Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts
Abstract
:1. Introduction
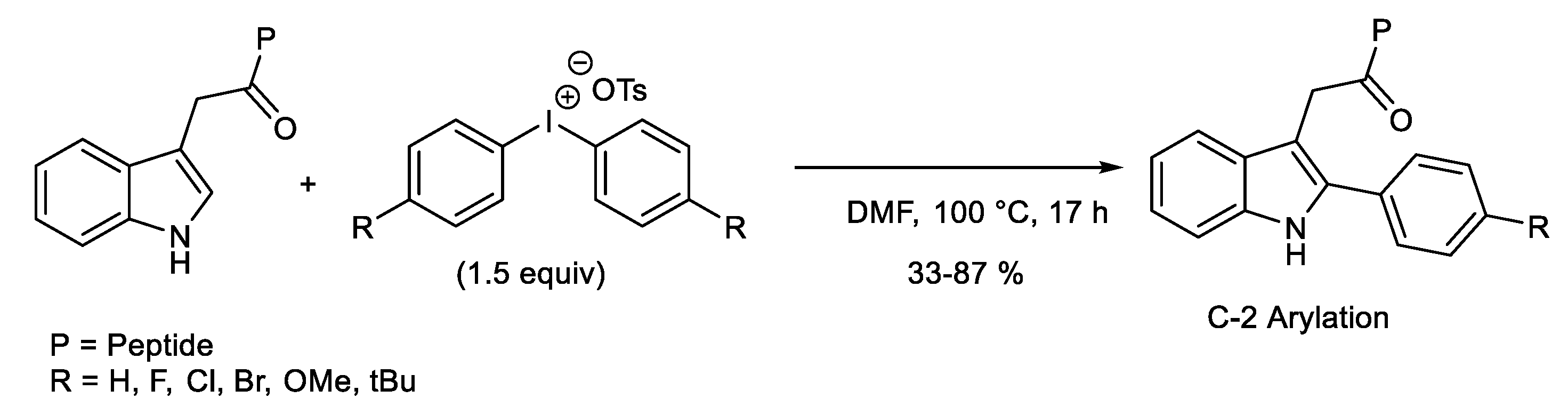
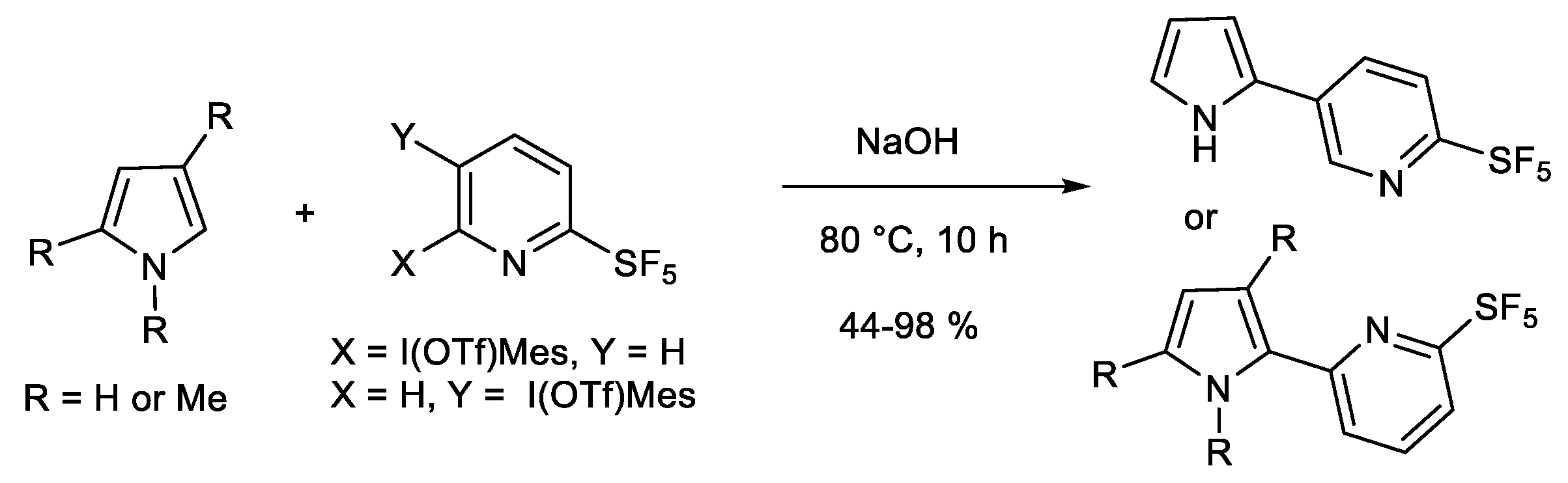
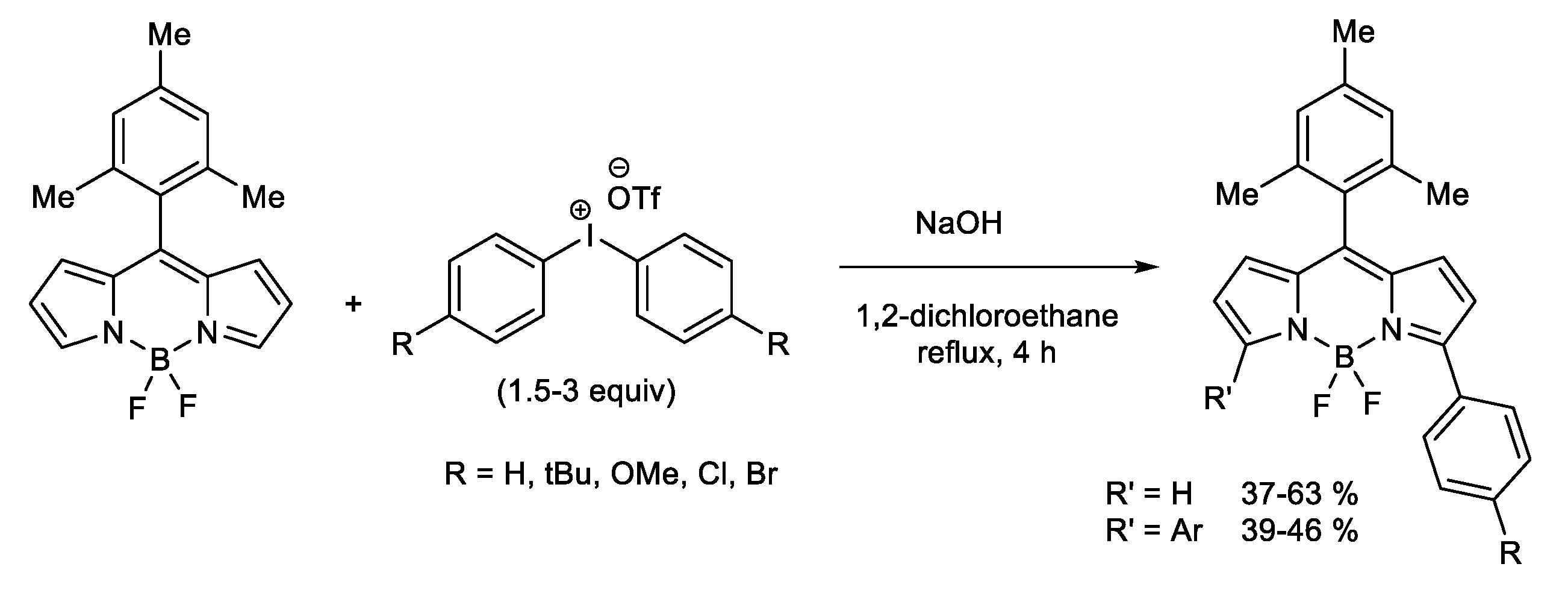
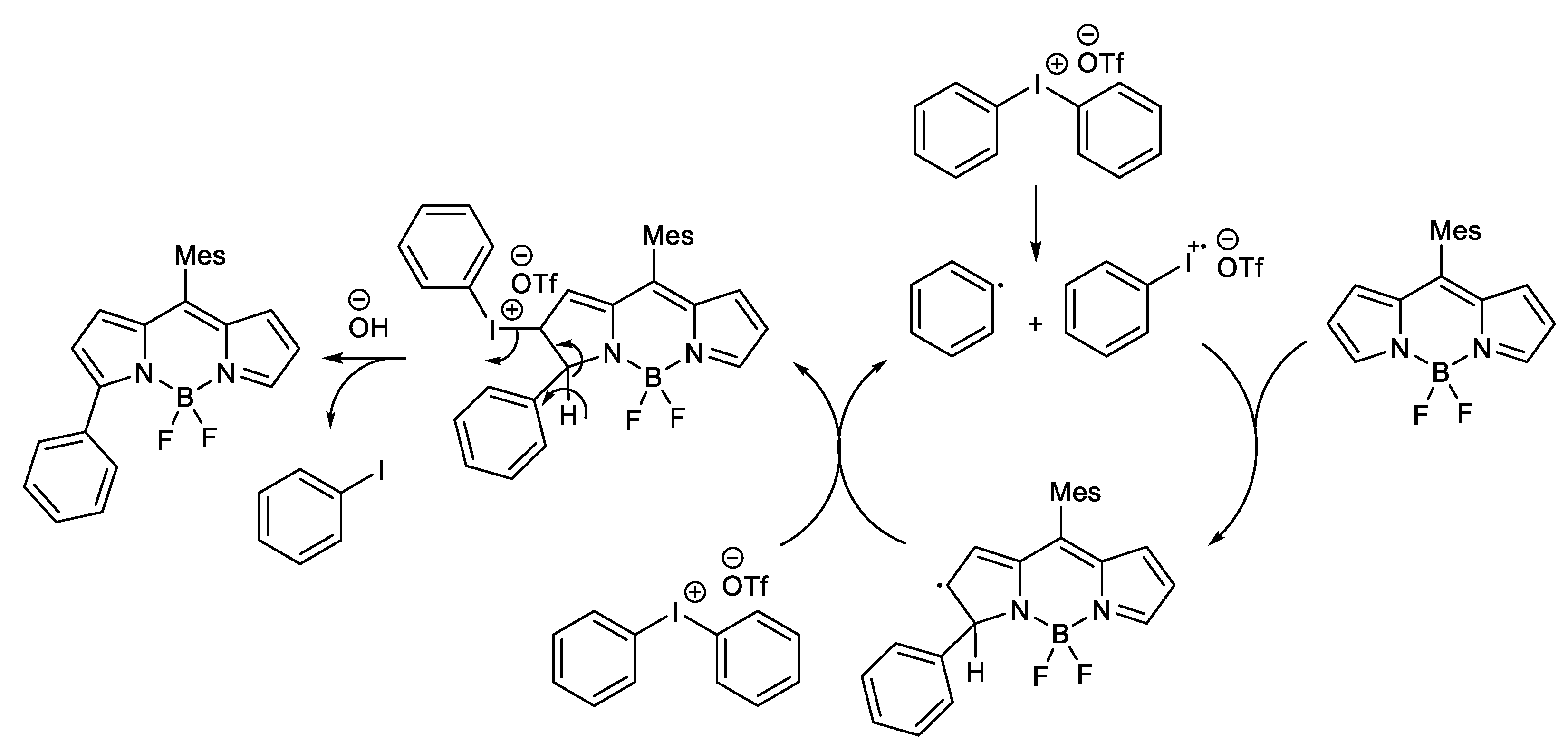
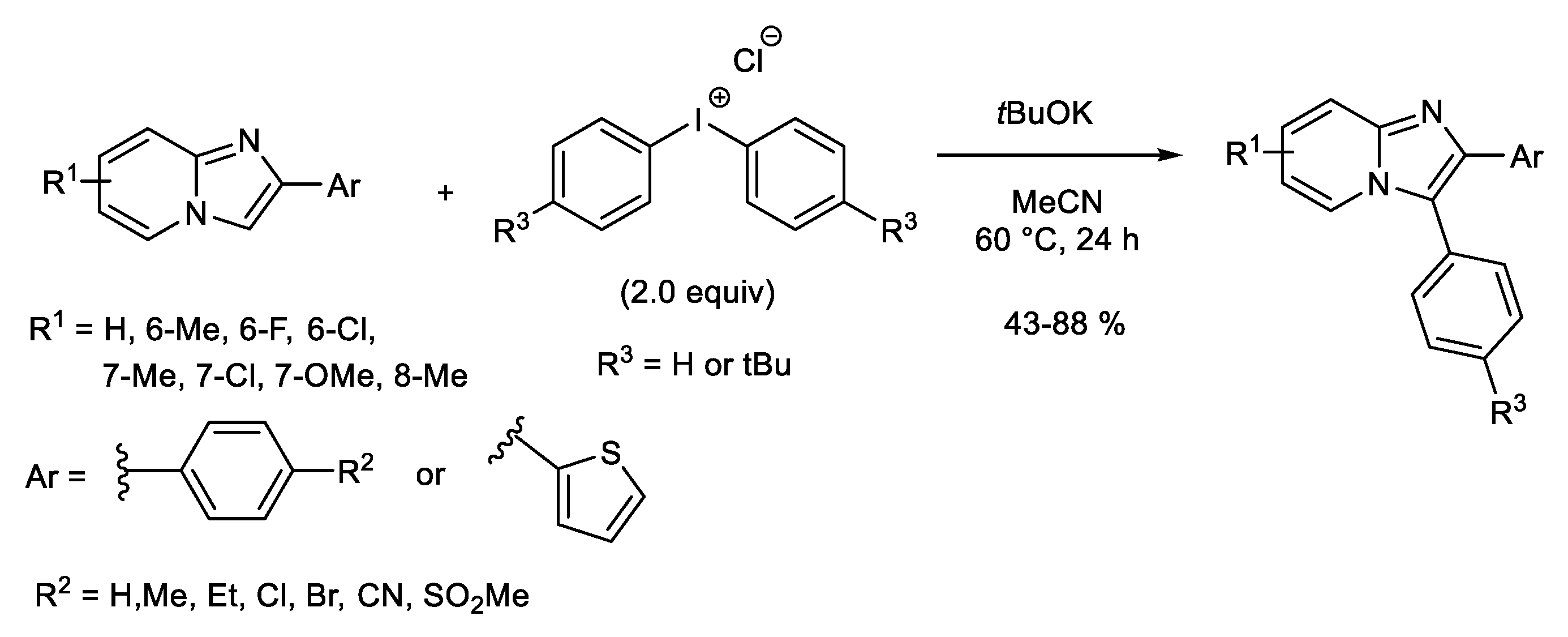
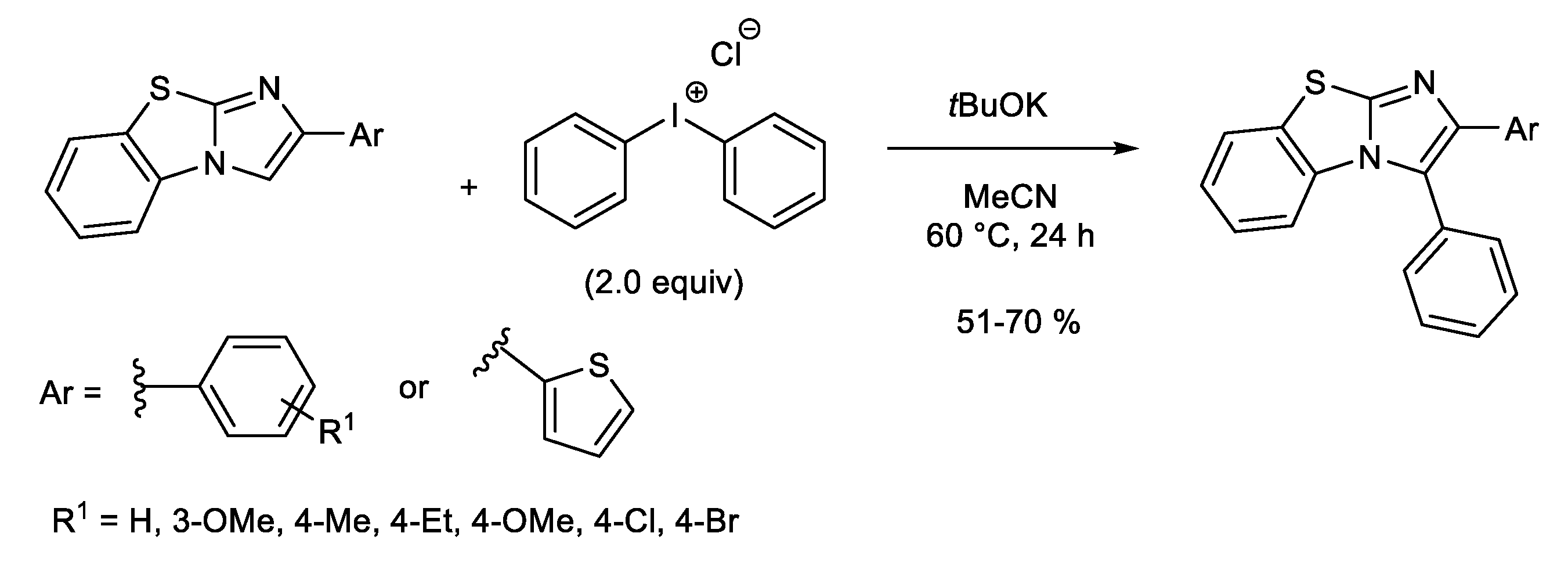
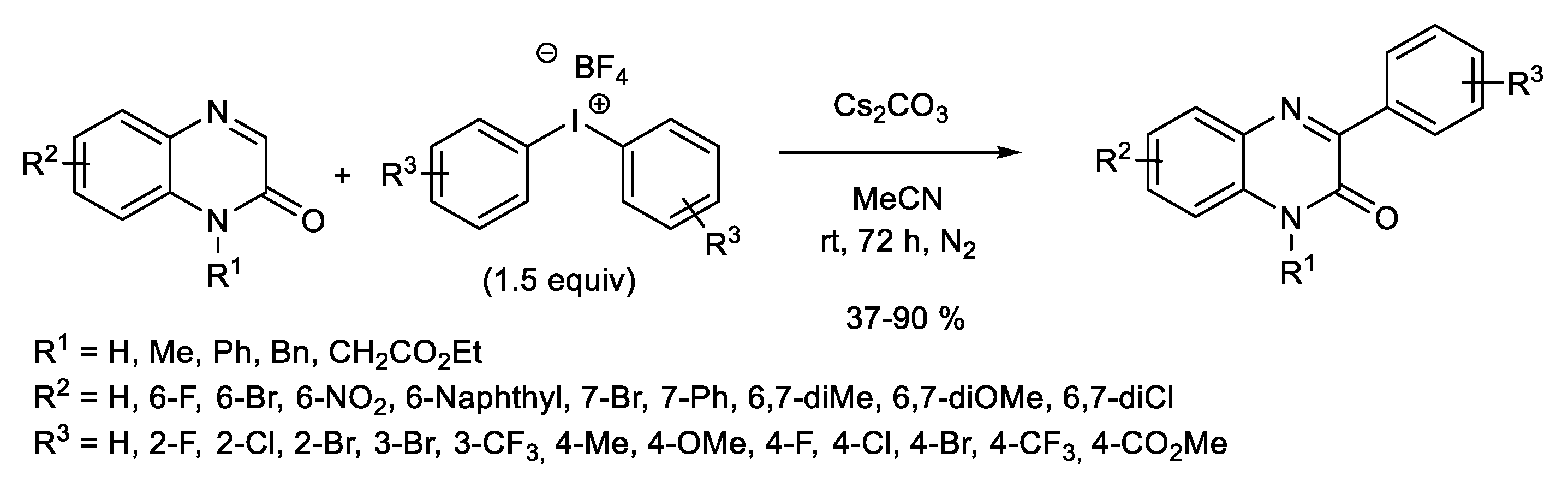
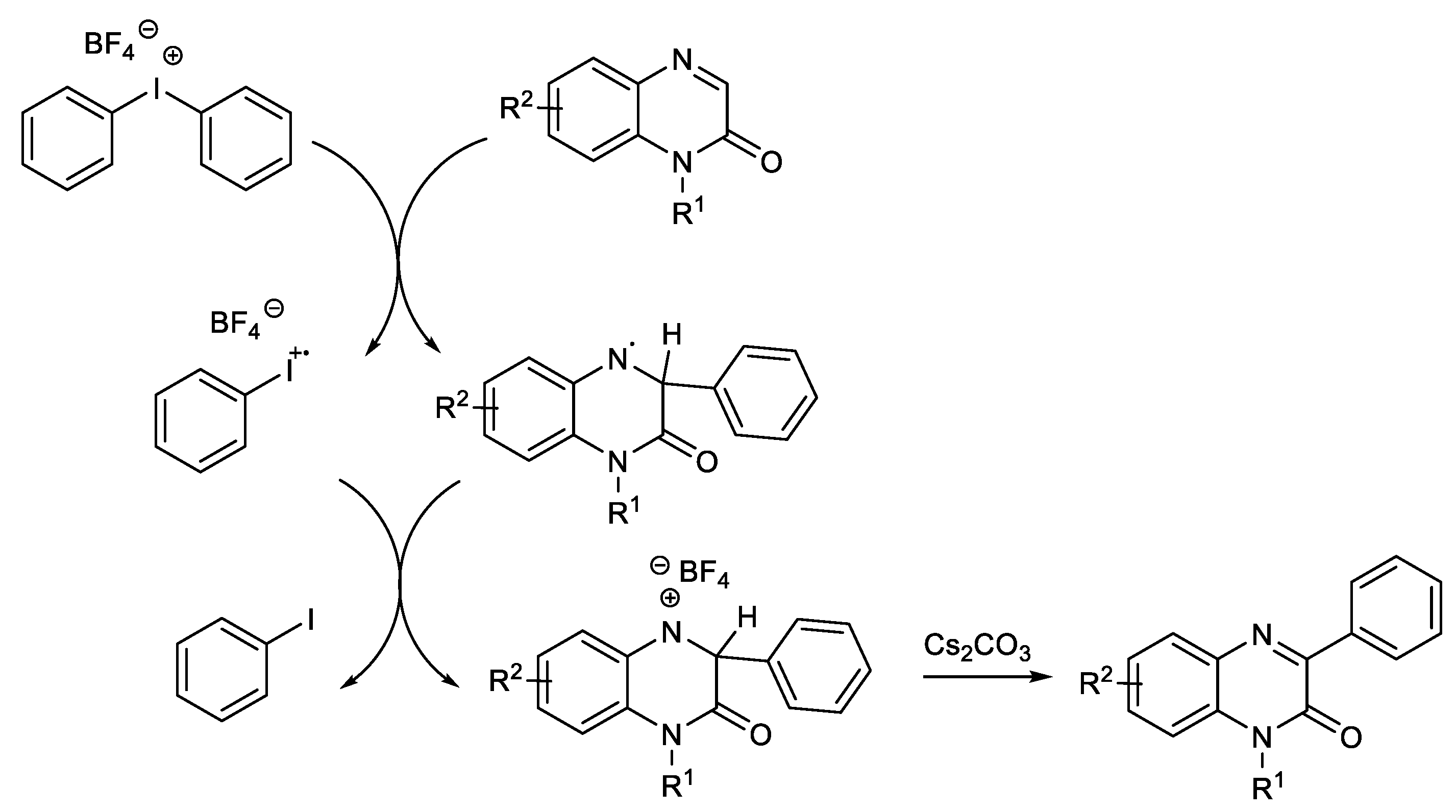
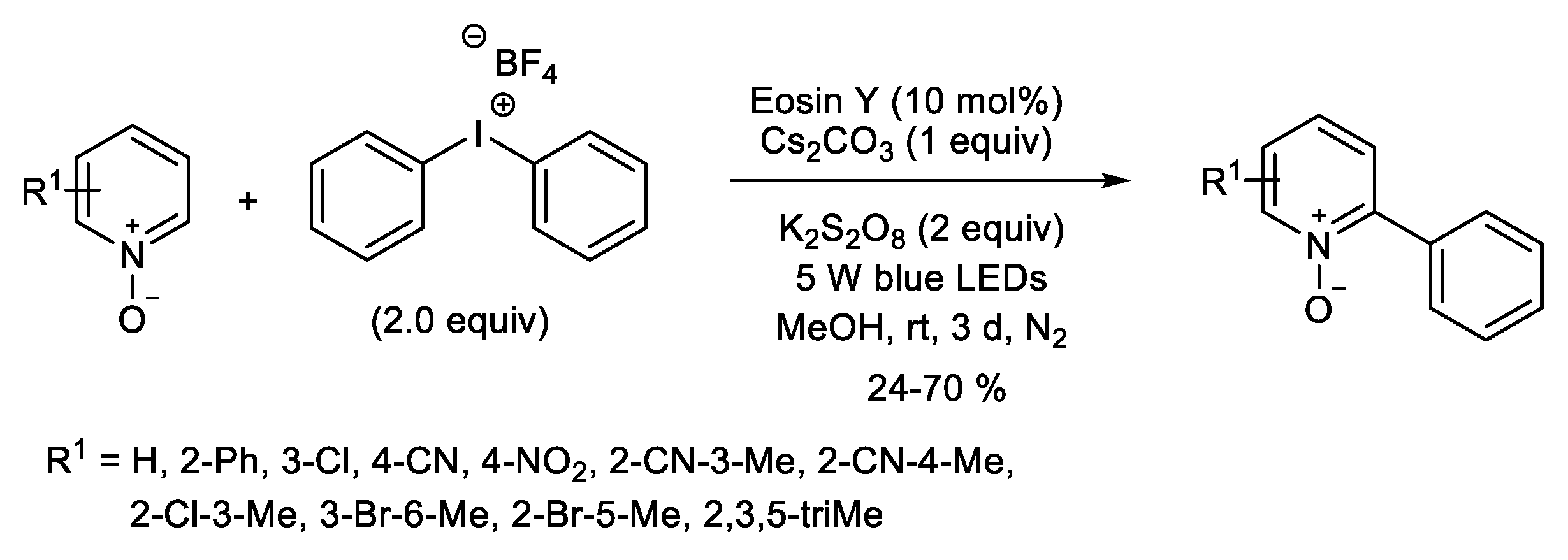
1.1. Metal-Free C-H Arylation of Heteroarenes
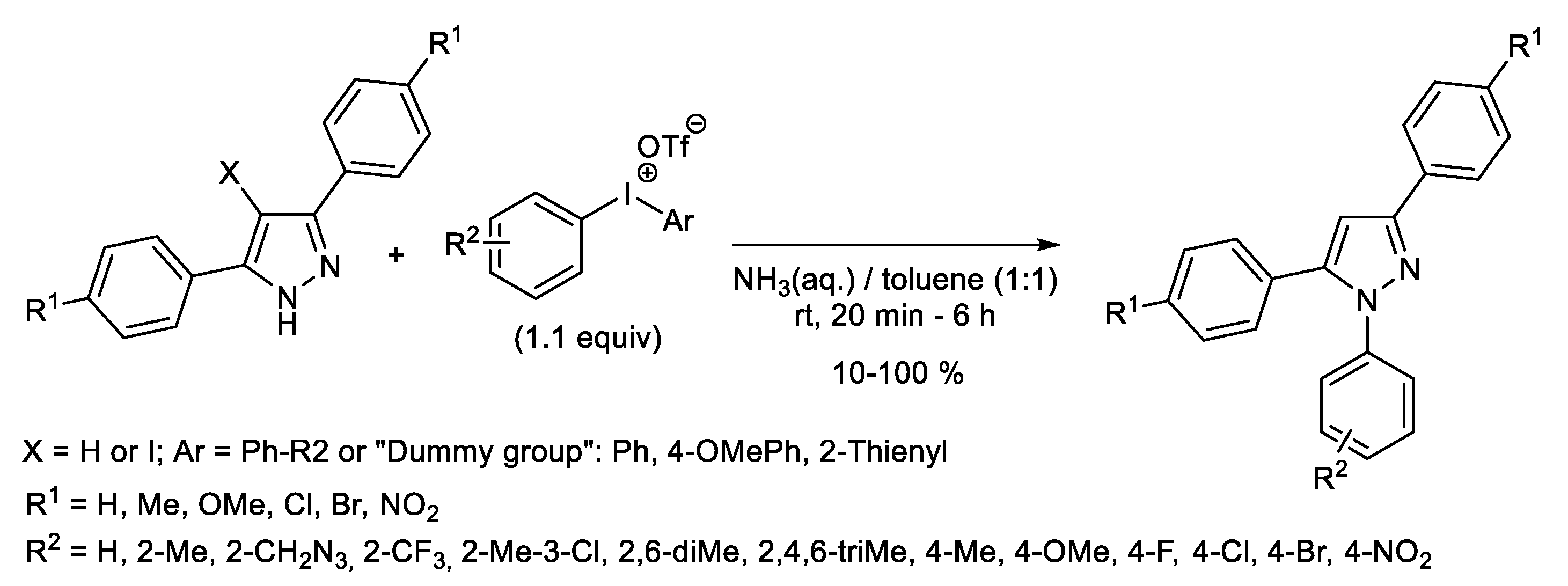
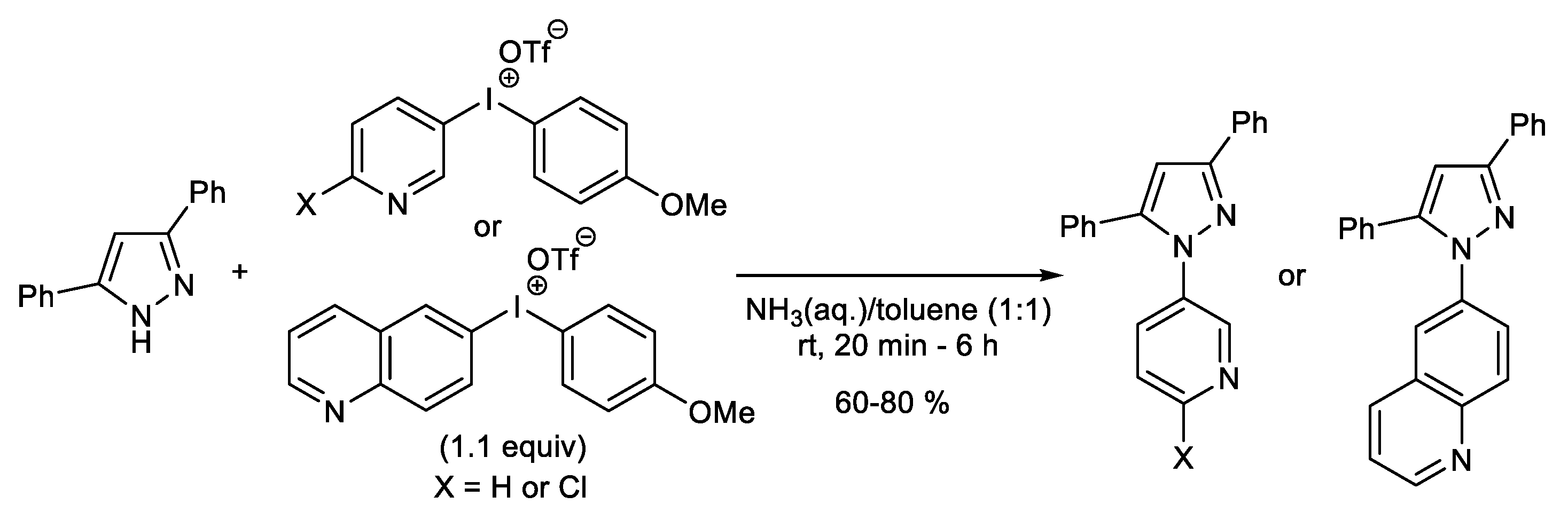
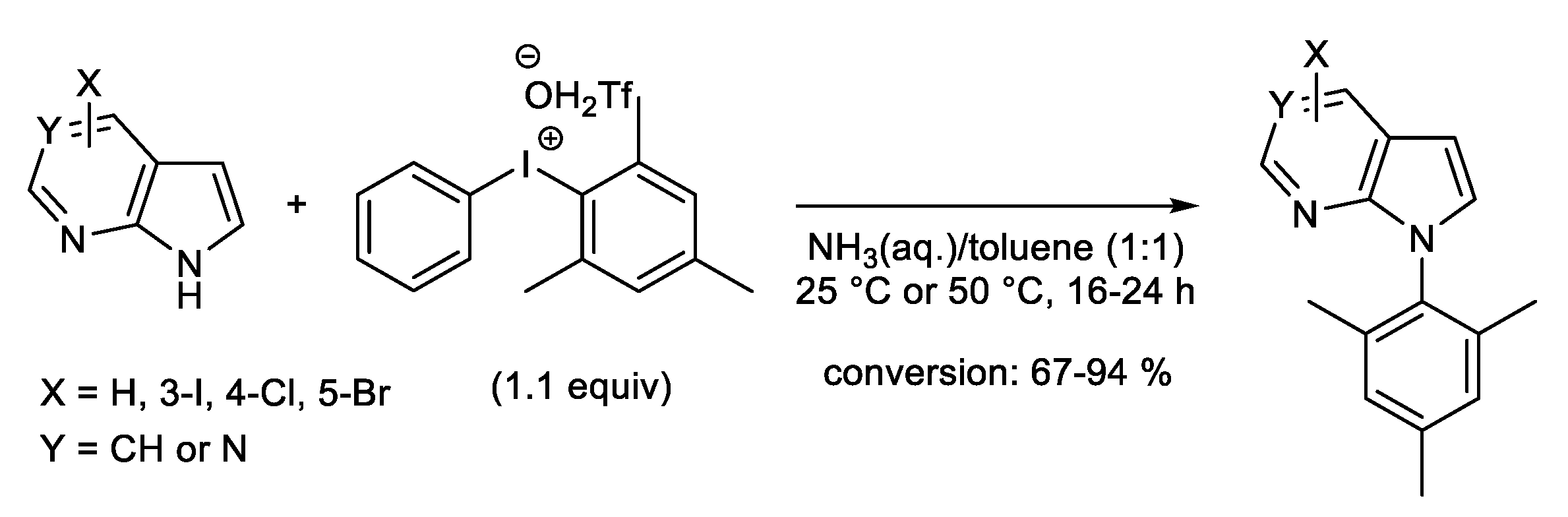
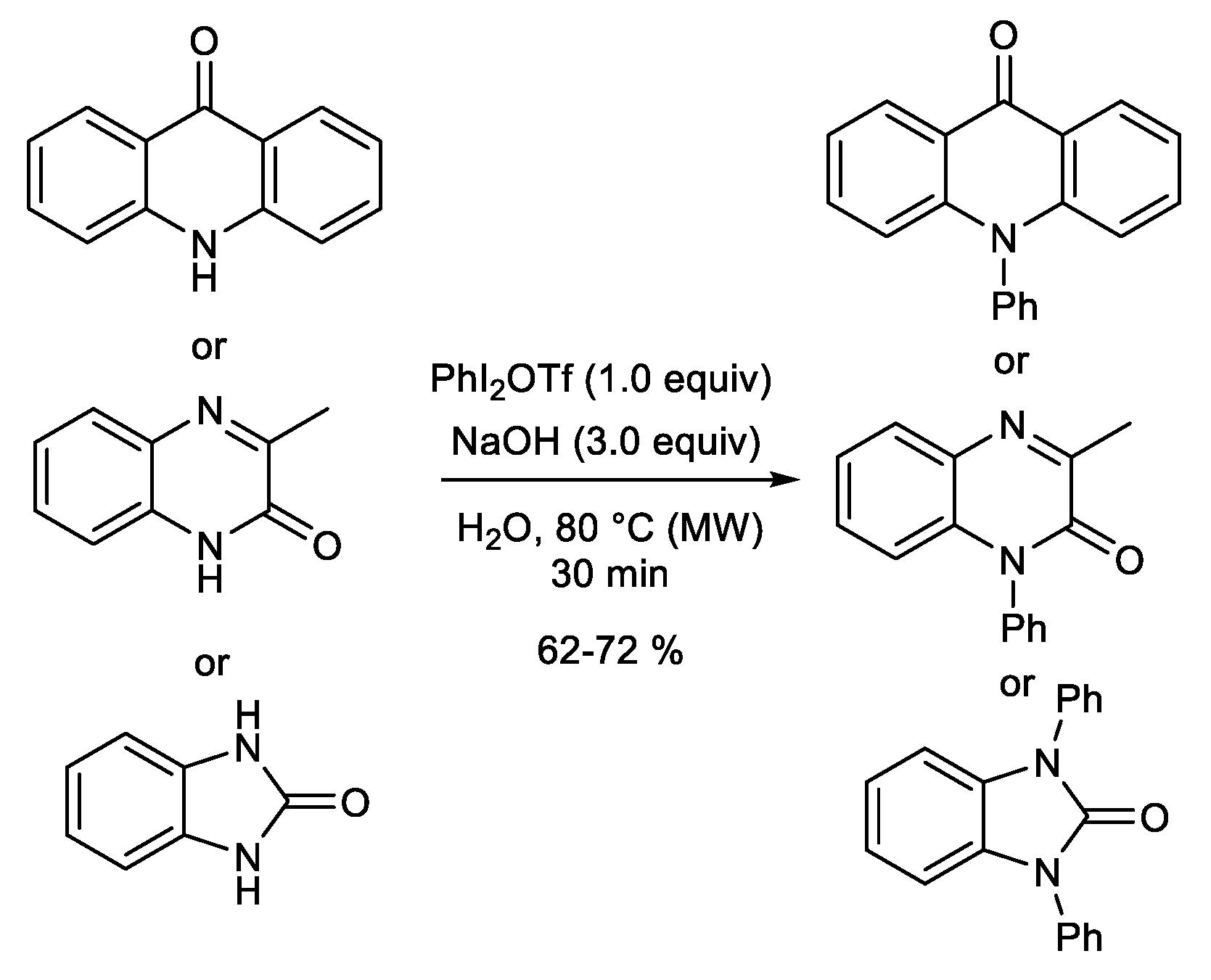
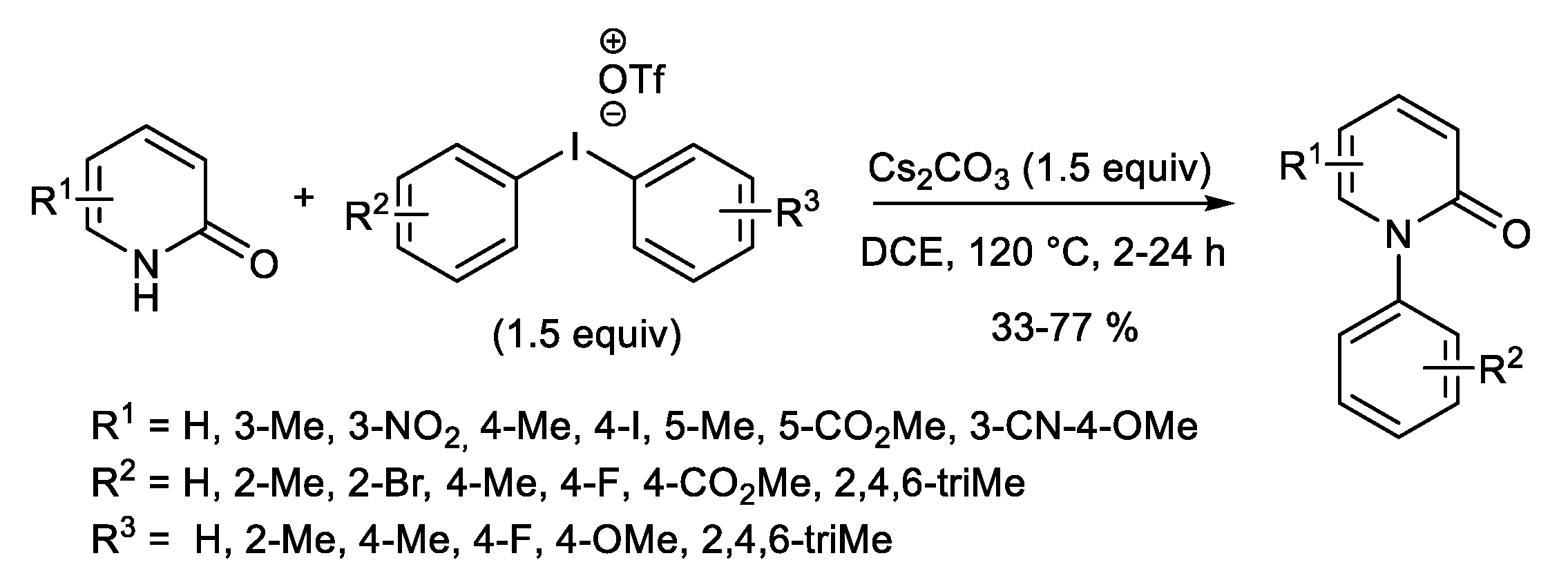
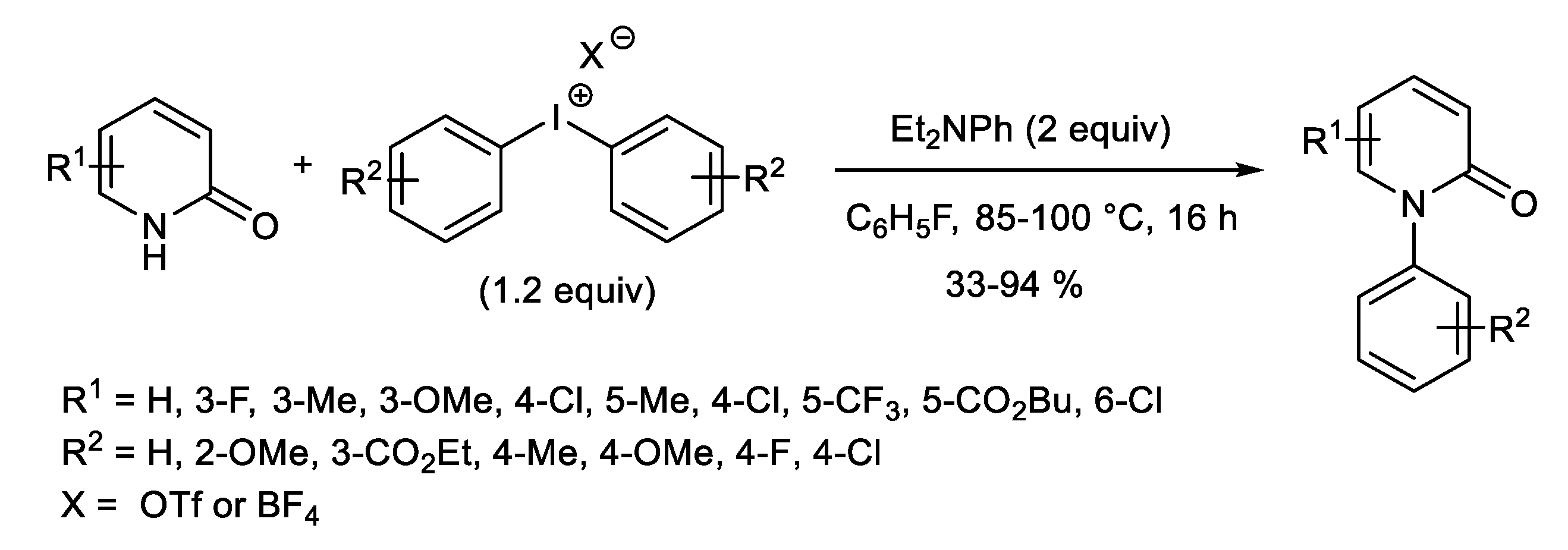
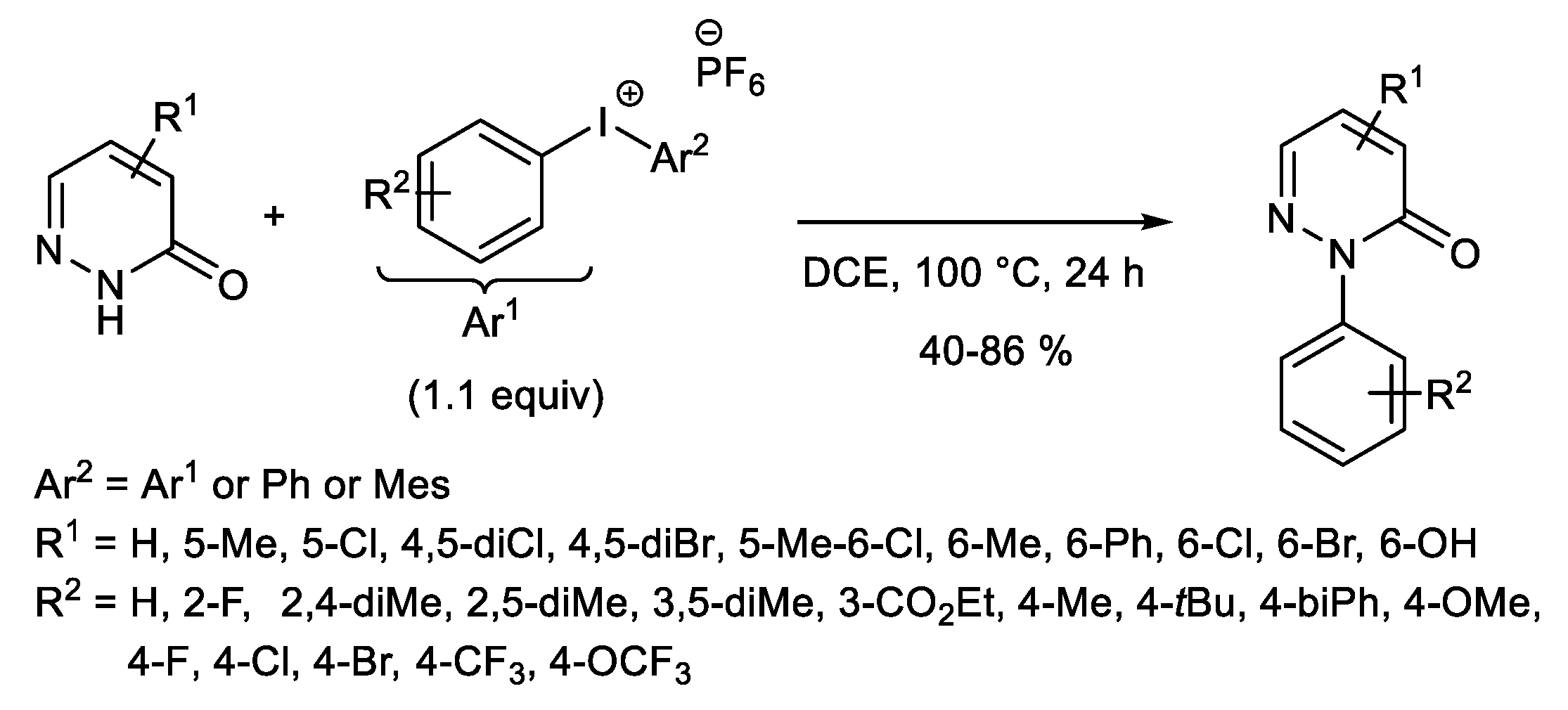
1.2. Metal-Free N-H Arylation of Heteroarenes
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, B.F.; Snodgrass, H.M.; Jones, K.A.; Andorfer, M.C.; Lewis, J.C. Site-Selective C–H Halogenation Using Flavin-Dependent Halogenases Identified via Family-Wide Activity Profiling. ACS Cent. Sci. 2019, 5, 1844–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struble, T.J.; Coley, C.W.; Jensen, K.F. Multitask prediction of site selectivity in aromatic C–H functionalization reactions. React. Chem. Eng. 2020, 5, 896–902. [Google Scholar] [CrossRef] [Green Version]
- Cardoza, S.; Shrivash, M.K.; Das, P.; Tandon, V. Strategic Advances in Sequential C-Arylations of Heteroarenes. J. Org. Chem. 2021, 86, 1330–1356. [Google Scholar] [CrossRef] [PubMed]
- Wencel-Delord, J.; Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 2013, 5, 369–375. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Durak, L.J.; Payne, J.T.; Lewis, J.C. Late-Stage Diversification of Biologically Active Molecules via Chemoenzymatic C–H Functionalization. ACS Catal. 2016, 6, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Liu, Y.; Tyagarajan, S.; Streckfuss, E.; Reibarkh, M.; Chen, K.; Zamora, I.; Fontaine, F.; Goracci, L.; Helmy, R.; et al. Enabling Efficient Late-Stage Functionalization of Drug-Like Molecules with LC-MS and Reaction-Driven Data Processing. Eur. J. Org. Chem. 2017, 2017, 7122–7126. [Google Scholar] [CrossRef]
- Shugrue, C.R.; Miller, S.J. Applications of Non enzymatic Catalysts to the Alteration of Natural Products. Chem. Rev. 2017, 117, 11894–11951. [Google Scholar] [CrossRef]
- Kuttruff, C.A.; Haile, M.; Kraml, J.; Tautermann, C.S. Late-Stage Functionalization of Drug-Like Molecules Using Diversinates. ChemMedChem 2018, 13, 983–987. [Google Scholar] [CrossRef]
