GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients
Abstract
:1. Introduction
2. Results
2.1. Demography
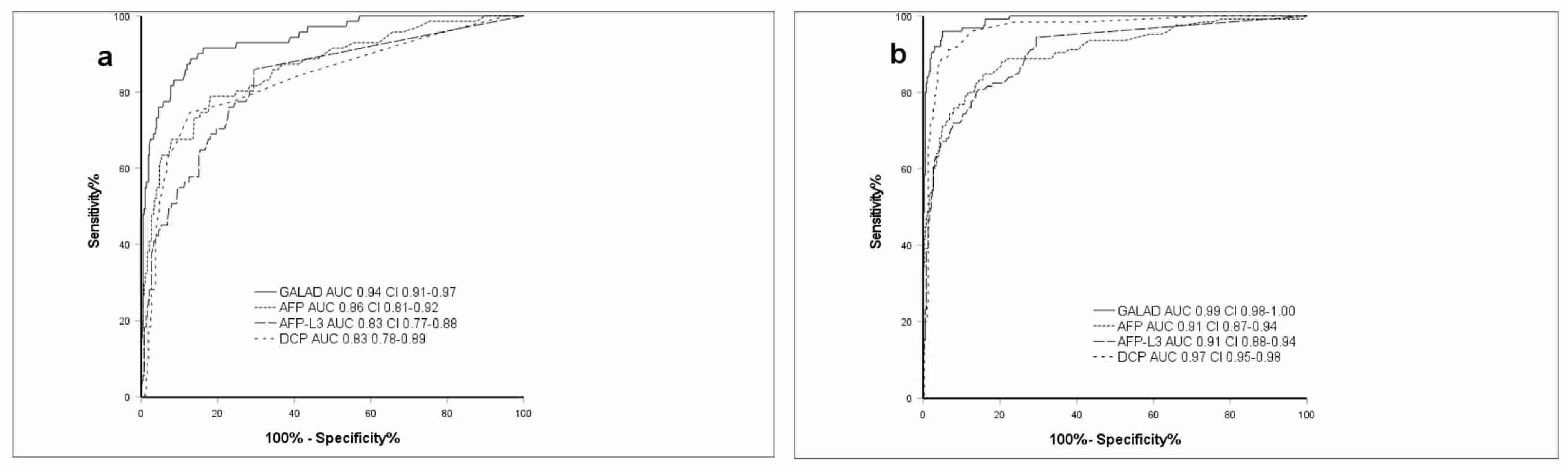
2.2. Performance of GALAD Compared to Individual Markers in All Etiologies
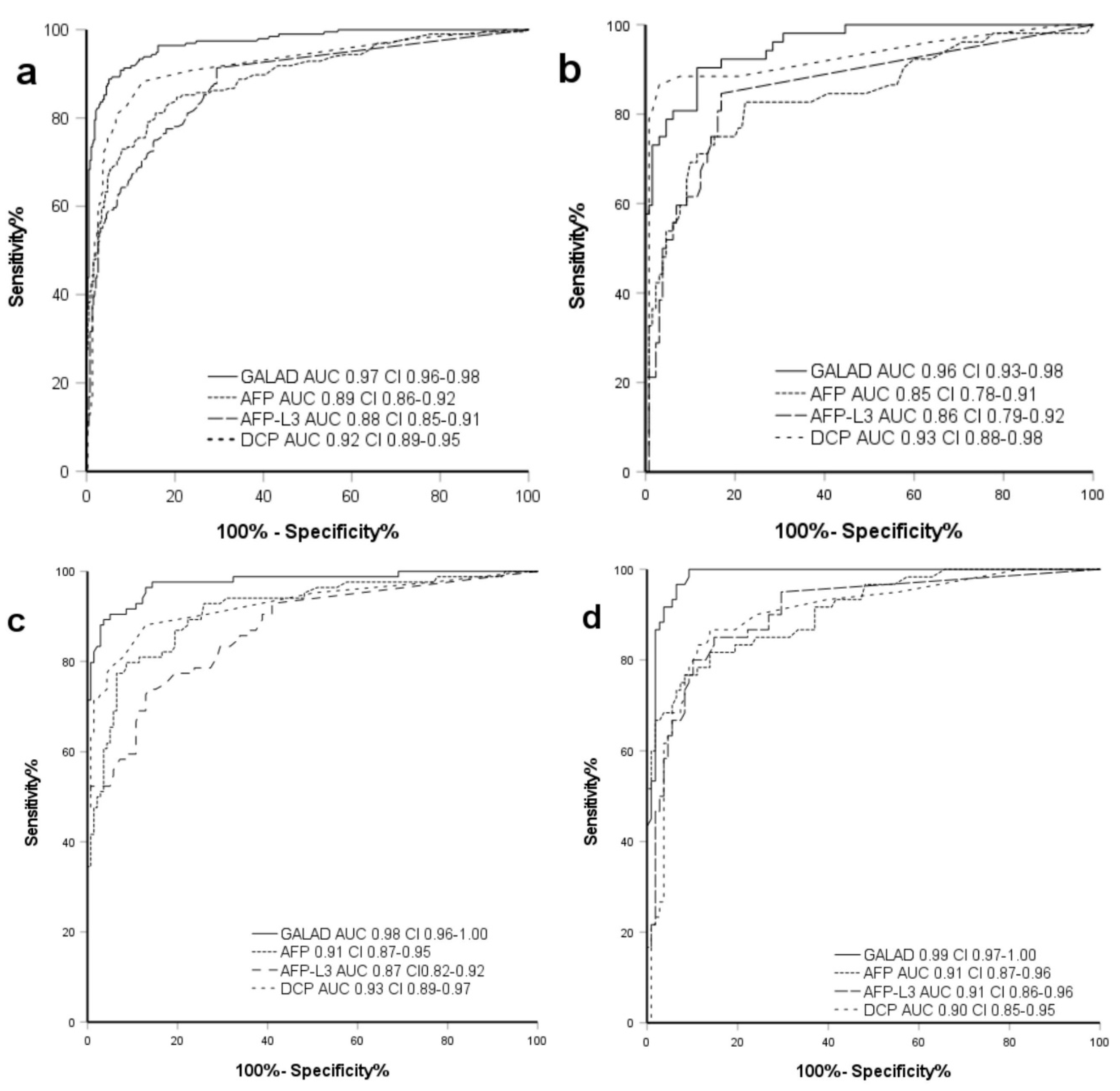
2.3. Demographics and GALAD Performance in Chronic HBV
2.4. Demographics and GALAD Performance in Chronic HCV
2.5. Demographics and GALAD Performance in Other Etiologies of Chronic Liver Disease
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Retrospective Cohort Study
4.3. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Serag, H.B. Hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kanwal, F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014, 60, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, R.; Colombo, M. Should surveillance for liver cancer be modified in hepatitis C patients after treatment-related cirrhosis regression? Liver Int. 2016, 36, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Arends, J.; Bartenstein, P.; Bechstein, W.; Bernatik, T.; Bitzer, M.; Chavan, A.; Dollinger, M.; Domagk, D.; Drognitz, O.; Düx, M.F.S. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik und Therapie des Hepatozellulären Karzinoms, Kurzversion 1.0, AWMF Registrierungsnummer: 032-053OL. 2013. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/hcc-und-biliaere-karzinome/ (accessed on 27 July 2021).
