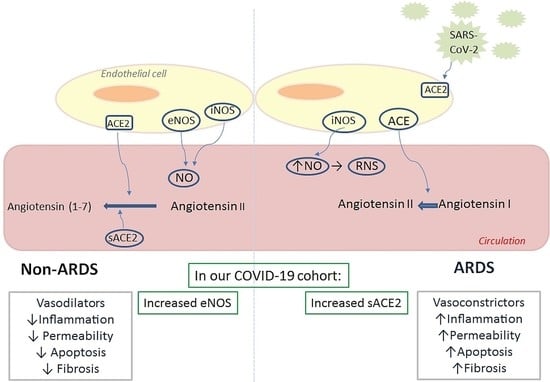
Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS)
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vane, J.R.; Anggard, E.E.; Botting, R.M. Regulatory functions of the vascular endothelium. N. Engl. J. Med. 1990, 323, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.D.; Evans, T.W. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur. Respir. J. 2003, 21, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Maniatis, N.A.; Kotanidou, A.; Catravas, J.D.; Orfanos, S.E. Endothelial pathomechanisms in acute lung injury. Vasc. Pharmacol. 2008, 49, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef]
- Guan, S.P.; Seet, R.C.S.; Kennedy, B.K. Does eNOS derived nitric oxide protect the young from severe COVID–19 complications? Ageing Res. Rev. 2020, 64, 101201. [Google Scholar] [CrossRef]
- Abdih, H.; Kelly, C.J.; Bouchier–Hayes, D.; Watson, R.W.; Redmond, H.P.; Burke, P.; Bouchier–Hayes, D.J. Nitric oxide (endothelium–derived relaxing factor) attenuates revascularization–induced lung injury. J. Surg. Res. 1994, 57, 39–43. [Google Scholar] [CrossRef]
- Garrean, S.; Gao, X.P.; Brovkovych, V.; Shimizu, J.; Zhao, Y.Y.; Vogel, S.M.; Malik, A.B. Caveolin–1 regulates NF–kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J. Immunol. 2006, 177, 4853–4860. [Google Scholar] [CrossRef]
- Kaminski, A.; Pohl, C.B.; Sponholz, C.; Ma, N.; Stamm, C.; Vollmar, B.; Steinhoff, G. Up–regulation of endothelial nitric oxide synthase inhibits pulmonary leukocyte migration following lung ischemia–reperfusion in mice. Am. J. Pathol. 2004, 164, 2241–2249. [Google Scholar] [CrossRef]
- Kaminski, A.; Kasch, C.; Zhang, L.; Kumar, S.; Sponholz, C.; Choi, Y.H.; Ma, N.; Liebold, A.; Ladilov, Y.; Steinhoff, G.; et al. Endothelial nitric oxide synthase mediates protective effects of hypoxic preconditioning in lungs. Respir. Physiol. Neurobiol. 2007, 155, 280–285. [Google Scholar] [CrossRef]
- Takenaka, K.; Nishimura, Y.; Nishiuma, T.; Sakashita, A.; Yamashita, T.; Kobayashi, K.; Satouchi, M.; Ishida, T.; Kawashima, S.; Yokoyama, M. Ventilator–induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L1078–L1086. [Google Scholar] [CrossRef]
- Yamashita, T.; Kawashima, S.; Ohashi, Y.; Ozaki, M.; Ueyama, T.; Ishida, T.; Inoue, N.; Hirata, K.; Akita, H.; Yokoyama, M. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation 2000, 101, 931–937. [Google Scholar] [CrossRef]
- Zhang, L.; Kumar, S.; Kaminski, A.; Kasch, C.; Sponholz, C.; Stamm, C.; Ladilov, Y.; Steinhoff, G. Importance of endothelial nitric oxide synthase for the hypothermic protection of lungs against ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2006, 131, 969–974. [Google Scholar] [CrossRef][Green Version]