- Blakemore, D.C.; Castro, L.; Churcher, I.; Rees, D.C.; Thomas, A.W.; Wilson, D.M.; Wood, A. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018, 10, 383–394. [Google Scholar] [CrossRef]
- Boström, J.; Brown, D.G.; Young, R.J.; Keserü, G.M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 2018, 17, 709–727. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lorion, M.M.; Shah, J.; Kapdi, A.R.; Ackermann, L. Late-Stage Peptide Diversification by Position-Selective C−H Activation. Angew. Chem. Int. Ed. 2018, 57, 14700–14717. [Google Scholar] [CrossRef] [PubMed]
- Moir, M.; Danon, J.J.; Reekie, T.A.; Kassiou, M. An overview of late-stage functionalization in today’s drug discovery. Expert Opin. Drug Discov. 2019, 14, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Fessner, N.D. P450 Monooxygenases Enable Rapid Late-Stage Diversification of Natural Products via C-H Bond Activation. ChemCatChem 2019, 11, 2226–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capaldo, L.; Lafayette Quadri, L.; Ravelli, D. Photocatalytic hydrogen atom transfer: The philosopher’s stone for late-stage functionalization? Green Chem. 2020, 22, 3376–3396. [Google Scholar] [CrossRef]
- Hong, B.; Luo, T.; Lei, X. Late-Stage Diversification of Natural Products. ACS Cent. Sci. 2020, 6, 622–635. [Google Scholar] [CrossRef] [Green Version]
- Cannalire, R.; Pelliccia, S.; Sancineto, L.; Novellino, E.; Tron, G.C.; Giustiniano, M. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 2021, 50, 766–897. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C–H/C–H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef]
- Murakami, K.; Yamada, S.; Kaneda, T.; Itami, K. C–H Functionalization of Azines. Chem. Rev. 2017, 117, 9302–9332. [Google Scholar] [CrossRef]
- Kaur, M.; Van Humbeck, J.F. Recent trends in catalytic sp3 C–H functionalization of heterocycles. Org. Biomol. Chem. 2020, 18, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-Y.; Benzai, A.; Shi, X.; Doucet, H. Effective Tools for the Metal-Catalyzed Regiodivergent Direct Arylations of (Hetero)arenes. Chem. Rec. 2021, 21, 343–356. [Google Scholar] [CrossRef]
- Basak, S.; Dutta, S.; Maiti, D. Accessing C2-Functionalized 1,3-(Benz)azoles through Transition Metal-Catalyzed C−H Activation. Chem. Eur. J. 2021, 27, 475. [Google Scholar] [CrossRef]
- Mehta, V.V.; Punji, B. Recent advances in transition-metal-free direct C–C and C–heteroatom bond forming reactions. RSC Adv. 2013, 3, 11957–11986. [Google Scholar] [CrossRef]
- Samanta, R.; Matcha, K.; Antonchick, A.P. Metal-Free Oxidative Carbon-Heteroatom Bond Formation Through C–H Bond Functionalization. Eur. J. Org. Chem. 2013, 2013, 5769–5804. [Google Scholar] [CrossRef]
