Essential Oil from the Leaves of Chromolaena odorata, and Sesquiterpene Caryophyllene Oxide Induce Sedative Activity in Mice
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of COEO
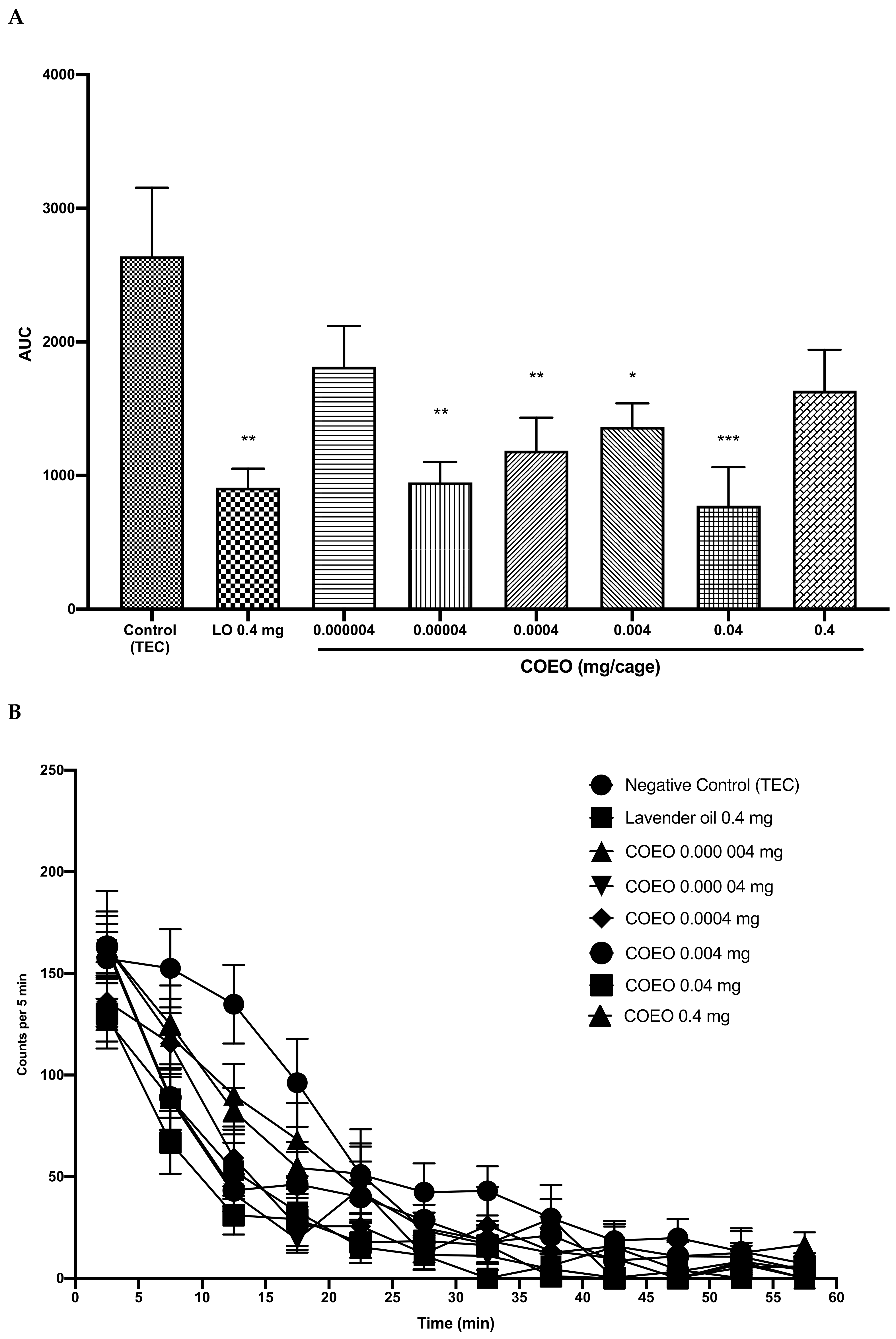
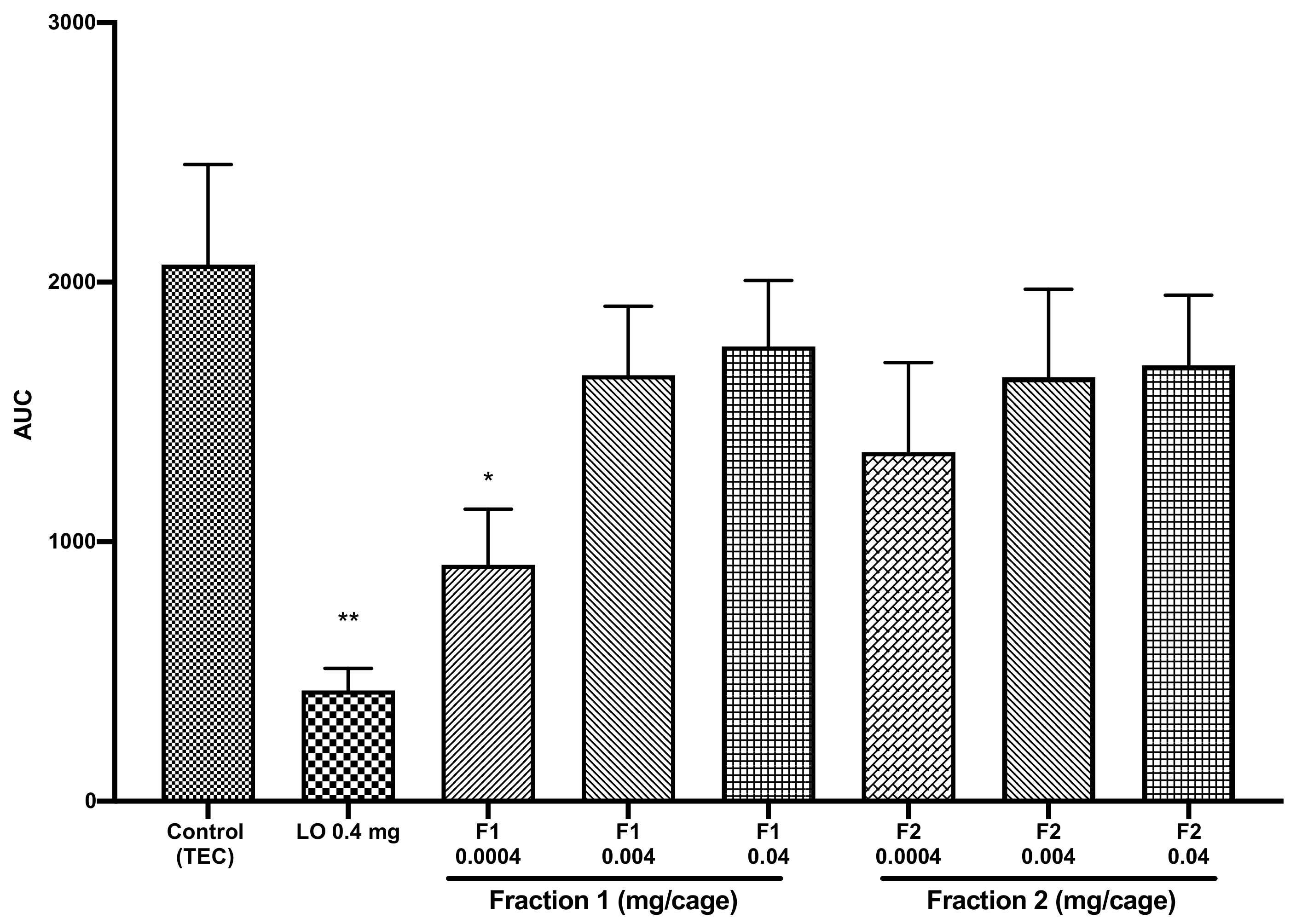
2.2. Sedative Activity and Bioactivity-Guided Fractionation of COEO
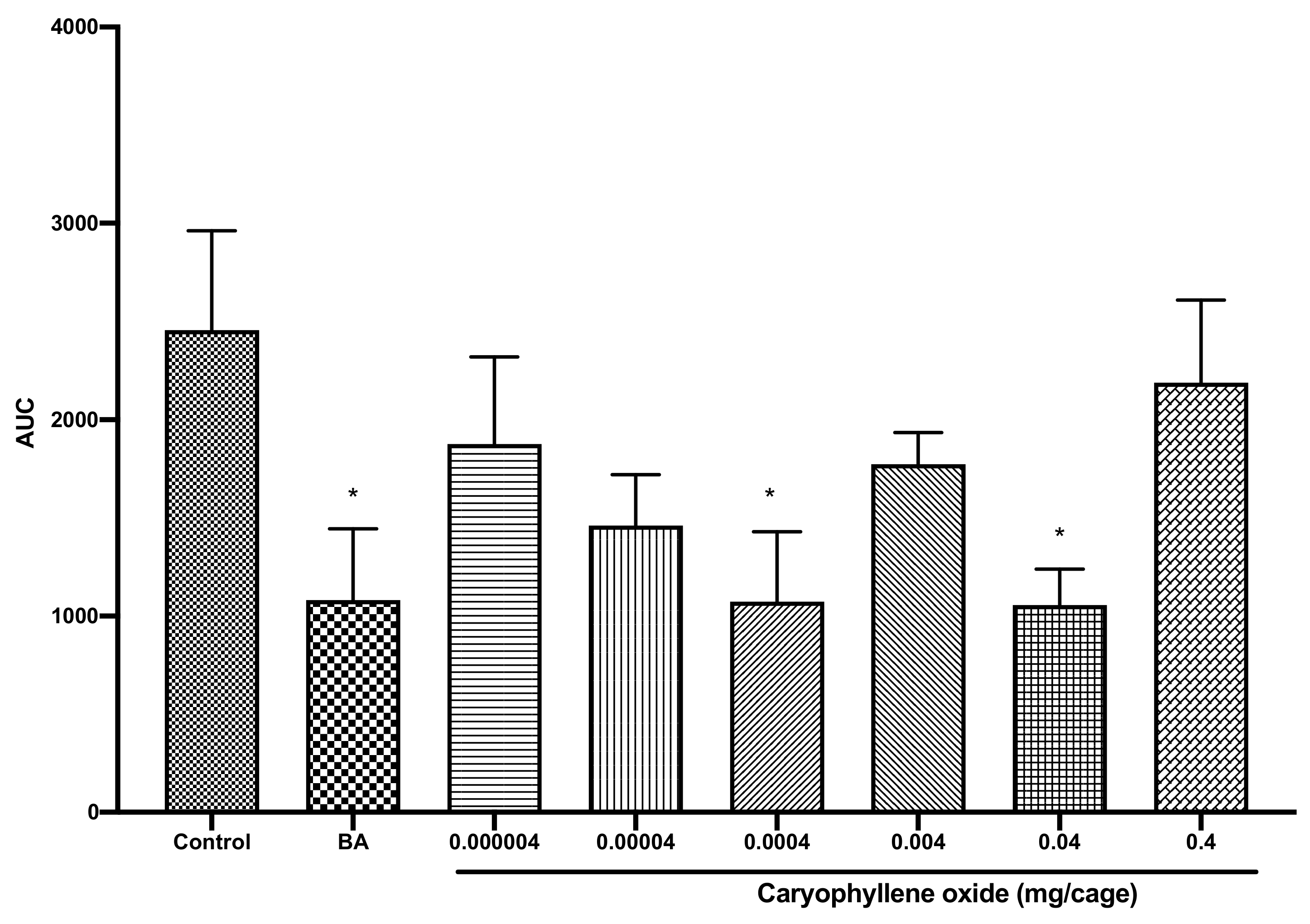
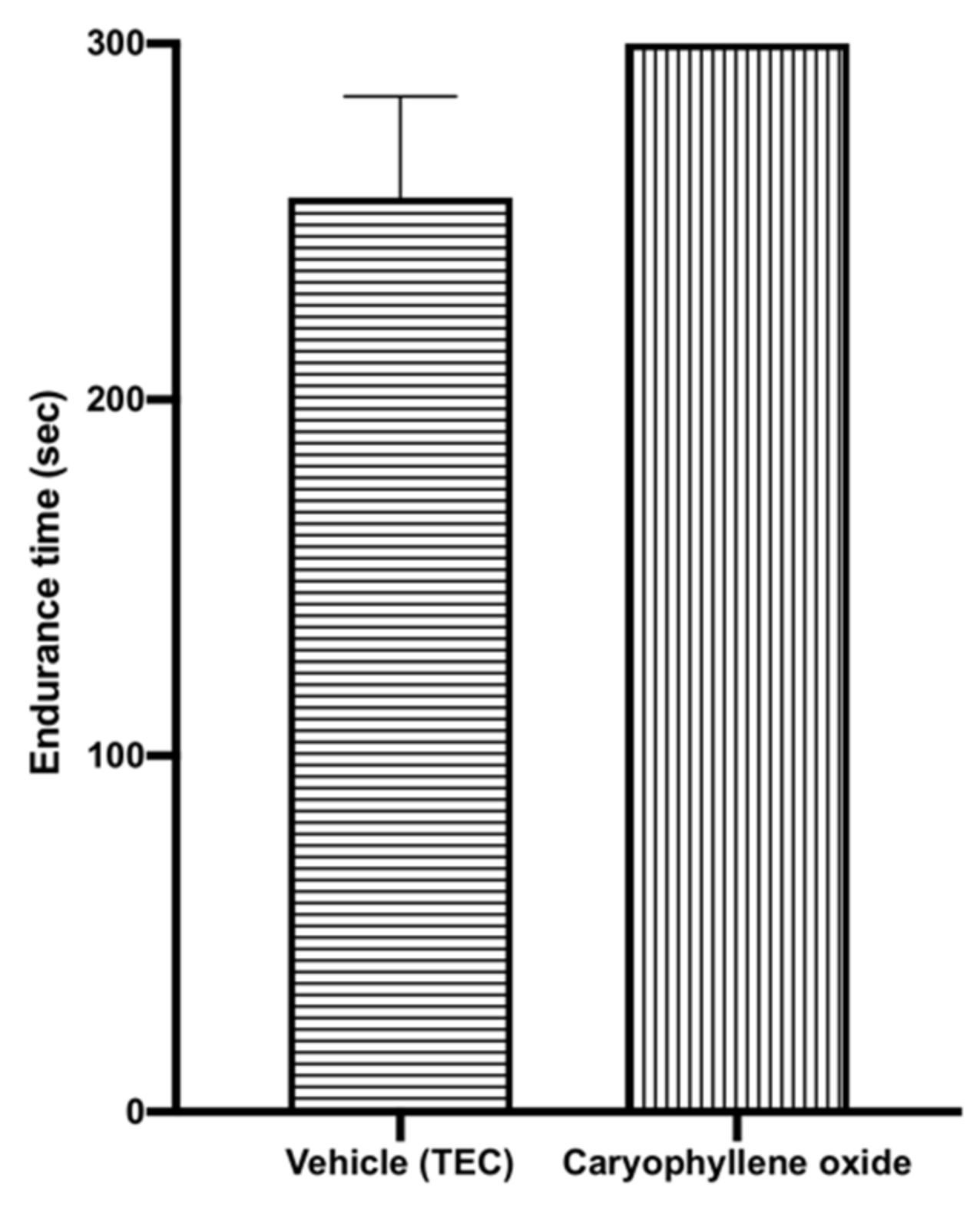
2.3. Caryophyllene Oxide Induced Sedative Activity in Mice
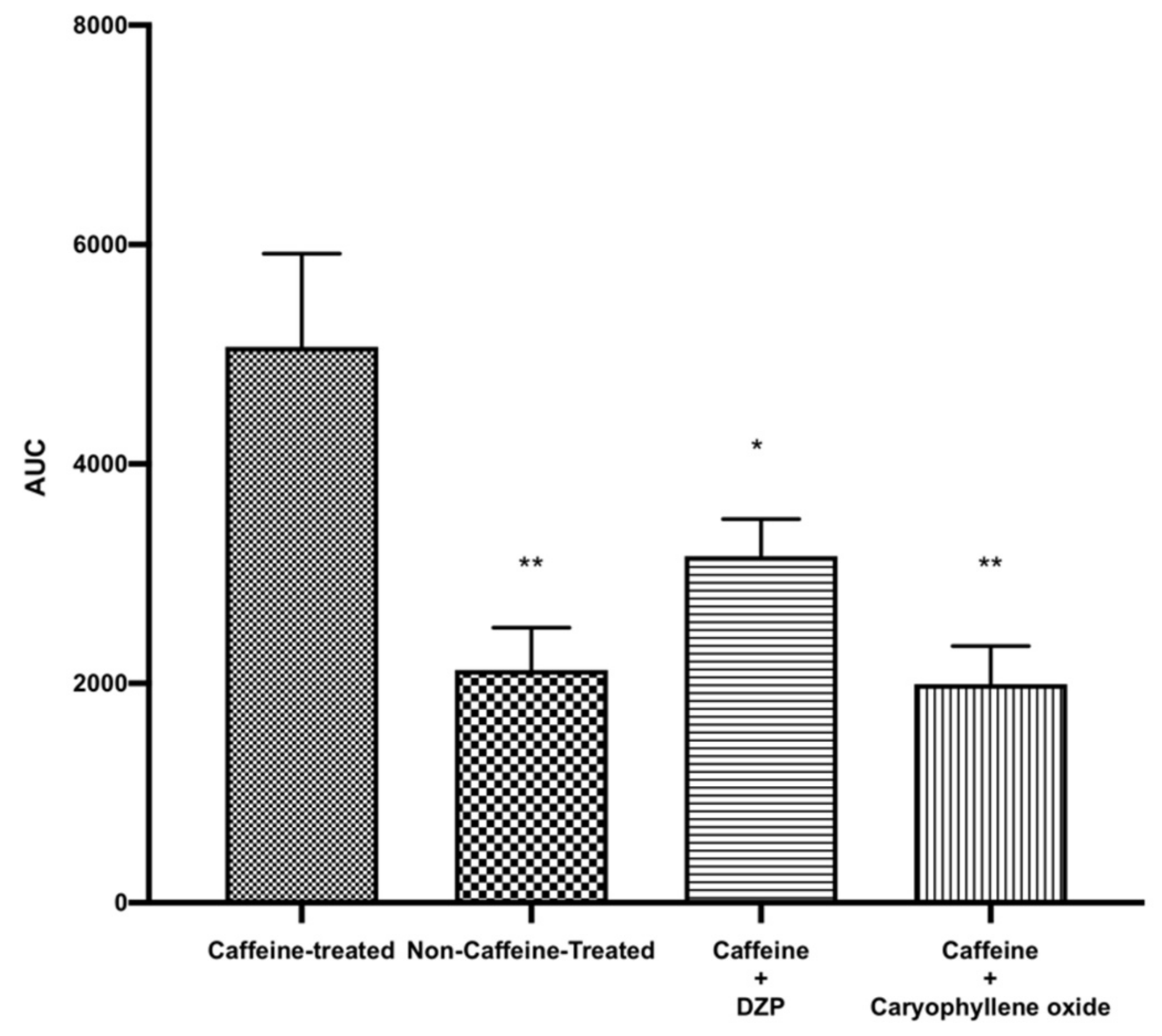
2.4. Effect of Caryophyllene Oxide on Pre-Excited Mice with Caffeine
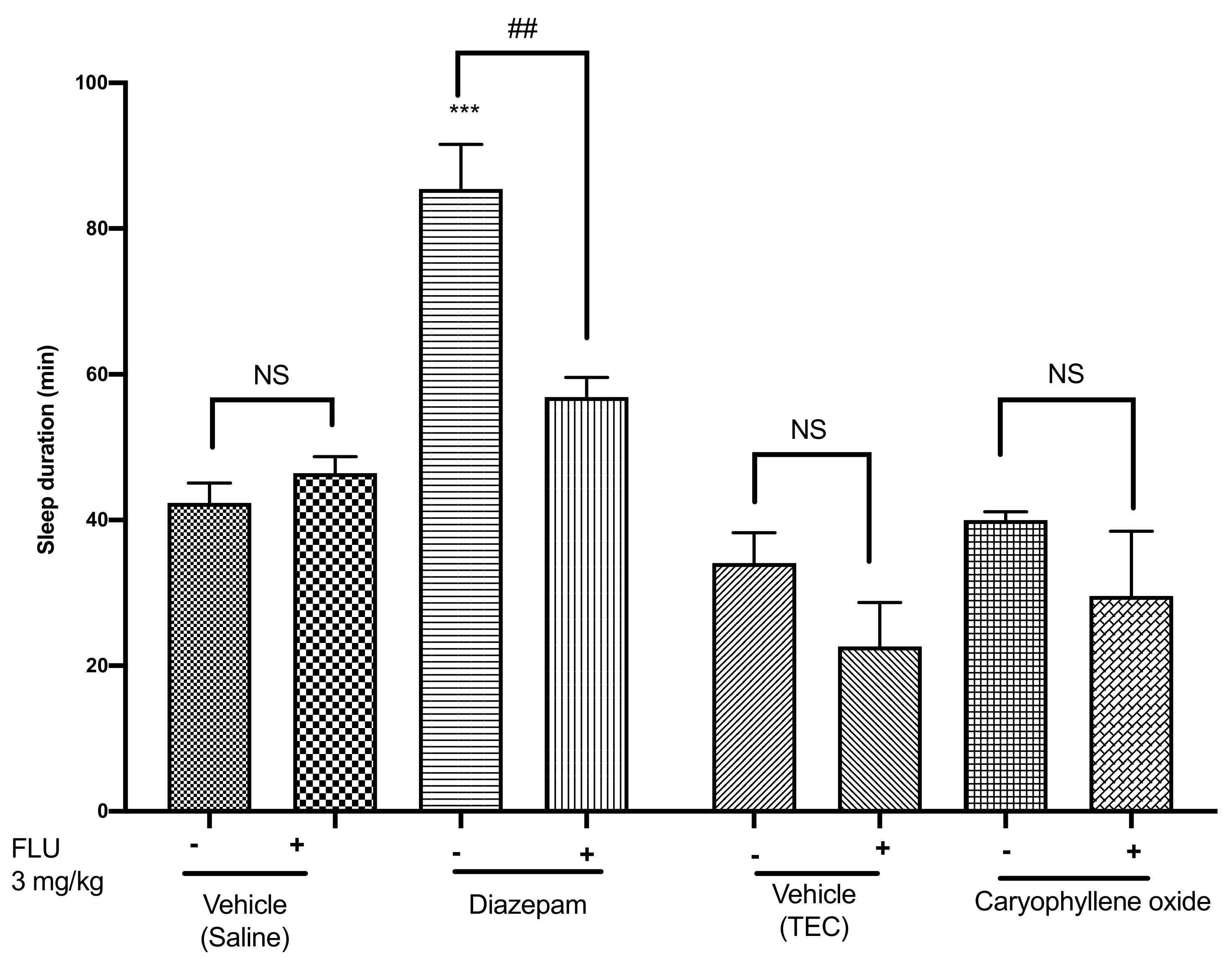
2.5. Effects of Caryophyllene Oxide on the Pentobarbital-Induced Sleeping Test in Mice
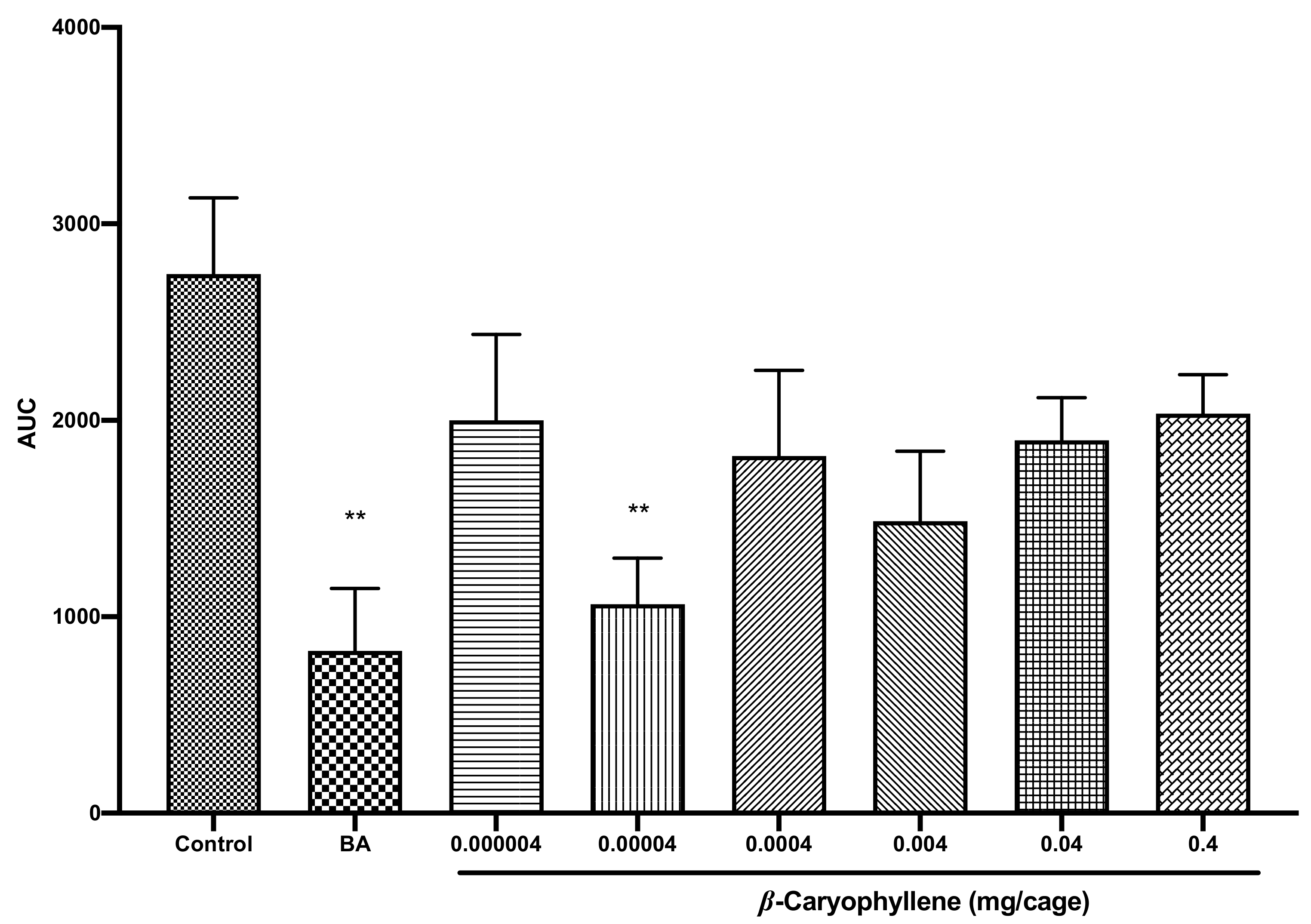
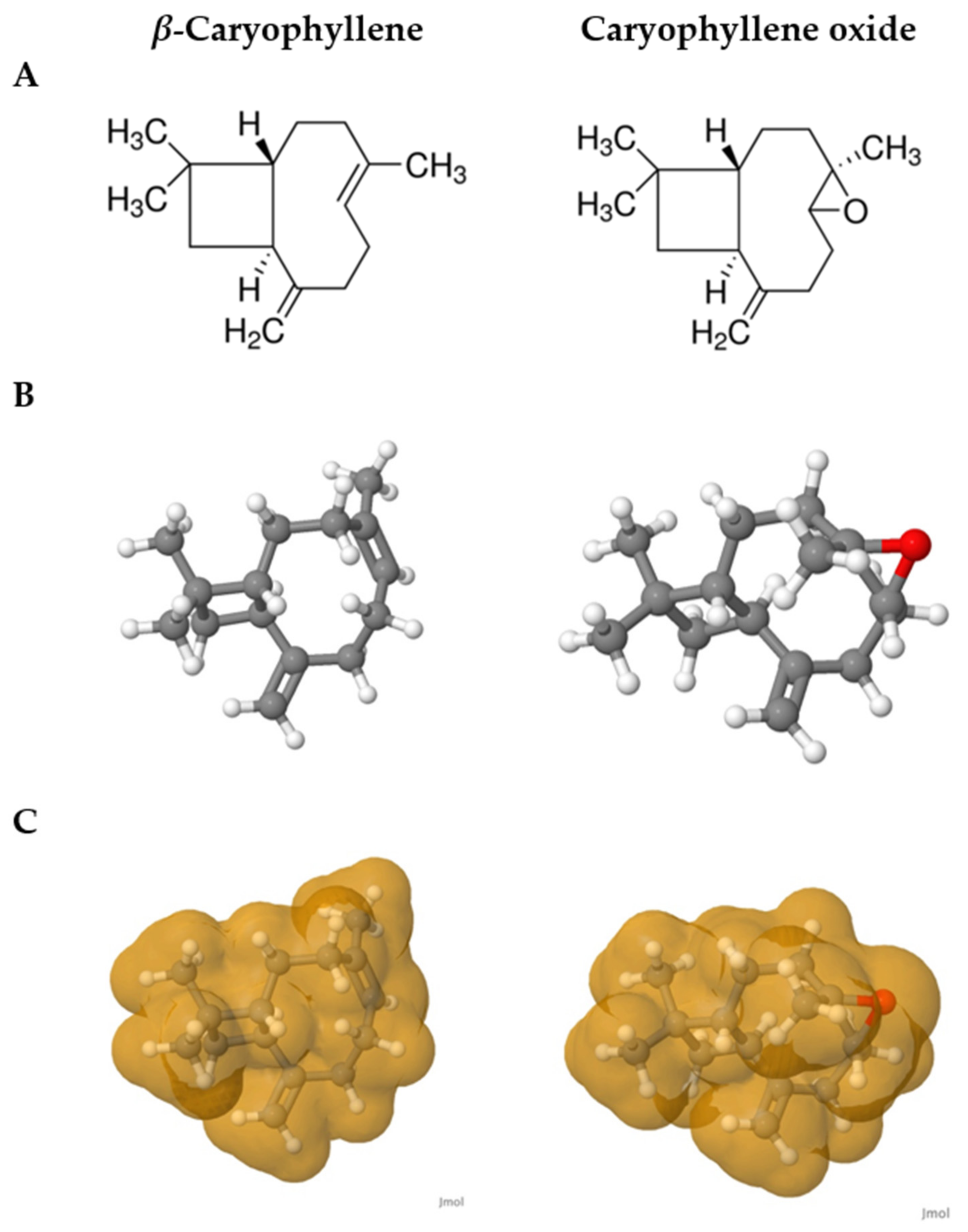
2.6. Structure–Activity Relationships of Caryophyllene Oxide and Its Precursor Β-Caryophyllene
3. Materials and Methods
3.1. Plant Material
3.2. Preparation and Fractionation of COEO
3.3. Drugs and Reagents
3.4. Animals
3.5. GC and GC-MS Analyses
3.6. Open-Field Test
3.7. Caffeine-Induced Stimulation of Locomotor Activity in Mice
3.8. Pentobarbital-Induced Sleeping Test
3.9. Rota-Rod Test
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.-H.; Sun, H.-L.; Chen, S.-Y.; Zeng, L.; Wang, T.-T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Almeida Bezerra, J.W.; Rodrigues Costa, A.; de Freitas, M.A.; Rodrigues, F.C.; de Souza, M.A.; da Silva, A.R.P.; dos Santos, A.T.L.; Vieiralves Linhares, K.; Melo Coutinho, H.D.; de Lima Silva, J.R.; et al. Chemical composition, antimicrobial, modulator and antioxidant activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 58–64. [Google Scholar] [PubMed]