- Gielis, J.F.; Quirynen, L.; Briede, J.J.; Roelant, E.; Cos, P.; Van Schil, P.E.Y. Pathogenetic role of endothelial nitric oxide synthase uncoupling during lung ischaemia–reperfusion injury. Eur. J. Cardio Thorac. Surg. 2017, 52, 256–263. [Google Scholar] [CrossRef]
- Drucker, N.A.; Jensen, A.R.; Te Winkel, J.P.; Ferkowicz, M.J.; Markel, T.A. Loss of endothelial nitric oxide synthase exacerbates intestinal and lung injury in experimental necrotizing enterocolitis. J. Pediatric Surg. 2018, 53, 1208–1214. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef]
- Gow, A.J.; Thom, S.R.; Ischiropoulos, H. Nitric oxide and peroxynitrite–mediated pulmonary cell death. Am. J. Physiol. 1998, 274, L112–L118. [Google Scholar] [CrossRef]
- Weinberger, B.; Laskin, D.L.; Heck, D.E.; Laskin, J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. Off. J. Soc. Toxicol. 2001, 59, 5–16. [Google Scholar] [CrossRef]
- Müller, H.C.; Witzenrath, M.; Tschernig, T.; Gutbier, B.; Hippenstiel, S.; Santel, A.; Suttorp, N.; Rosseau, S. Adrenomedullin attenuates ventilator–induced lung injury in mice. Thorax 2010, 65, 1077–1084. [Google Scholar] [CrossRef][Green Version]
- Itoh, T.; Obata, H.; Murakami, S.; Hamada, K.; Kangawa, K.; Kimura, H.; Nagaya, N. Adrenomedullin ameliorates lipopolysaccharide–induced acute lung injury in rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L446–L452. [Google Scholar] [CrossRef]
- Pagliaro, P.; Thairi, C.; Alloatti, G.; Penna, C. Angiotensin–converting enzyme 2: A key enzyme in key organs. J. Cardiovasc. Med. 2021. [Google Scholar] [CrossRef]
- Orfanos, S.E.; Armaganidis, A.; Glynos, C.; Psevdi, E.; Kaltsas, P.; Sarafidou, P.; Catravas, J.D.; Dafni, U.G.; Langleben, D.; Roussos, C. Pulmonary capillary endothelium–bound angiotensin–converting enzyme activity in acute lung injury. Circulation 2000, 102, 2011–2018. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Janssen Bonás, M.; Vorselaars, A.D.M.; Veltkamp, M. Biomarkers in the Diagnosis and Prognosis of Sarcoidosis: Current Use and Future Prospects. Front. Immunol. 2020, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID–19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef]
- Wood, K.C.; Cortese–Krott, M.M.; Kovacic, J.C.; Noguchi, A.; Liu, V.B.; Wang, X.; Raghavachari, N.; Boehm, M.; Kato, G.J.; Kelm, M.; et al. Circulating blood endothelial nitric oxide synthase contributes to the regulation of systemic blood pressure and nitrite homeostasis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Muellenbach, R.M.; Meybohm, P.; Mutlak, H.; Lepper, P.M.; Rolfes, C.B.; Peivandi, A.; Stumpner, J.; Kredel, M.; Kranke, P.; et al. Effects of inhaled nitric oxide in COVID–19–induced ARDS–Is it worthwhile? Acta Anaesthesiol. Scand. 2021, 65, 629–632. [Google Scholar] [CrossRef]