- Sun, C.-L.; Shi, Z.-J. Transition-Metal-Free Coupling Reactions. Chem. Rev. 2014, 114, 9219–9280. [Google Scholar] [CrossRef]
- Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G.; Bellina, F. Transition Metal-Free Direct C-H (Hetero)arylation of Heteroarenes: A Sustainable Methodology to Access (Hetero)aryl-Substituted Heteroarenes. Adv. Synth. Catal. 2015, 357, 3777–3814. [Google Scholar] [CrossRef]
- Roopan, S.M.; Palaniraja, J. Synthetic journey towards transition metal-free arylations. Res. Chem. Intermed. 2015, 41, 8111–8146. [Google Scholar] [CrossRef]
- Narayan, R.; Matcha, K.; Antonchick, A.P. Metal-Free Oxidative C-C Bond Formation through C-H Bond Functionalization. Chem. Eur. J. 2015, 21, 14678–14693. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Marder, T.B. A leap ahead for activating C-H bonds. Science 2015, 349, 473–474. [Google Scholar] [CrossRef]
- Légaré, J.L.; Courtemanche, M.-A.; Rochette, E.; Fontaine, F.-G. Metal-free catalytic C-H bond activation and borylation of heteroarenes. Science 2015, 349, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Toutov, A.A.; Liu, W.-B.; Betz, K.N.; Fedorov, A.; Stoltz, B.M.; Grubbs, R.H. Silylation of C–H bonds in aromatic heterocycles by an Earth-abundant metal catalyst. Nature 2015, 518, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Légaré Lavergne, J.L.; Jayaraman, A.; Castro, L.C.M.; Rochette, E.; Fontaine, F.-G. Metal-Free Borylation of Heteroarenes Using Ambiphilic Aminoboranes: On the Importance of Sterics in Frustrated Lewis Pair C–H Bond Activation. J. Am. Chem. Soc. 2017, 139, 14714–14723. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Chen, X.; Xue, X.-S.; Zhao, B.; Liang, Y.; Wang, M.; Jin, L.; Yuan, Y.; Han, Y.; Zhao, Y.; et al. Metal-free directed sp2-C–H borylation. Nature 2019, 575, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Tian, S.; Wan, J.-P.; Liu, Y. Recent Advances in Transition Metal-Free Halogenation of C(sp2)-H Bonds. Curr. Org. Chem. 2021, 25, 1180–1193. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Chen, X.; Wu, L.; Wong, J.J.; Liang, Y.; Zhao, Y.; Houk, K.N.; Shi, Z. Metal-Free Directed C−H Borylation of Pyrroles. Angew. Chem. Int. Ed. 2021, 60, 8500–8504. [Google Scholar] [CrossRef] [PubMed]
- Budhwan, R.; Yadava, S.; Murarka, S. Late stage functionalization of heterocycles using hypervalent iodine(III) reagents. Org. Biomol. Chem. 2019, 17, 6326–6341. [Google Scholar] [CrossRef]
- Kandimalla, S.R.; Parvathaneni, S.P.; Sabitha, G.; Reddy, B.V.S. Recent Advances in Intramolecular Metal-Free Oxidative C–H Bond Aminations Using Hypervalent Iodine(III) Reagents. Eur. J. Org. Chem. 2019, 1687–1714. [Google Scholar] [CrossRef]