- Dougnon, G.; Ito, M. Role of Ascaridole and p-Cymene in the Sleep-Promoting Effects of Dysphania ambrosioides Essential Oil via the GABAergic System in a ddY Mouse Inhalation Model. J. Nat. Prod. 2021, 84, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, G.; Ito, M. Inhalation administration of the bicyclic ethers 1,8- and 1,4-cineole prevent anxiety and depressive-like behaviours in mice. Molecules 2020, 25, 1884. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A.; Jaffal, S.M.; Al-Najjar, B.O. Analgesic and anxiolytic activities of Achillea biebersteinii: Evidence for the involvement of GABAergic systems. Orient. J. Chem. 2019, 35, 1433–1442. [Google Scholar] [CrossRef]
- Bagci, E.; Aydin, E.; Mihasan, M.; Maniu, C.; Hritcu, L. Anxiolytic and antidepressant-like effects of Ferulago angulata essential oil in the scopolamine rat model of Alzheimer’s disease. Flavour Fragr. J. 2016, 31, 70–80. [Google Scholar] [CrossRef]
- Miyoshi, T.; Ito, M.; Kitayama, T.; Isomori, S.; Yamashita, F. Sedative effects of inhaled benzylacetone and structural features contributing to its activity. Biol. Pharm. Bull. 2013, 36, 1474–1481. [Google Scholar] [CrossRef]
- Abdelhakim, A.M.; Hussein, A.S.; Doheim, M.F.; Sayed, A.K. The effect of inhalation aromatherapy in patients undergoing cardiac surgery: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102256. [Google Scholar] [CrossRef]
- Sugawara, Y.; Hara, C.; Tamura, K.; Fujii, T.; Nakamura, K.; Masujima, T.; Aoki, T. Sedative effect on humans of inhalation of essential oil of linalool. Anal. Chim. Acta 1998, 365, 293–299. [Google Scholar] [CrossRef]
- Muniappan, R.; Reddy, G.V.P.; Raman, A. Biological Control of Tropical Weeds Using Arthropods; Cambridge University Press: New York, NY, USA, 2009; ISBN 9780511576348. [Google Scholar]
- Zahara, M. Description of Chromolaena odorata L. R.M King and H. Robinson as medicinal plant: A Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Aceh, Indonesia, 2019; Volume 506. [Google Scholar]