- European Association for the Study of the Liver. Electronic address, e.e.e. and L. European Association for the Study of the, EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.M.; Berhane, S.; de Groot, C.E.; Toyoda, H.; Tada, T.; Kumada, T.; Satomura, S.; Nishida, N.; Kudo, M.; Kimura, T.; et al. Serum Levels of α-Fetoprotein Increased More Than 10 Years Before Detection of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2021, 19, 162–170.e4. [Google Scholar] [CrossRef]
- Trevisani, F.; D’Intino, P.E.; Morselli/labate, A.M.; Mazzella, G.; Accogli, E.; Caraceni, P.; Domenicali, M.; De Notariis, S.; Roda, E.; Bernardi, M. Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: Influence of HBsAg and anti-HCV status. J. Hepatol. 2001, 34, 570–575. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Hoofnagle, J.H. Elevations in serum alpha-fetoprotein levels in patients with chronic hepatitis B. Cancer 1989, 64, 2117–2120. [Google Scholar] [CrossRef]
- Di Bisceglie, A.M.; Sterling, R.K.; Chung, R.T.; Everhart, J.E.; Dienstag, J.; Bonkovsky, H.L.; Wright, E.C.; Everson, G.T.; Lindsay, K.L.; Lok, A.S.; et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: Results from the HALT-C Trial. J. Hepatol. 2005, 43, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Kumada, T.; Kiriyama, S.; Sone, Y.; Tanikawa, M.; Hisanaga, Y.; Yamaguchi, A.; Isogai, M.; Kaneoka, Y.; Washizu, J. Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2006, 4, 111–117. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Ertle, J.M.; Heider, D.; Wichert, M.; Keller, B.; Kueper, R.; Hilgard, P.; Gerken, G.; Schlaak, J.F. A combination of alpha-fetoprotein and des-gamma-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion 2013, 87, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y. Diagnostic value of AFP-L3 and PIVKA-II in hepatocellular carcinoma according to total-AFP. World J. Gastroenterol. 2013, 19, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef]
- Johnson, P.J.; Pirrie, S.J.; Cox, T.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886.e6. [Google Scholar] [CrossRef] [Green Version]
- Best, J.; Bechmann, L.P.; Sowa, J.-P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzaferro, V.M.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. New Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Yang, J.D.; Dai, J.; Singal, A.G.; Gopal, P.; Addissie, B.D.; Nguyen, M.H.; Befeler, A.S.; Reddy, K.R.; Schwartz, M.; Harnois, D.M.; et al. Improved Performance of Serum Alpha-Fetoprotein for Hepatocellular Carcinoma Diagnosis in HCV Cirrhosis with Normal Alanine Transaminase. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lok, A.S.; Seeff, L.B.; Morgan, T.R.; di Bisceglie, A.M.; Sterling, R.K.; Curto, T.M.; Everson, G.T.; Lindsay, K.L.; Lee, W.M.; Bonkovsky, H.L.; et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009, 136, 138–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asselah, T.; Rubbia-Brandt, L.; Marcellin, P.; Negro, F. steatosis in chronic hepatitis C: Why does it really matter? Gut 2006, 55, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma: An update. Hepatology 2011, 53, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Bilgi, H.; Heider, D.; Schotten, C.; Manka, P.; Bedreli, S.; Gorray, M.; Ertle, J.; van Grunsven, L.; Dechêne, A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z. Gastroenterol. 