- Mudithanapelli, C.; Kim, M. Metal-free late-stage C(sp2)–H functionalization of N-aryl amines with various sodium salts. Org. Biomol. Chem. 2020, 18, 450–464. [Google Scholar] [CrossRef]
- Merritt, E.A.; Olofsson, B. Diaryliodonium Salts: A Journey from Obscurity to Fame. Angew. Chem. Int. Ed. 2009, 48, 9052–9070. [Google Scholar] [CrossRef]
- Olofsson, B. Arylation with Diaryliodonium Salts. In Hypervalent Iodine Chemistry. Topics in Current Chemistry; Wirth, T., Ed.; Springer: Cham, Switzerland, 2015; Volume 373. [Google Scholar] [CrossRef]
- Aradi, K.; Tóth, B.L.; Tolnai, G.L.; Novák, Z. Diaryliodonium Salts in Organic Syntheses: A Useful Compound Class for Novel Arylation Strategies. Synlett 2016, 27, 1456–1485. [Google Scholar] [CrossRef]
- Pacheco-Benichou, A.; Besson, T.; Fruit, C. Diaryliodoniums Salts as Coupling Partners for Transition-Metal Catalyzed C- and N-Arylation of Heteroarenes. Catalysts 2020, 10, 483. [Google Scholar] [CrossRef]
- Seidl, T.L.; Sundalam, S.K.; McCullough, B.; Stuart, D.R. Unsymmetrical Aryl(2,4,6-trimethoxyphenyl)iodonium Salts: One-Pot Synthesis, Scope, Stability, and Synthetic Studies. J. Org. Chem. 2016, 81, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.R. Aryl Transfer Selectivity in Metal-Free Reactions of Unsymmetrical Diaryliodonium Salts. Chem. Eur. J. 2017, 23, 15852–15863. [Google Scholar] [CrossRef]
- Carreras, V.; Sandtorv, A.H.; Stuart, D.R. Synthesis of Aryl(2,4,6-trimethoxyphenyl)iodonium Trifluoroacetate Salts. J. Org. Chem. 2017, 82, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, E.; Reitti, M.; Olofsson, B. One-Pot Synthesis of Unsymmetric Diaryliodonium Salts from Iodine and Arenes. J. Org. Chem. 2017, 82, 11909–11914. [Google Scholar] [CrossRef]
- Seidl, T.L.; Moment, A.; Orella, C.; Vickery, T.; Stuart, D.R. Synthesis of 4-Methylbenzoate(2′,4′,6′-trimethoxyphenyl)iodonium Tosylate. Org. Synth. 2019, 96, 137–149. [Google Scholar] [CrossRef]
- Dohi, T.; Hayashi, T.; Ueda, S.; Shoji, T.; Komiyama, K.; Takeuchi, H.; Kita, Y. Recyclable synthesis of mesityl iodonium(III) salts. Tetrahedron 2019, 75, 3617–3627. [Google Scholar] [CrossRef]
- Tóth, B.L.; Béke, F.; Egyed, O.; Bényei, A.; Stirling, A.; Novák, Z. Synthesis of Multifunctional Aryl(trifloxyalkenyl)iodonium Triflate Salts. ACS Omega 2019, 4, 9188–9197. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, R.T.; Basu, S.; Stuart, D.R. Trimethoxyphenyl (TMP) as a Useful Auxiliary for in situ Formation and Reaction of Aryl(TMP)iodonium Salts: Synthesis of Diaryl Ethers. Adv. Synth. Catal. 2020, 362, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Komiyama, K.; Kobayashi, S.; Shoji, T.; Kikushima, K.; Dohi, T.; Kita, Y. Practical synthesis of diaryliodonium(iii) triflates using ArI(OAc)2/TfOH/MeCN reaction system. Russ. Chem. Bull. 2020, 69, 2328–2332. [Google Scholar] [CrossRef]