- Phan, T.T.; Hughes, M.A.; Cherry, G.W. Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plast. Reconstr. Surg. 1998, 101, 756–765. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Ibukun, E.O.; Dan-Ologe, I.A. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Sci. Res. Essays 2007, 2, 191–194. [Google Scholar]
- Idu, M.; Onyibe, H.I. Medicinal Plants of Edo State, Nigeria. Res. J. Med. Plant. 2007, 1, 32–41. [Google Scholar]
- Nanadini, N.; Nagababu, P.; Rao, U.; Venugopal, N. Phytochemical, antimicrobial and antioxidant properties of an invasive weed-Chromolaena odorata (L.) King & Robinson. Int. J. Phytomed. 2014, 6, 286–292. [Google Scholar]
- Lamaty, G.; Menut, C.; Zollo, P.H.A.; Kuiate, J.R.; Bessiere, J.M.; Ouamba, J.M.; Silou, T. Aromatic plants of tropical central Africa IV. Essential oils of Eupatorium odoratum L. from Cameroon and Congo. J. Essent. Oil Res. 1992, 4, 101–105. [Google Scholar] [CrossRef]
- Gogoi, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. North-East Indian Chromolaena odorata (L. King Robinson) Aerial Part Essential Oil Chemical Composition, Pharmacological Activities—Neurodegenerative Inhibitory and Toxicity Study. J. Essent. Oil Bear. Plants 2020, 23, 1173–1191. [Google Scholar] [CrossRef]
- Tonzibo, Z.F.; Wognin, E.; Chalchat, J.C.; N’Guessan, Y.T. Chemical investigation of Chromolaena odorata L. King Robinson from ivory coast. J. Essent. Oil Bear. Plants 2007, 10, 94–100. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Medicinal uses, thin-layer chromatography and high-performance liquid chromatography profiles of plant species from Abomey-Calavi and Dantokpa Market in the Republic of Benin. J. Nat. Med. 2020, 74, 311–322. [Google Scholar] [CrossRef]
