46, S98–S99. [Google Scholar]
- Gummert, J.F.; Haverich, A.; Schmitto, J.D.; Potapov, E.; Schramm, R.; Falk, V. Permanent Implantable Cardiac Support Systems. Dtsch. Arztebl. Int. 2019, 116, 843–848. [Google Scholar] [CrossRef]
- Schramm, R.; Zittermann, A.; Morshuis, M.; Schoenbrodt, M.; Von Roessing, E.; Von Dossow, V.; Koster, A.; Fox, H.; Hakim-Meibodi, K.; Gummert, J.F. Comparing short-term outcome after implantation of the HeartWare® HAVAD® and Abbott® HeartMate3®. ESC Heart Fail. 2020, 7, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Gyoten, T.; Morshuis, M.; Rojas, S.V.; Deutsch, M.-A.; Schramm, R.; Gummert, J.F.; Fox, H. Identification of characteristics, risk factors, and predictors of recurrent LVAD thrombosis: Conditions in HeartWare devices. J. Artif. Organs 2021, 24, 173–181. [Google Scholar] [CrossRef]
- Gyoten, T.; Rojas, S.V.; Irimie, A.; Schramm, R.; Morshuis, M.; Gummert, J.F.; Sitzer, M.; Fox, H. Patients with ventricular assist device and cerebral entrapment—Supporting skullcap reimplantation. Artif. Organs. 2021, 45, 473–478. [Google Scholar] [CrossRef]
- Hilal, T.; Mudd, J.; DeLoughery, T.G. Hemostatic complications associated with ventricular assist devices. Res. Pract. Thromb. Haemost. 2019, 3, 589–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capodanno, D.; Petronio, A.S.; Prendergast, B.; Eltchaninoff, H.; Vahanian, A.; Modine, T.; Lancellotti, P.; Søndergaard, L.; Ludman, P.F.; Tamburino, C.; et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: A consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2017, 38, 3382–3390. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, J.R.; Foster, T.J.; Cox, D. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 2006, 4, 445–457. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; EDQM Council of Europe: Strasbourg, France, 2021.
- Chaly, T.; Dahl, J.R. Thin layer chromatographic detection of kryptofix 2.2.2 in routine synthesis of [18F]2-fluoro-2-deoxy-D-glucose. Int. J. Rad. Appl. Instrum. B 1989, 16, 385–387. [Google Scholar] [CrossRef]
- EudraLex-Volume 4. EU Guidelines of Good Manufacturing Practice (GMP) for Medicinal Products for Human and Veterinary Use. In Annex 15: Qualification and Validation; European Commission: B-1049 Brussels, Belgium, 2015. [Google Scholar]
- EudraLex-Volume 4: EU Guidelines of Good Manufacturing Practice (GMP) for Medicinal Products for Human and Veterinary Use. Annex 3: Manufacture of Radiopharmaceuticals; European Commission: Brussels, Belgium, 2008. [Google Scholar]
| Parameter | Acceptance criteria | Method | Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 |
|---|---|---|---|---|---|---|---|
| Appearance | Clear, free of particles | Visual inspection | conforms | conforms | conforms | conforms | conforms |
| Radiochemical identity | Rt([18F]GP1) = Rt (GP1 reference) ± 10% | HPLC/ γ-detector | conforms | conforms | conforms | conforms | conforms |
| Radiochemical purity | [18F]GP1 ≥ 90% | HPLC/ γ-detector | 99.6% | 99.4% | 99.6% | > 99.9% | 98.6% |
| Chemical purity | cGP1:([18F]GP1 + [19F]GP1): ≤ 10.0 µg/dose 1 | HPLC/UV-detector | 0.12 µg/mL | 0.34 µg/mL | 0.14 µg/mL | 0.04 µg/mL | 0.46 µg/mL |