- Takenaga, N.; Kumar, R.; Dohi, T. Heteroaryliodonium(III) Salts as Highly Reactive Electrophiles. Front. Chem. 2020, 8, 599026. [Google Scholar] [CrossRef]
- Eastman, K.; Baran, P.S. A simple method for the direct arylation of indoles. Tetrahedron 2009, 65, 3149–3154. [Google Scholar] [CrossRef]
- Kita, Y.; Morimoto, K.; Ito, M.; Ogawa, C.; Goto, A.; Dohi, T. Metal-Free Oxidative Cross-Coupling of Unfunctionalized Aromatic Compounds. J. Am. Chem. Soc. 2009, 131, 1668–1669. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Yamaoka, N.; Ogawa, C.; Nakae, T.; Fujioka, H.; Dohi, T.; Kita, Y. Metal-Free Regioselective Oxidative Biaryl Coupling Leading to Head-to-Tail Bithiophenes: Reactivity Switching, a Concept Based on the Iodonium(III) Intermediate. Org. Lett. 2010, 12, 3804–3807. [Google Scholar] [CrossRef]
- Ackermann, L.; Dell’Acqua, M.; Fenner, S.; Vicente, R.; Sandmann, R. Metal-Free Direct Arylations of Indoles and Pyrroles with Diaryliodonium Salts. Org. Lett. 2011, 13, 2358–2360. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Zhang, R.-Y.; Chen, S.-Y.; Zhang, J.; Yu, X.-Q. Direct Arylation of Arene and N-Heteroarenes with Diaryliodonium Salts without the Use of Transition Metal Catalyst. J. Org. Chem. 2012, 77, 766–771. [Google Scholar] [CrossRef]
- Guo, F.; Wang, L.; Wang, P.; Yu, J.; Han, J. Transition-Metal-Free N-Arylation of Carbazoles and C-Arylation of Tetrahydrocarbazoles by using Diaryliodonium Salts. Asian J. Org. Chem. 2012, 1, 218–221. [Google Scholar] [CrossRef]
- Zhu, Y.; Bauer, M.; Ploog, J.; Ackermann, L. Late-Stage Diversification of Peptides by Metal-Free C-H Arylation. Chem. Eur. J. 2014, 20, 13099–13102. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Ueda, S.; Hirai, A.; Kojima, Y.; Morimoto, K.; Kita, Y. Selective Aryl Radical Transfers into N-Heteroaromatics from Diaryliodonoium Salts with Trimethoxybenzene Auxiliary. Heterocycles 2017, 95, 1272–1284. [Google Scholar] [CrossRef]
- Das, P.; Takada, M.; Matsuzaki, K.; Saito, N.; Shibata, N. SF5-pyridylaryl-λ3-iodonium salts and their utility as electrophilic reagents to access SF5 pyridine derivatives in the late-stage of synthesis. Chem. Commun. 