- Pereira da Silva, H.N.; dos Santos Machado, S.D.; de Andrade Siqueira, A.M.; Cardoso Costa da Silva, E.; de Oliveira Canto, M.Â.; Jensen, L.; Vargas Flores da Silva, L.; Sena Fugimura, M.M.; de Sousa Barroso, A.; Veras Mourão, R.H.; et al. Sedative and anesthetic potential of the essential oil and hydrolate from the fruit of Protium heptaphyllum and their isolated compounds in Colossoma macropomum juveniles. Aquaculture 2020, 529, 735629. [Google Scholar] [CrossRef]
- Judzentiene, A.; Budiene, J.; Butkiene, R.; Kupcinskiene, E.; Laffont-Schwob, I.; Masotti, V. Caryophyllene oxide-rich essential oils of lithuanian Artemisia campestris ssp. campestris and their toxicity. Nat. Prod. Commun. 2010, 5, 1981–1984. [Google Scholar]
- Farag, R.S.; Shalaby, A.S.; El-Baroty, G.A.; Ibrahim, N.A.; Ali, M.A.; Hassan, E.M. Chemical and Biological Evaluation of the Essential Oils of Different Melaleuca Species. Phyther. Res. 2004, 18, 30–35. [Google Scholar] [CrossRef]
- de Lima Silva, L.; da Silva, D.T.; Garlet, Q.I.; Cunha, M.A.; Mallmann, C.A.; Baldisserotto, B.; Longhi, S.J.; Pereira, A.M.S.; Heinzmann, B.M. Anesthetic activity of Brazilian native plants in silver catfish (Rhamdia quelen). Neotrop. Ichthyol. 2013, 11, 443–451. [Google Scholar] [CrossRef]
- Dougnon, G.; Ito, M. Sedative effects of the essential oil from the leaves of Lantana camara occurring in the Republic of Benin via inhalation in mice. J. Nat. Med. 2020, 74, 159–169. [Google Scholar] [CrossRef]
- Takemoto, H.; Omameuda, Y.; Ito, M.; Fukuda, T.; Kaneko, S.; Akaike, A.; Kobayashi, Y. Inhalation administration of valerena-4,7(11)-diene from Nardostachys chinensis roots ameliorates restraint stress-induced changes in murine behavior and stress-related factors. Biol. Pharm. Bull. 2014, 37, 1050–1055. [Google Scholar] [CrossRef]
- Oshima, T.; Ito, M. Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. J. Nat. Med. 2021, 75, 319–325. [Google Scholar] [CrossRef]