| Highest unspecific impurities 2: ≤1.5 µg/dose 1 | HPLC/UV-detector | <0.15 µg/mL | <0.15 µg/mL | <0.15 µg/mL | <0.15 µg/mL | <0.15 µg/mL | |
| K222 ≤ 50 µg/mL | TLC | <50 µg/mL | <50 µg/mL | <50 µg/mL | <50 µg/mL | <50 µg/mL | |
| Acetonitrile: ≤410 µg/mL | GC | <410 µg/mL | <410 µg/mL | <410 µg/mL | <410 µg/mL | <410 µg/mL | |
| Ethanol: ≤79,000 µg/mL | GC | <79,000 µg/mL | <79,000 µg/mL | <79,000 µg/mL | <79,000 µg/mL | <79,000 µg/mL | |
| Radionuclidic purity | ≥99.9% | HPGe detector | >99.9% | >99.9% | >99.9% | >99.9% | >99.9% |
| Radionuclidic identity | Deviation: <±4.5% | Dose calibrator | −0.49% | −2.56% | −0.27% | 2.46% | 0.01% |
| pH | 4.0 ≤ pH ≤ 8.5 | Potentiometry | 7.7 | 7.5 | 7.4 | 7.7 | 7.2 |
| Bacterial endotoxins | ≤17.5 EU/mL | Ph. Eur. | <0.25 EU/mL | <0.25 EU/mL | <0.25 EU/mL | <0.25 EU/mL | <0.25 EU/mL |
| Sterile filter integrity | ≥3500 mbar | Bubble point test | conforms | conforms | conforms | conforms | conforms |
| Sterility 3 | sterile | Ph. Eur. | sterile | sterile | sterile | sterile | sterile |
| Strength at EOS | cEOS ≥ 50 MBq/mL | Dose calibrator | 612 MBq/mL | 676 MBq/mL | 721 MBq/mL | 788 MBq/mL | 1330 MBq/mL |
| Specific molar activity | ; | 2440 GBq/µmol | 952 GBq/µmol | 2465 GBq/µmol | 9428 GBq/µmol | 1388 GBq/µmol | |
| Radiochemical yield (d.c.) 4 | 34.0% | 32.0% | 37.2% | 43.6% | 35.3% | ||
| Synthesis time | 69 min | 72 min | 76 min | 68 min | 76 min |
| Vial No | Preparation Steps |
|---|---|
| 1-1 | 1.5 mL of K222/K2CO2 solution (1.0 mg K2CO3, 5.0 mg K222, 0.25 mL WFI, 1.25 mL acetonitrile) |
| 1-3 | 1 mL of anhydrous acetonitrile |
| 1-2 | 7 mg GP1 precursor dissolved in 0.5 mL anhydrous acetonitrile |
| 1-5 | 3 mL WFI + 0.25 mL 3 N NaOH |
| 1-4 | 1.0 mL 1N HCl |
| 1-6 | 5 mL WFI |
| 1-7 | 1.5 mL ethanol |
| 1-8 | 16.5 mL sodium ascorbic acid solution (27.3 mg/mL in WFI) (Product) |
| 2-1 | 70 mL WFI (Collect) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugenberg, V.; Zerna, M.; Berndt, M.; Zabel, R.; Preuss, R.; Rolfsmeier, D.; Wegener, J.; Fox, H.; Kassner, A.; Milting, H.; et al. GMP-Compliant Radiosynthesis of [18F]GP1, a Novel PET Tracer for the Detection of Thrombi. Pharmaceuticals 2021, 14, 739. https://doi.org/10.3390/ph14080739
Hugenberg V, Zerna M, Berndt M, Zabel R, Preuss R, Rolfsmeier D, Wegener J, Fox H, Kassner A, Milting H, et al. GMP-Compliant Radiosynthesis of [18F]GP1, a Novel PET Tracer for the Detection of Thrombi. Pharmaceuticals. 2021; 14(8):739. https://doi.org/10.3390/ph14080739
Chicago/Turabian StyleHugenberg, Verena, Marion Zerna, Mathias Berndt, Reinhard Zabel, Rainer Preuss, Dirk Rolfsmeier, Janet Wegener, Henrik Fox, Astrid Kassner, Hendrik Milting, and et al. 2021. "GMP-Compliant Radiosynthesis of [18F]GP1, a Novel PET Tracer for the Detection of Thrombi" Pharmaceuticals 14, no. 8: 739. https://doi.org/10.3390/ph14080739
APA StyleHugenberg, V., Zerna, M., Berndt, M., Zabel, R., Preuss, R., Rolfsmeier, D., Wegener, J., Fox, H., Kassner, A., Milting, H., Koglin, N., Stephens, A. W., Gummert, J. F., Burchert, W., & Deutsch, M.-A. (2021). GMP-Compliant Radiosynthesis of [18F]GP1, a Novel PET Tracer for the Detection of Thrombi. Pharmaceuticals, 14(8), 739. https://doi.org/10.3390/ph14080739