2017, 53, 3850–3853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, Q.; Yu, Y.; Yu, C.; Hao, E.; Wei, Y.; Mu, X.; Jiao, L. Metal-Free Direct α-Selective Arylation of Boron Dipyrromethenes via Base-Mediated C−H Functionalization. Org. Lett. 2016, 18, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Malmgren, J.; Santoro, S.; Jalalian, N.; Himo, F.; Olofsson, B. Arylation with unsymmetrical diaryliodonium salts: A chemoselectivity study. Chemistry 2013, 19, 10334–10342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Ravi, C.; Rawat, D.; Adimurth, S. Base-Promoted Transition-Metal-Free Arylation of Imidazo-Fused Heterocycles with Diaryliodonium Salts. Eur. J. Org. Chem. 2018, 2018, 1665–1673. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdavi, M. C3-Functionalization of Imidazo[1,2-a]pyridines. Eur. J. Org. Chem. 2020, 269–284. [Google Scholar] [CrossRef]
- Gundlewad, G.B.; Wagh, S.S.; Patil, B.R. Catalyst and Solvent Free Synthesis and Biological Activities of Imidazo[1,2-a]pyridine. Asian J. Org. Med. Chem. 2020, 5, 221–226. [Google Scholar] [CrossRef]
- Mohana Roopan, S.; Patil, S.M.; Palaniraja, J. Recent synthetic scenario on imidazo[1,2-a]pyridines chemical intermediate. Res. Chem. Intermed. 2016, 42, 2749–2790. [Google Scholar] [CrossRef]
- Seidl, T.L.; Stuart, D.R. An Admix Approach to Determine Counter Anion Effects on Metal-Free Arylation Reactions with Diaryliodonium Salts. J. Org. Chem. 2017, 82, 11765–11771. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Nandwana, N.K.; Legoabe, L.J.; Das, B.C.; Kumar, A. Recent Advances in Radical C−H Bond Functionalization of Imidazoheterocycles. Adv. Synth. Catal. 2020, 362, 4226–4255. [Google Scholar] [CrossRef]
- Ke, Q.; Yan, G.; Yu, J.; Wu, X. Recent advances in the direct functionalization of quinoxalin-2(1H)-ones. Org. Biomol. Chem. 2019, 17, 5863–5881. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, J.; Zhang, H.-Y.; Zhang, Y.; Zhao, J. The C3-H Bond Functionalization of Quinoxalin-2(1H)-Ones With Hypervalent Iodine(III) Reagents. Front. Chem. 2020, 8, 582. [Google Scholar] [CrossRef]
- Yin, K.; Zhang, R. Transition-Metal-Free Direct C–H Arylation of Quinoxalin-2(1H)-ones with Diaryliodonium Salts at Room Temperature. Org. Lett. 2017, 19, 1530–1533. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Jiang, Z.; Zhang, J.; Zhuo, W.-T.; Zou, F.-Y.; Wang, W.-P.; Gao, G.-L.; Song, J. Visible-Light-Promoted C2 Selective Arylation of Quinoline and Pyridine N Oxides with Diaryliodonium Tetrafluoroborate. J. Org. Chem. 2020, 85, 2733–2742. [Google Scholar] [CrossRef]
- Hari, D.P.; Hering, T.; Koenig, B. Chapter 8: Arene functionalization by visible light photoredox catalysis. In Visible Light Photocatalysis in Organic Chemistry; Yoon, T.P., Stephenson, C.R.J., MacMillan, D.W.C., Eds.; Whiley-VCH Verlag GmbH & Co. KGaA: Wheinheim, Germany, 2018; pp. 253–281. [Google Scholar] [CrossRef]