- Takemoto, H.; Ito, M.; Shiraki, T.; Yagura, T.; Honda, G. Sedative effects of vapor inhalation of agarwood oil and spikenard extract and identification of their active components. J. Nat. Med. 2008, 62, 41–46. [Google Scholar] [CrossRef]
- Ogawa, K.; Yabe, H.; Kitayama, T.; Ito, M. Locomotor-reducing activity of sesquiterpenes related to Zingiber zerumbet essential oil and hexahydrozerumbone derivatives. Biol. Pharm. Bull. 2016, 39, 1077–1080. [Google Scholar] [CrossRef][Green Version]
- Ascari, J.; Sens, S.L.; Nunes, D.S.; Wisniewski, A.; Arbo, M.D.; Linck, V.M.; Lunardi, P.; Leal, M.B.; Elisabetsky, E. Sedative effects of essential oils obtained from Baccharis uncinella. Pharm. Biol. 2012, 50, 113–119. [Google Scholar] [CrossRef]
- Benovit, S.C.; Silva, L.L.; Salbego, J.; Loro, V.L.; Mallmann, C.A.; Baldisserotto, B.; Flores, E.M.M.; Heinzmann, B.M. Anesthetic activity and bioguided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An. Acad. Bras. Cienc. 2015, 87, 1675–1689. [Google Scholar]
- Parodi, T.V.; Gressler, L.T.; de Lima Silva, L.; Becker, A.G.; Schmidt, D.; Caron, B.O.; Heinzmann, B.M.; Baldisserotto, B. Chemical composition of the essential oil of Aloysia triphylla under seasonal influence and its anaesthetic activity in fish. Aquac. Res. 2020, 51, 2515–2524. [Google Scholar] [CrossRef]
- Hammami, S.; Jmii, H.; El Mokni, R.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; El Aouni, M.H.; Aouni, M.; Joshi, R.K. Antiviral activities of Teucrium pseudochamaepitys growing spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.Q.; Kenney, P.M.; Lam, L.K.T. Sesquiterpenes from clove (Eugenia caryophyllata) as potential anticarcinogenic agents. J. Nat. Prod. 1992, 55, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—Natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.; Kunde, R. Die Leitsubstanzen der Haschisch-Suchhunde. Krim. Z Gesamte Krim. Wiss Prax 1973, 27, 385–389. [Google Scholar]