- Longobardo, A.; Montanari, C.; Shulman, R.; Benhalim, S.; Singer, M.; Arulkumaran, N. Inhaled nitric oxide minimally improves oxygenation in COVID–19 related acute respiratory distress syndrome. Br. J. Anaesth. 2021, 126, e44–e46. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pozzi, M.; Mongodi, S.; Dammassa, V.; Romito, G.; Mojoli, F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID–19 pneumonia. Crit. Care 2020, 24, 508. [Google Scholar] [CrossRef]
- Ferrari, M.; Santini, A.; Protti, A.; Andreis, D.T.; Iapichino, G.; Castellani, G.; Rendiniello, V.; Costantini, E.; Cecconi, M. Inhaled nitric oxide in mechanically ventilated patients with COVID–19. J. Crit. Care 2020, 60, 159–160. [Google Scholar] [CrossRef]
- Hupf, J.; Mustroph, J.; Hanses, F.; Evert, K.; Maier, L.S.; Jungbauer, C.G. RNA–expression of adrenomedullin is increased in patients with severe COVID–19. Crit. Care 2020, 24, 527. [Google Scholar] [CrossRef]
- Wilson, D.C.; Schefold, J.C.; Baldirà, J.; Spinetti, T.; Saeed, K.; Elke, G. Adrenomedullin in COVID–19 induced endotheliitis. Crit. Care 2020, 24, 411. [Google Scholar] [CrossRef]
- Chan, K.K.; Dorosky, D.; Sharma, P.; Abbasi, S.A.; Dye, J.M.; Kranz, D.M.; Herbert, A.S.; Procko, E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science 2020, 369, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Lockey, R.F.; Kolliputi, N. Soluble ACE2 as a potential therapy for COVID–19. Am. J. Physiol. Cell Physiol. 2021, 320, C279–C281. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B., Jr.; Fejes, Z.; Szentkereszty, Z.; Sütő, R.; Várkonyi, I.; Ajzner, É.; Kappelmayer, J.; Papp, Z.; Tóth, A.; Fagyas, M. A dramatic rise in serum ACE2 activity in a critically ill COVID–19 patient. Int. J. Infect. Dis. 2021, 103, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hasan, M.; Ahmed, A. Potential detrimental role of soluble ACE2 in severe COVID–19 comorbid patients. Rev. Med Virol. 2021. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Trusa, S.; Olson, S.C. Angiotensin type 1 receptor is linked to inhibition of nitric oxide production in pulmonary endothelial cells. Regul. Pept. 2005, 132, 113–122. [Google Scholar] [CrossRef]
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. The potential role of serum angiotensin–converting enzyme in coronavirus disease 2019. BMC Infect. Dis. 2020, 20, 883. [Google Scholar] [CrossRef]
- Avanoglu Guler, A.; Tombul, N.; Aysert Yıldız, P.; Özger, H.S.; Hızel, K.; Gulbahar, O.; Tufan, A.; Erbaş, G.; Aygencel, G.; Guzel Tunçcan, O.; et al. The assessment of serum ACE activity in COVID–19 and its association with clinical features and severity of the disease. Scand. J. Clin. Lab. Investig. 2021, 81, 160–165. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]

| Characteristics | ARDS | Non-ARDS | P-value | Reference Values |
|---|---|---|---|---|
| Number of patients, N | 68 | 21 | ||
| Age (years), (mean ± SD) | 62 ± 13 | 61 ± 13 | 0.8 | |
| Sex, N (%) | >0.9 | |||
| Male | 53 (77.9) | 17 (80.9) | ||
| Female | 15 (22.1) | 4 (19.1) | ||