2016, 54, 1296–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.; Mo, F.; Johnson, P.J.; Siu, D.Y.W.; Chan, M.H.M.; Lau, W.Y.; Lai, P.B.-S.; Lam, C.W.K.; Yeo, W.; Yu, S.C.H. Performance of serum α-fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB 2014, 16, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.K.; Song, M.J.; Kim, S.H.; Park, M. Comparing various scoring system for predicting overall survival according to treatment modalities in hepatocellular carcinoma focused on Platelet-albumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grade: A nationwide cohort study. PLoS ONE 2019, 14, e0216173. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2016. J. Hepatol. 2017, 66, 153–194. [Google Scholar] [CrossRef] [Green Version]
| Parameter | Units | HBV | HCV | OE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCC (n = 52) | Non-HCC (n = 130) | p-Value | HCC (n = 84) | Non-HCC (n = 139) | p-Value | HCC (n = 60) | Non-HCC (n = 108) | p-Value | ||
| Age (SD) | (Years) | 62.5 (11.8) | 47.2 (13.9) | ≤0.05 b | 64.3 (9.4) | 57.3 (13.2) | ≤0.05 b | 69.6 (7.9) | 59.7 (14.5) | ≤0.05 b |
| Sex m/f | N | 44/8 | 91/39 | 0.06 a | 59/25 | 79/60 | ≤0.05 a | 49/11 | 45/63 | ≤0.05 a |
| Child-Pugh grade n (%) | No cirrhosis (n%) | 16 (30.8) | 114 (87.7) | ≤0.05 a | 7 (8.3) | 81 (58.3) | ≤0.05 a | 11 (18.3) | 59 (54.6) | ≤0.05 a |
| A (n%) | 25 (48.1) | 13 (10.0) | 0.60 a | 54 (64.3) | 49 (35.4) | 0.16 a | 31 (51.7) | 35 (32.4) | 0.50 a | |
| B (n%) | 10 (19.2) | 3 (2.3) | 23 (27.4) | 9 (6.5) | 15 (25.0) | 10 (9.3) | ||||
| C (n%) | 1 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (6.7) | 4 (3.7) | ||||
| MELD Score | Mean (SD) | 11.00 (3.06) | 10.60 (6.47) | 0.90 b | 12.64 (3.92) | 11.20 (4.21) | 0.16 b | 11.86 (4.06) | 11.65 (4.35) | 0.90 b |
| BMI (SD) | (kg/m2) | 25.91 (3.20) | 26.34 (4.49) | 0.59 b | 25.24 (4.69) | 27.9 (4.99) | ≤0.05 b | 29.53 (4.58) | 27.81 (5.95) | ≤0.05 b |
| ALBI Score (IQR) | (−2.60) | −2.92 (0.67) | −3.08 (0.38) | ≤0.05 c | −2.71 (0.65) | −3.05 (0.58) | ≤0.05 c | −2.69 (0.73) | −3.08 (0.67) | ≤0.05 c |
| ALBI grade, n (%) | 1 (n%) | 32 (68.1) | 113 (92.6) | 0.68 a | 53 (63.9) | 110 (82.1) | 0.60 a | 30 (55.6) | 24 (77.4) | 0.84 a |
| 2 (n%) | 11 (23.4) | 8 (6.6) | 29 (34.9) | 24 (17.9) | 20 (37.0) | 6 (19.4) | ||||
| 3 (n%) | 4 (8.5) | 1 (0.8) | 1 (1.2) | 0 (0.0) | 4 (7.4) | 1 (3.2) | ||||
| BCLC Stage | 0 (%) | 5 (9.6) | 8 (9.5) | 1 (1.7) | ||||||
| A (%) | 13 (25.0) | 34 (40.5) | 9 (15.0) | |||||||
| B (%) | 26 (50.0) | 27 (32.1) | 40 (66.7) | |||||||
| C (%) | 7 (13.5) | 15 (17.9) | 9 (15.0) | |||||||
| D (%) | 1 (1.9) | 0 (0.0) | 1 (1.7) | |||||||
| Nodules | (number) (SD) | 1.79 (1.07) | 1.93 (1.11) | 1.92 (1.10) | ||||||
| Tumor size (major nodule) | (cm) (SD) | 5.11 (3.28) | 4.19 (2.85) | 6.64 (4.05) | ||||||
| Parameter (Normal Values) | HBV | HCV | OE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HCC (n = 52) | Non-HCC (n = 130) | p-Value | HCC (n = 84) | Non-HCC (n = 139) | p-Value | HCC (n = 60) | Non-HCC (n = 108) | p-Value | |
| AST (SD) (U/L; m < 50/f < 35) | 71.8 (57.87) | 46.7 (105.35) | ≤0.05 | 95.8 (91.81) | 50.8 (51.01) | ≤0.05 | 111.4 (250.43) | 42.4 (25.04) | ≤0.05 |