- Kohn, M.C.; Melnick, R.L. Biochemical origins of the non-monotonic receptor-mediated dose-response. J. Mol. Endocrinol. 2002, 29, 113–123. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Ito, M. Synergistic effect of fragrant herbs in Japanese scent sachets. Planta Med. 2015, 81, 193–199. [Google Scholar] [CrossRef]
- Kagawa, D.; Jokura, H.; Ochiai, R.; Tokimitsu, I.; Tsubone, H. The sedative effects and mechanism of action of cedrol inhalation with behavioral pharmacological evaluation. Planta Med. 2003, 69, 637–641. [Google Scholar]
- Meckes, M.; Calzada, F.; Tortoriello, J.; Gonzalez, J.L.; Martínez, M. Terpenoids isolated from Psidium guajava hexane extract with depressant activity on central nervous system. Phyther. Res. 1996, 10, 600–603. [Google Scholar] [CrossRef]
- Çiçek, S.S. Structure-dependent activity of natural GABA(A) receptor modulators. Molecules 2018, 23, 1512. [Google Scholar] [CrossRef]
- Takemoto, H.; Ito, M.; Asada, Y.; Kobayashi, Y. Inhalation administration of the sesquiterpenoid aristolen-1(10)-en-9-ol from Nardostachys chinensis has a sedative effect via the GABAergic system. Planta Med. 2015, 81, 343–347. [Google Scholar] [CrossRef]
- Oppong-Damoah, A.; Blough, B.E.; Makriyannis, A.; Murnane, K.S. The sesquiterpene beta-caryophyllene oxide attenuates ethanol drinking and place conditioning in mice. Heliyon 2019, 5, e01915. [Google Scholar] [CrossRef]
- The Good Scents Company. Caryophyllene, 13877-93-5. Available online: http://www.thegoodscentscompany.com/data/rw1011551.html#tosafty (accessed on 30 April 2021).
- The Good Scents Company. Beta-Caryophyllene Oxide, 1139-30-6. Available online: http://www.thegoodscentscompany.com/data/rw1023631.html#tonotes (accessed on 30 April 2021).