- Dixneuf, P.H.; Soulé, J.F. Functionalization of C(sp2)–H bonds of arenes and heteroarenes assisted by photoredox catalysts for the C–C Bond Formation. In Organometallics for Green Catalysis. Topics in Organometallic Chemistry; Dixneuf, P., Soulé, J.F., Eds.; Springer: Cham, Switzerland, 2018; Volume 63, pp. 225–265. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Yang, T. Recent Synthetic Applications of the Hypervalent Iodine(III) Reagents in Visible-Light-Induced Photoredox Catalysis. Front. Chem. 2020, 8, 849–870. [Google Scholar] [CrossRef]
- Wang, D.; Désaubry, L.; Li, G.; Huang, M.; Zheng, S. Recent Advances in the Synthesis of C2-Functionalized Pyridines and Quinolines Using N-Oxide Chemistry. Adv. Synth. Catal. 2021, 363, 2–39. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef] [PubMed]
- Dorababu, A. Report on Recently (2017–20) Designed Quinoline-Based Human Cancer Cell Growth Inhibitors. ChemistrySelect 2020, 5, 13902–13915. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Weyesa, A.; Mulugeta, E. Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: A review. RSC Adv. 2021, 10, 20784–20793. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Wu, W.; Li, Q.; Wei, W.-T. Visible-Light Induced C(sp3)−H Functionalization for the Formation of C−N Bonds under Metal Catalyst-Free Conditions. Adv. Synth. Catal. 2020, 362, 2770–2777. [Google Scholar] [CrossRef]
- Halder, P.; Roya, T.; Das, P. Recent developments in selective N-arylation of azoles. Chem. Commun. 2021, 57, 5235–5249. [Google Scholar] [CrossRef]
- Neha, D.; Ashish, R.; Kumar, R.; Kumar, V. Recent Synthetic Strategies for Monocyclic Azole Nucleus and Its Role in Drug Discovery and Development. Curr. Org. Synth. 2018, 15, 321–340. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Gonda, Z.; Novák, Z. Transition-Metal-Free N-Arylation of Pyrazoles with Diaryliodonium Salts. Chem. Eur. J. 2015, 21, 16801–16806. [Google Scholar] [CrossRef] [PubMed]
- Bihari, T.; Babinszki, B.; Gonda, Z.; Kovács, S.; Novák, Z.; Stirling, A. Understanding and Exploitation of Neighboring Heteroatom Effect for the Mild N-Arylation of Heterocycles with Diaryliodonium Salts under Aqueous Conditions: A Theoretical and Experimental Mechanistic Study. J. Org. Chem. 2016, 81, 5417–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Huang, L.; Xie, L.; Cheng, J. Transition-Metal-Free Selective Iodoarylation of Pyrazoles via Heterocyclic Aryliodonium Ylides. Eur. J. Org. Chem. 2018, 2018, 3437–3443. [Google Scholar] [CrossRef]
- Riedmüllera, S.; Nachtsheim, B.J. Metal-Free N-Arylation of Indolines with Diaryliodonium Salts. Synlett 2015, 26, 651–655. [Google Scholar] [CrossRef]