| ALT (SD) (U/ML; m < 50/f < 35) | 64.5 (46.63) | 60.7 (138.46) | 0.33 | 74.8 (60.05) | 56.9 (67.32) | ≤0.05 | 57.8 (45.21) | 48.8 (37.85) | 0.18 |
| De Ritis (IQR) (0.6–0.8) | 1.1 (0.6) | 0.8 (0.5) | ≤0.05 | 1.2 (0.8) | 1.0 (0.6) | ≤0.05 | 1.3 (0.2) | 0.9 (0.6) | ≤0.05 |
| GGT (SD) (U/L; m < 55/f < 35) | 237.3 (441.32) | 44.8 (61.10) | ≤0.05 a | 187.5 (194.67) | 81.0 (118.50) | ≤0.05 a | 322.6 (318.18) | 152.3 (222.38) | ≤0.05 a |
| Bilirubin (IQR) (mg/dL; 0.3–1.2) | 0.8 (0.88) | 0.7 (0.40) | ≤0.05 b | 0.9 (0.70) | 0.65 (0.60) | ≤0.05 b | 0.9 (0.80) | 0.7 (0.80) | ≤0.05 b |
| Albumin (SD) (g/dL; 3.4–4.8) | 4.0 (0.68) | 4.5 (0.41) | ≤0.05 a | 4.1 (0.56) | 4.3 (0.46) | ≤0.05 a | 3.9 (0.65) | 4.3 (0.58) | ≤0.05 a |
| Creatinin (IQR) (mg/dL; 0.6–1.1) | 0.99 (0.17) | 0.93 (0.19) | 0.08 b | 0.94 (0.24) | 0.93 (0.21) | 0.68 b | 1.02 (0.33) | 0.98 (0.21) | 0.32 b |
| Parameter | Units | HBV | HCV | OE | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCC (n = 52) | non-HCC (n = 130) | p-Value | HCC (n = 84) | non-HCC (n = 139) | p-Value | HCC (n = 60) | non-HCC (n = 108) | p-Value | ||
| AFP (>10 ng/mL) | n (%) | 31 (59.6) | 11 (8.5) | ≤0.05 a | 67 (79.8) | 16 (11.5) | ≤0.05 a | 45 (75.0) | 8 (7.4) | ≤0.05 a |
| AFP (>20 ng/mL) | n (%) | 25 (48.1) | 6 (4.6) | ≤0.05 a | 54 (64.3) | 7 (5.0) | ≤0.05 a | 40 (66.7) | 2 (1.9) | ≤0.05 a |
| AFP (>50 ng/mL) | n (%) | 20 (38.5) | 3 (2.3) | ≤0.05 a | 41 (48.8) | 3 (2.2) | ≤0.05 a | 30 (50.0) | 0 (0.0) | ≤0.05 a |
| AFP (>100 ng/mL) | n (%) | 21 (38.5) | 1 (0.8) | ≤0.05 a | 35 (41.7) | 1 (0.7) | ≤0.05 a | 23 (38.3) | 0 (0.0) | ≤0.05 a |
| AFP (IQR) | ng/mL (20) | 22 (38.5) | 2.8 (2.0) | ≤0.05 c | 43.3 (616.5) | 4.0 (3.8) | ≤0.05 c | 50.0 (510.7) | 2.7 (2.8) | ≤0.05 c |
| AFP-L3 (IQR) | % (<10) | 23 (38.5) | 0.3 (0.0) | ≤0.05 c | 16.8 (47.1) | 0.3 (6.1) | ≤0.05 c | 25.4 (42.5) | 0.3 (5.9) | ≤0.05 c |
| DCP (IQR) | ng/mL (7.5) | 24 (38.5) | 0.3 (0.1) | ≤0.05 c | 3.2 (37.5) | 0.4 (0.2) | ≤0.05 c | 55.8 (194.3) | 0.4 (0.2) | ≤0.05 c |
| GALAD (SD) | (−0.63) | 25 (38.5) | −4.38 (1.96) | ≤0.05 b | 3.66 (4.21) | −3.21 (1.52) | ≤0.05 b | 5.51 (4.55) | −3.81 (2.15) | ≤0.05 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schotten, C.; Ostertag, B.; Sowa, J.-P.; Manka, P.; Bechmann, L.P.; Hilgard, G.; Marquardt, C.; Wichert, M.; Toyoda, H.; Lange, C.M.; et al. GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients. Pharmaceuticals 2021, 14, 735. https://doi.org/10.3390/ph14080735
Schotten C, Ostertag B, Sowa J-P, Manka P, Bechmann LP, Hilgard G, Marquardt C, Wichert M, Toyoda H, Lange CM, et al. GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients. Pharmaceuticals. 2021; 14(8):735. https://doi.org/10.3390/ph14080735
Chicago/Turabian StyleSchotten, Clemens, Bastian Ostertag, Jan-Peter Sowa, Paul Manka, Lars P. Bechmann, Gudrun Hilgard, Claudio Marquardt, Marc Wichert, Hidenori Toyoda, Christian M. Lange, and et al. 2021. "GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients" Pharmaceuticals 14, no. 8: 735. https://doi.org/10.3390/ph14080735
APA StyleSchotten, C., Ostertag, B., Sowa, J.-P., Manka, P., Bechmann, L. P., Hilgard, G., Marquardt, C., Wichert, M., Toyoda, H., Lange, C. M., Canbay, A., Johnson, P., Wedemeyer, H., & Best, J. (2021). GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients. Pharmaceuticals, 14(8), 735. https://doi.org/10.3390/ph14080735