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Chichester, UK, 2002; ISBN 9780471496410. [Google Scholar]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Amjad Kamal, M.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Klauke, A.L.; Racz, I.; Pradier, B.; Markert, A.; Zimmer, A.M.; Gertsch, J.; Zimmer, A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014, 24, 608–620. [Google Scholar] [CrossRef]
- Sabulal, B.; Dan, M.; J, A.J.; Kurup, R.; Pradeep, N.S.; Valsamma, R.K.; George, V. Caryophyllene-rich rhizome oil of Zingiber nimmonii from South India: Chemical characterization and antimicrobial activity. Phytochemistry 2006, 67, 2469–2473. [Google Scholar] [CrossRef]
- Medeiros, R.; Passos, G.F.; Vitor, C.E.; Koepp, J.; Mazzuco, T.L.; Pianowski, L.F.; Campos, M.M.; Calixto, J.B. Effect of two active compounds obtained from the essential oil of Cordia verbenacea on the acute inflammatory responses elicited by LPS in the rat paw. Br. J. Pharmacol. 2007, 151, 618–627. [Google Scholar] [CrossRef]
- Galdino, P.M.; Nascimento, M.V.M.; Florentino, I.F.; Lino, R.C.; Fajemiroye, J.O.; Chaibub, B.A.; de Paula, J.R.; de Lima, T.C.M.; Costa, E.A. The anxiolytic-like effect of an essential oil derived from Spiranthera odoratissima A. St. Hil. leaves and its major component, β-caryophyllene, in male mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 38, 276–284. [Google Scholar] [CrossRef]
- Marco, E.M.; García-Gutiérrez, M.S.; Bermúdez-Silva, F.J.; Moreira, F.A.; Guimarães, F.; Manzanares, J.; Viveros, M.P. Endocannabinoid system and psychiatry: In search of a neurobiological basis for detrimental and potential therapeutic effects. Front. Behav. Neurosci. 2011, 5, 63. [Google Scholar] [CrossRef]
- The Jmol Team Jmol: An Open-Source Java Viewer for Chemical Structures in 3D. Available online: http://jmol.sourceforge.net/ (accessed on 30 April 2021).