- Purkait, N.; Kervefors, G.; Linde, E.; Olofsson, B. Regiospecific N-Arylation of Aliphatic Amines under Mild and Metal-Free Reaction Conditions. Angew. Chem. Int. Ed. 2018, 57, 11427–11431. [Google Scholar] [CrossRef]
- Mehra, M.K.; Tantak, M.P.; Arun, V.; Kumar, I.; Kumar, D. Metal-free regioselective formation of C–N and C–O bonds with the utilization of diaryliodonium salts in water: Facile synthesis of N-arylquinolones and aryloxyquinolines. Org. Biomol. Chem. 2017, 15, 4956–4961. [Google Scholar] [CrossRef]
- Dhiman, P.; Arora, N.; Veeraveedu, P.; Thanikachalam, P.V.; Monga, V. Recent advances in the synthetic and medicinal perspective of quinolones: A review. Bioorg. Chem. 2019, 92, 103291–103335. [Google Scholar] [CrossRef]
- Li, X.-H.; Ye, A.-H.; Liang, C.; Mo, D.-L. Substituent Effects of 2-Pyridones on Selective O-Arylation with Diaryliodonium Salts: Synthesis of 2-Aryloxypyridines under Transition-Metal-Free Conditions. Synthesis 2018, 50, 1699–1710. [Google Scholar] [CrossRef] [Green Version]
- Kuriyama, M.; Hanazawa, N.; Abe, Y.; Katagiri, K.; Ono, S.; Yamamoto, K.; Onomura, O. N- and O-arylation of pyridin-2-ones with diaryliodonium salts: Base-dependent orthogonal selectivity under metal-free conditions. Chem. Sci. 2020, 11, 8295–8300. [Google Scholar] [CrossRef] [PubMed]
- Tinnis, F.; Stridfeldt, E.; Lundberg, H.; Adolfsson, H.; Olofsson, B. Metal-Free N-Arylation of Secondary Amides at Room Temperature. Org. Lett. 2015, 17, 2688–2691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Han, J.; Wang, L. Metal- and Base-Free Direct N-Arylation of Pyridazinones by Using Diaryliodonium Salts: An Anion Effect. Asian J. Org. Chem. 2018, 7, 1674–1680. [Google Scholar] [CrossRef]




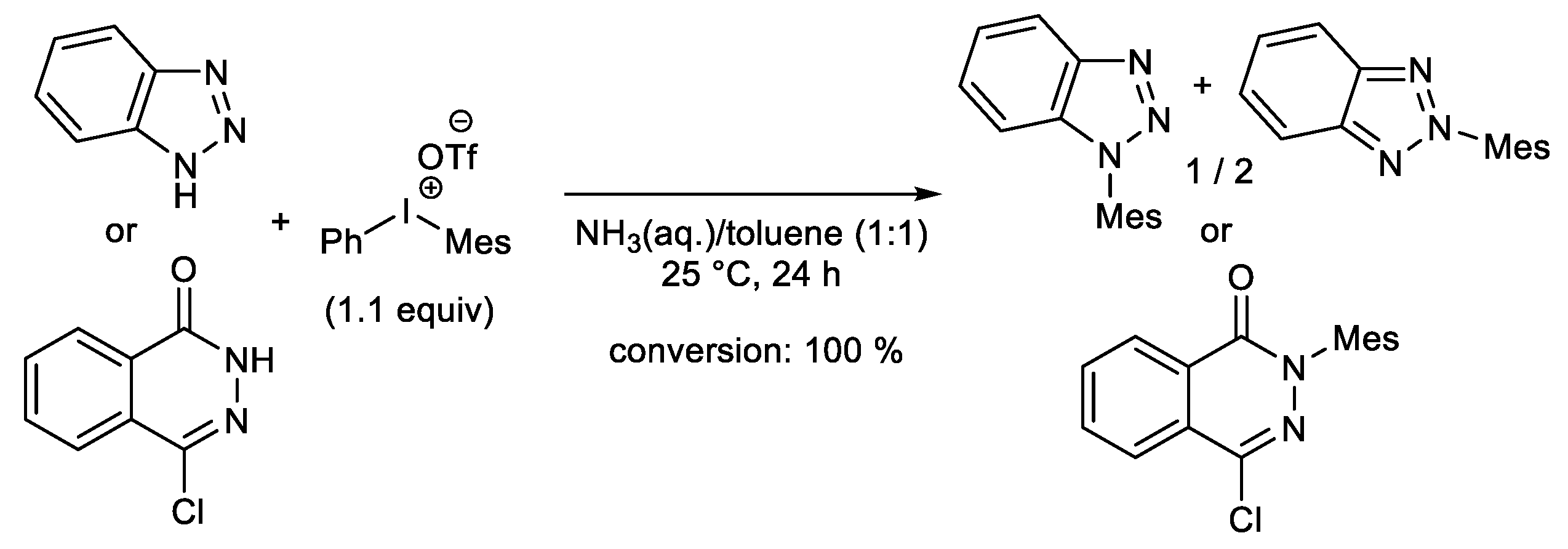
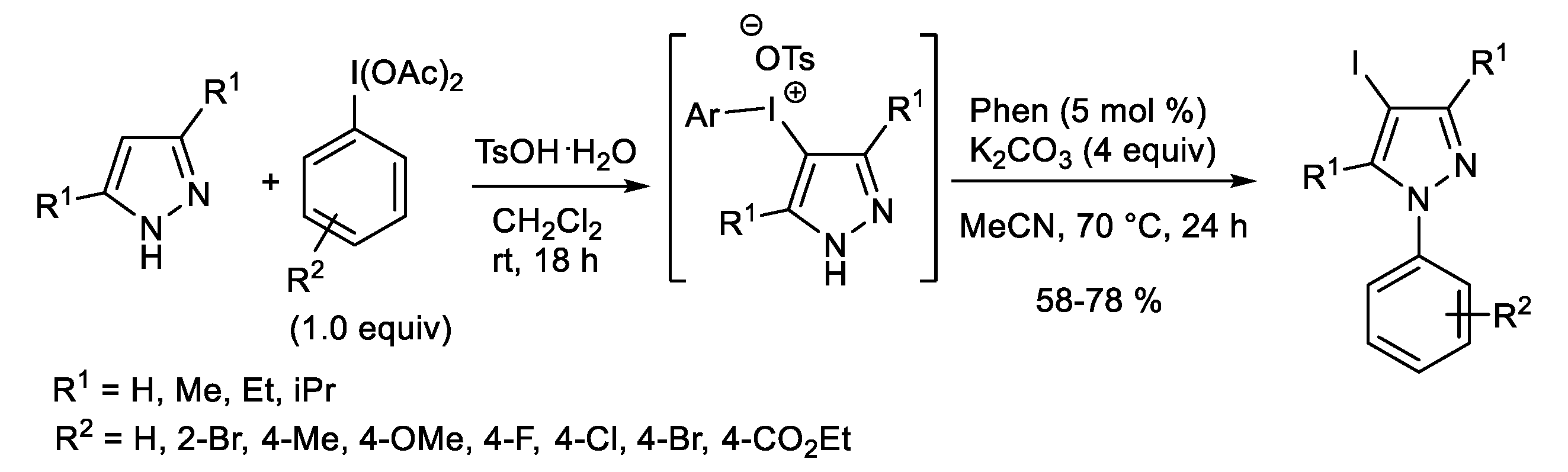
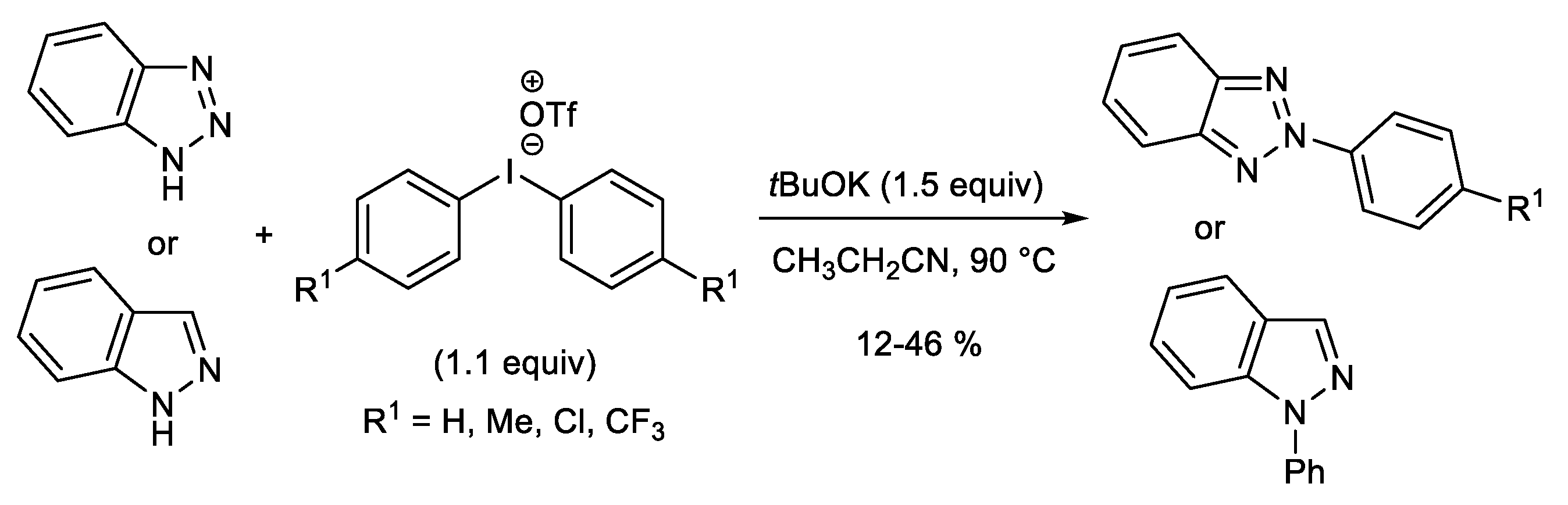
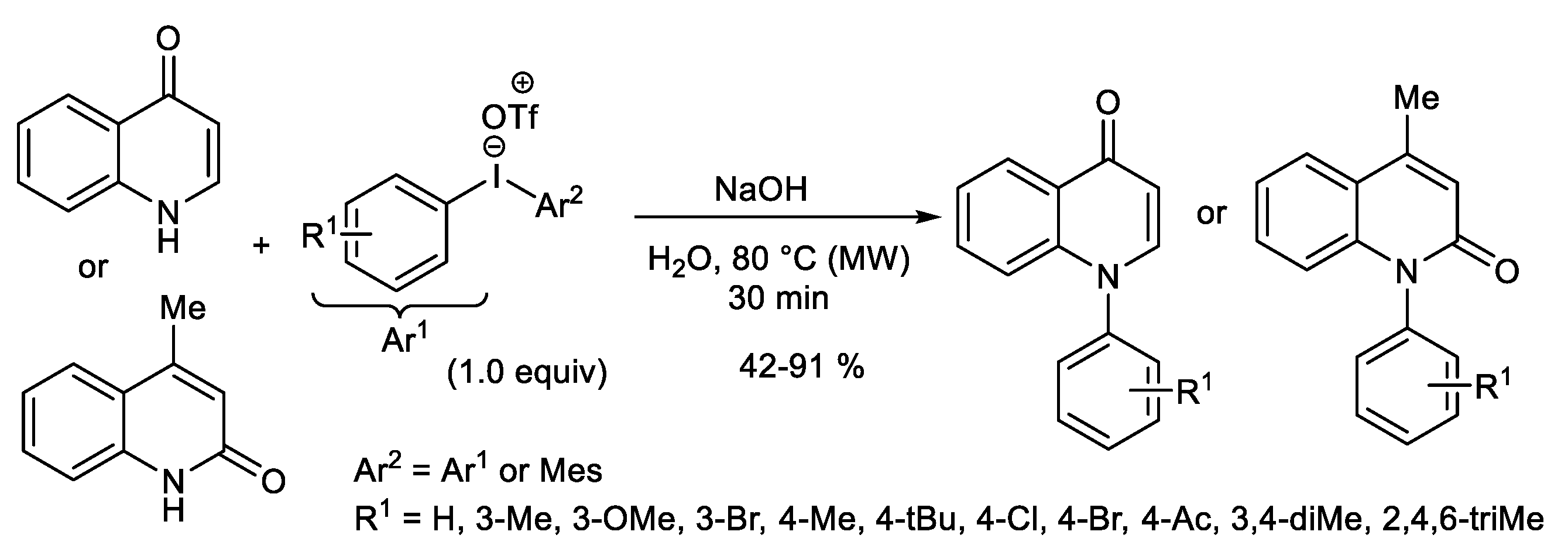
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besson, T.; Fruit, C. Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts. Pharmaceuticals 2021, 14, 661. https://doi.org/10.3390/ph14070661
Besson T, Fruit C. Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts. Pharmaceuticals. 2021; 14(7):661. https://doi.org/10.3390/ph14070661
Chicago/Turabian StyleBesson, Thierry, and Corinne Fruit. 2021. "Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts" Pharmaceuticals 14, no. 7: 661. https://doi.org/10.3390/ph14070661
APA StyleBesson, T., & Fruit, C. (2021). Recent Advances in Transition-Metal-Free Late-Stage C-H and N-H Arylation of Heteroarenes Using Diaryliodonium Salts. Pharmaceuticals, 14(7), 661. https://doi.org/10.3390/ph14070661