| Comorbidities, N (%) | 50 (73.5) | 17 (80.9) | 0.6 | |
| ICU vs. ward, N (%) | <0.0001 * | |||
| ICU | 60 (88.2) | 7 (33.3) | ||
| Ward | 8 (11.8) | 14 (66.7) | ||
| Sick days prior to admission, (mean ± SD) | 7 ± 3 | 6 ± 4 | 0.2 | |
| APACHE II, (mean ± SD) † | 14 ± 5 | 8 ± 6 | <0.0001 * | |
| SOFA, (median, IQR) † | 6 (4–8) | 3 (2–3) | <0.0001 * | |
| White blood cell count (per μL), (median, IQR) Neutrophils (%), (median, IQR) Lymphocytes (%), (median, IQR) Platelets (per μL), (median, IQR) CRP (mg/dL), (median, IQR) Fibrinogen (mg/dL), (mean ± SD) D-dimers (µg/mL), (median, IQR) LDH (U/L), (median, IQR) Ferritin (ng/mL), (median, IQR) Lactate (mmol/L), (mean ± SD) | 8760 (5915–11,370) 83 (76–88) 12 (7–17) 225,000 (169,000–27,000) 11.5 (5.4–20.4) 630 ± 179 1.05 (0.46–2.29) 434 (339–574) 682 (265–2006) 1.5 ± 0.6 | 5630 (4355–12,405) 83 (63–87) 13 (9–29) 195,000 (155,250–269,500) 4.8 (1.6–11.1) 548 ± 151 0.74 (0.51–1.36) 257 (211–391) 376 (164–868) 1.4 ± 0.5 | 0.2 0.6 0.2 0.3 0.002 * 0.1 0.2 <0.0001 * 0.2 0.8 | 4–10.5 × 103 40–70 25–45 140–450 × 103 <0.5 200–400 <0.5 <225 10–250 <2.0 |
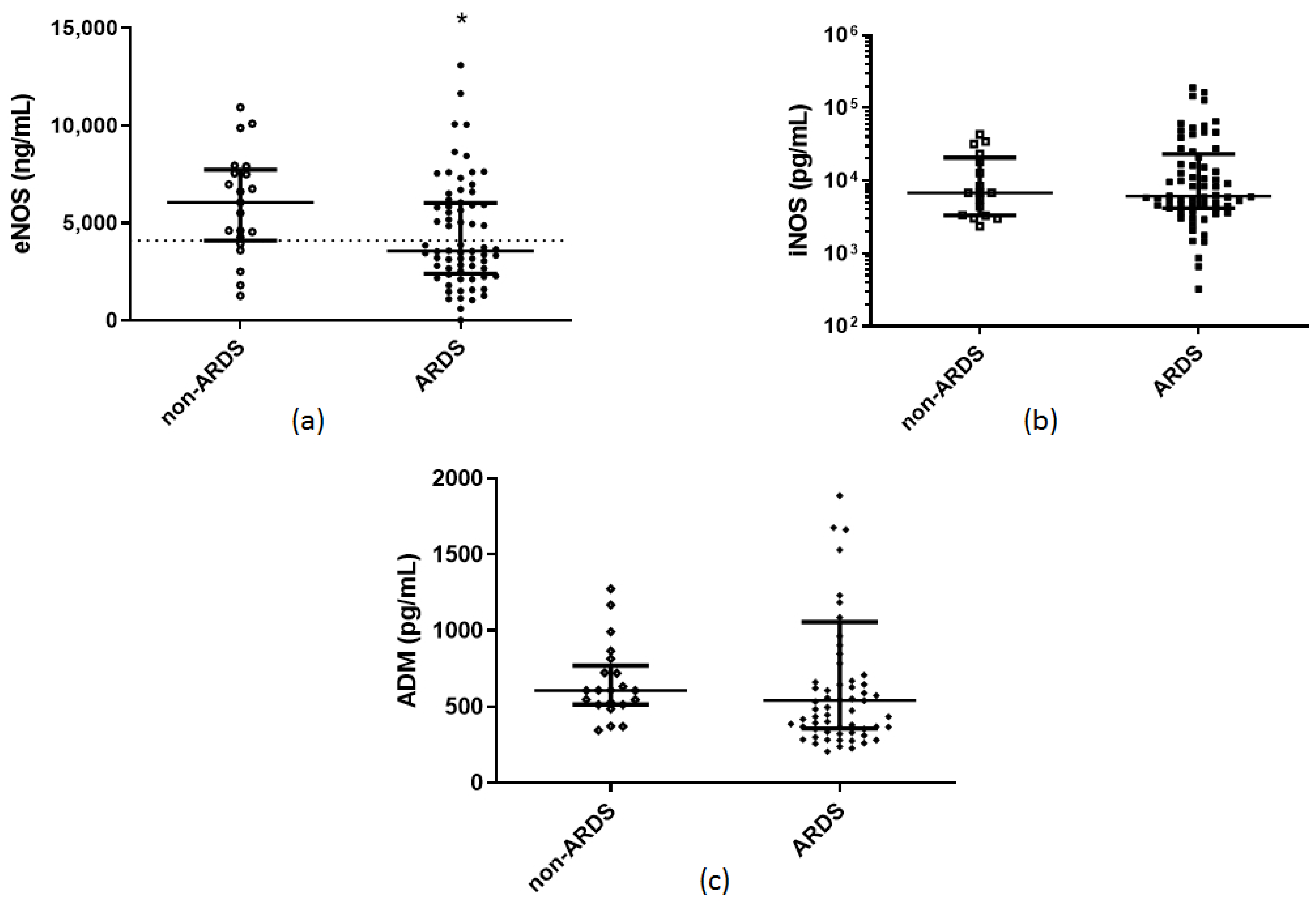
| Soluble endothelial–related molecules eNOS (ng/mL), (median, IQR) iNOS (pg/mL), (median, IQR) ADM (pg/mL), (median, IQR) sACE2 (pg/mL), (median, IQR) sACE (U/L), (median, IQR) | 3570 (2405–6030) 6138 (4200–23,131) 543 (357–1057) 8700 (4313–13,325) 30 (23–43) | 6070 (4025–7830) 6763 (3300–23,288) 608 (514–794) 125 (10–5225) 29 (18–42) | 0.02 * 0.7 0.4 <0.0001 * 0.5 | |
| Outcomes | ||||
| Mechanical ventilation, N (%) | 50 (73.5) | 4 (19.0) | <0.0001 * | |
| MV duration (days), (median, IQR) | 8 (1–22) | 0 (0–0) | <0.001 * | |
| LoS (days), (median, IQR) | 18 (13–30) | 8 (6–22) | 0.002 * | |
| In–hospital mortality, N (%) | 23 (33.8) | 3 (14.3) | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassiliou, A.G.; Zacharis, A.; Keskinidou, C.; Jahaj, E.; Pratikaki, M.; Gallos, P.; Dimopoulou, I.; Kotanidou, A.; Orfanos, S.E. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals 2021, 14, 695. https://doi.org/10.3390/ph14070695
Vassiliou AG, Zacharis A, Keskinidou C, Jahaj E, Pratikaki M, Gallos P, Dimopoulou I, Kotanidou A, Orfanos SE. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals. 2021; 14(7):695. https://doi.org/10.3390/ph14070695
Chicago/Turabian StyleVassiliou, Alice G., Alexandros Zacharis, Chrysi Keskinidou, Edison Jahaj, Maria Pratikaki, Parisis Gallos, Ioanna Dimopoulou, Anastasia Kotanidou, and Stylianos E. Orfanos. 2021. "Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS)" Pharmaceuticals 14, no. 7: 695. https://doi.org/10.3390/ph14070695
APA StyleVassiliou, A. G., Zacharis, A., Keskinidou, C., Jahaj, E., Pratikaki, M., Gallos, P., Dimopoulou, I., Kotanidou, A., & Orfanos, S. E. (2021). Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals, 14(7), 695. https://doi.org/10.3390/ph14070695