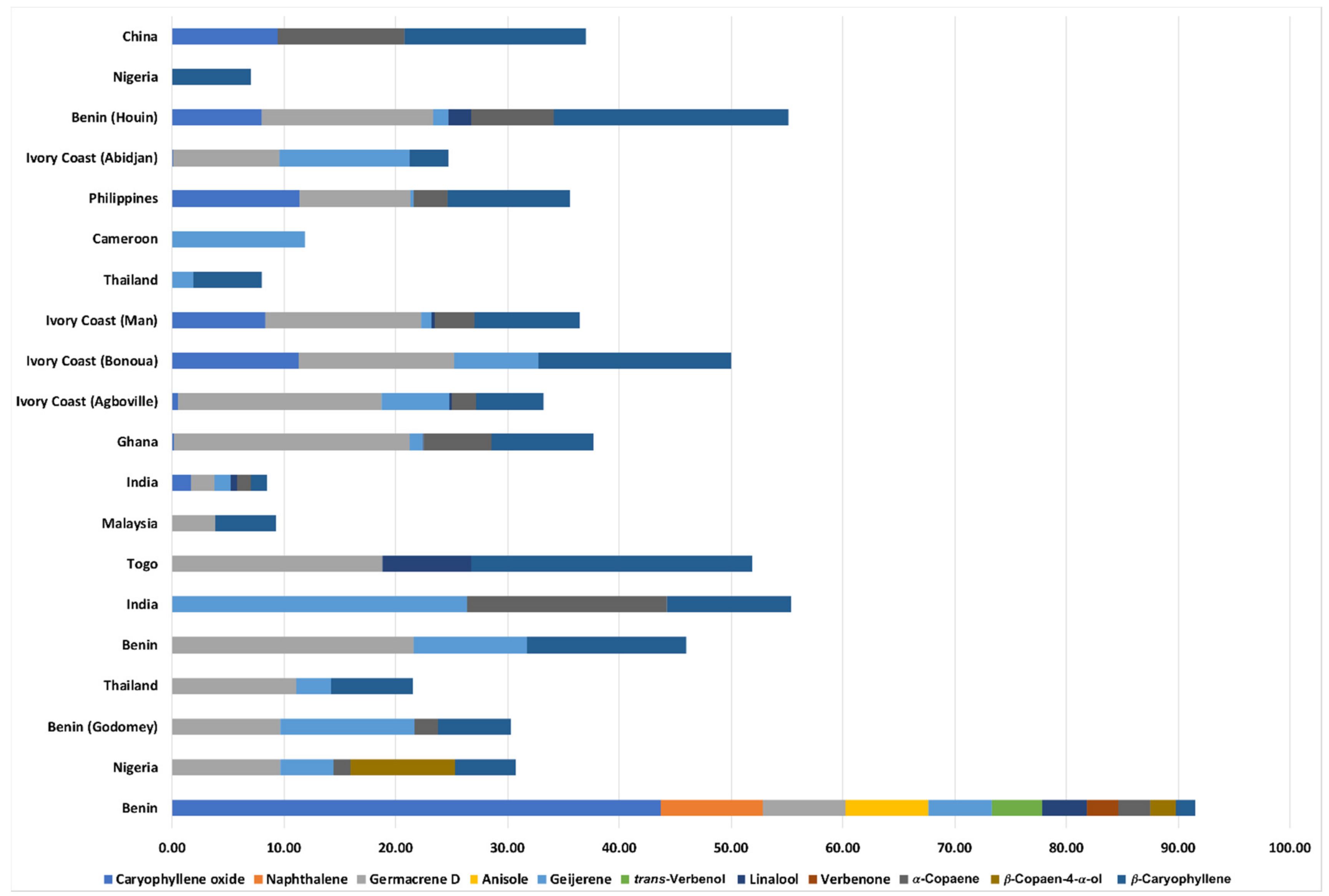
| N° | Compounds a | Peak Area (%) b | Calculated RI c | Literature RI d |
|---|---|---|---|---|
| 1 | Geijerene | 5.68 | 1298 | 1338 |
| 2 | α-Copaene | 2.84 | 1461 | 1491 |
| 3 | Benzene, 1,2,4-triethyl- | 1.14 | 1501 | 1501 |
| 4 | Linalool | 3.98 | 1504 | 1543 |
| 5 | Pinocarvone | 1.70 | 1535 | 1575 |
| 6 | β-Caryophyllene | 1.70 | 1559 | 1588 |
| 7 | β-Copaen-4-α--ol | 2.27 | 1571 | 1594 |
| 8 | trans-Pinocarveol | 0.57 | 1611 | 1661 |
| 9 | Anisole | 7.39 | 1627 | |
| 10 | trans-Verbenol | 4.55 | 1633 | 1680 |
| 11 | Germacrene D | 7.39 | 1661 | 1708 |
| 12 | Verbenone | 2.84 | 1664 | 1720 |
| 13 | p-Mentha-1,5-dien-8-ol | 0.57 | 1673 | 1738 |
| 14 | Naphthalene | 9.09 | 1690 | 1755 |
| 15 | Caryophyllene oxide | 43.75 | 1916 | 1986 |
| Unidentified | 4.55 | |||
| Total | 100 |
| β-Caryophyllene | Caryophyllene Oxide | |
|---|---|---|
| Appearance | Colorless to pale yellow clear oily liquid | Pale yellow white crystalline solid |
| Molecular Weight | 204.35 g/mol | 220.35 g/mol |
| Formula | C15 H24 | C15 H24 O |
| Odor | Spicy | Woody |
| Optical Rotation | −5.00 to −10.00 | −65.00 to −75.00 |
| Melting Point | 129.00 to 130.00 °C at 14.00 mm Hg | 60.00 to 62.00 °C at 760.00 mm Hg |
| Boiling Point | 254.00 to 257.00 °C at 760.00 mm Hg | 279.68 °C at 760.00 mm Hg |
| Vapor Pressure | 0.013000 mmHg at 25 °C | 0.007000 mmHg at 25 °C |
| Flash Point | 205.00 °F | >212.00 °F |
| logP (o/w) | 6.777 | 4.429 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougnon, G.; Ito, M. Essential Oil from the Leaves of Chromolaena odorata, and Sesquiterpene Caryophyllene Oxide Induce Sedative Activity in Mice. Pharmaceuticals 2021, 14, 651. https://doi.org/10.3390/ph14070651
Dougnon G, Ito M. Essential Oil from the Leaves of Chromolaena odorata, and Sesquiterpene Caryophyllene Oxide Induce Sedative Activity in Mice. Pharmaceuticals. 2021; 14(7):651. https://doi.org/10.3390/ph14070651
Chicago/Turabian StyleDougnon, Godfried, and Michiho Ito. 2021. "Essential Oil from the Leaves of Chromolaena odorata, and Sesquiterpene Caryophyllene Oxide Induce Sedative Activity in Mice" Pharmaceuticals 14, no. 7: 651. https://doi.org/10.3390/ph14070651
APA StyleDougnon, G., & Ito, M. (2021). Essential Oil from the Leaves of Chromolaena odorata, and Sesquiterpene Caryophyllene Oxide Induce Sedative Activity in Mice. Pharmaceuticals, 14(7), 651. https://doi.org/10.3390/ph14070651







