Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time
Abstract
1. Introduction
2. Results
2.1. Viability Curves for 12 Tested Cytotoxic Agents on 6 CRC Cell Lines (HCT116, HT29, Caco-2, SW48, SW480 and SW620)
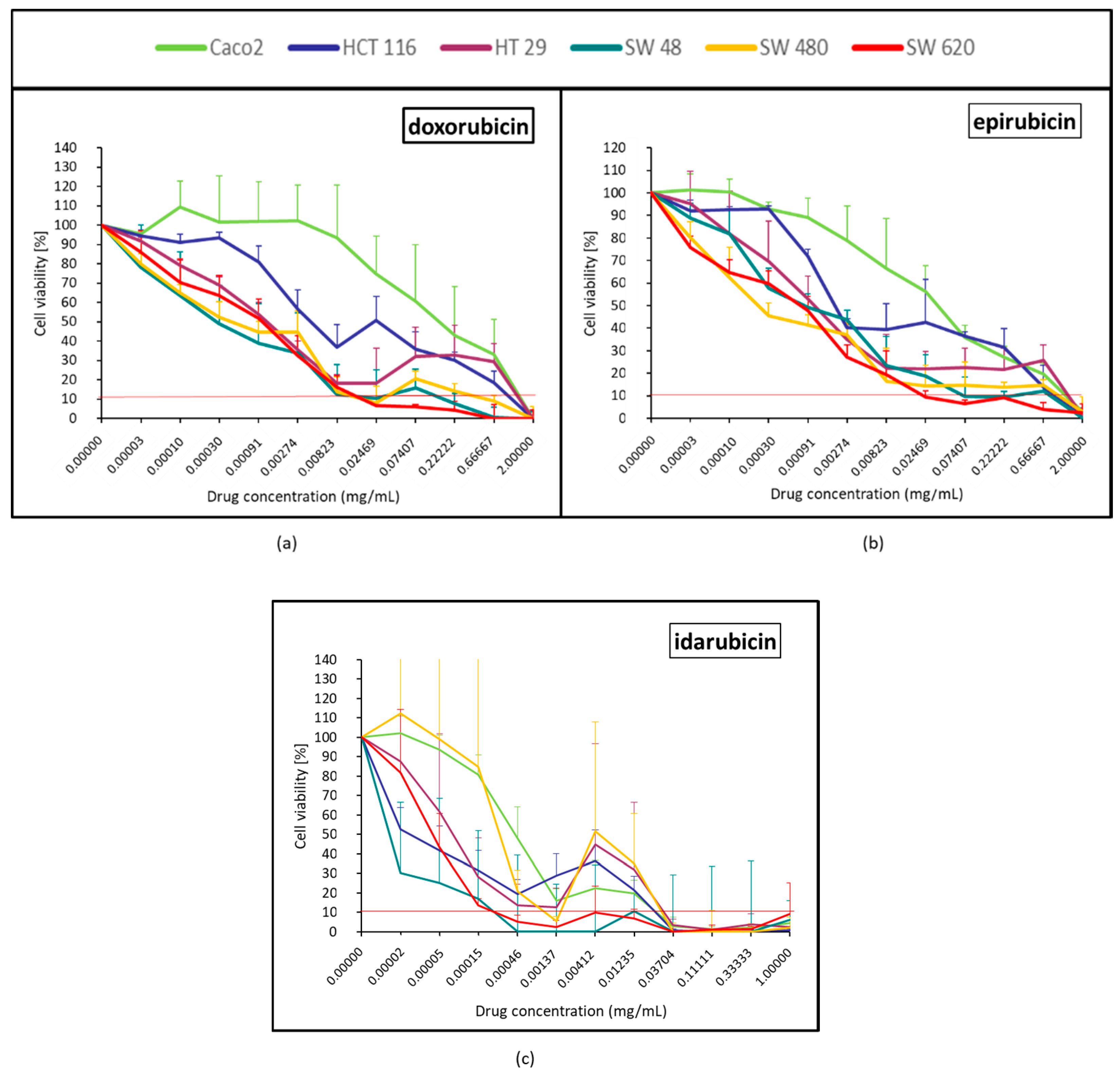
2.1.1. Topoisomerase II Inhibitor (Doxorubicin, Epirubicin and Idarubicin)
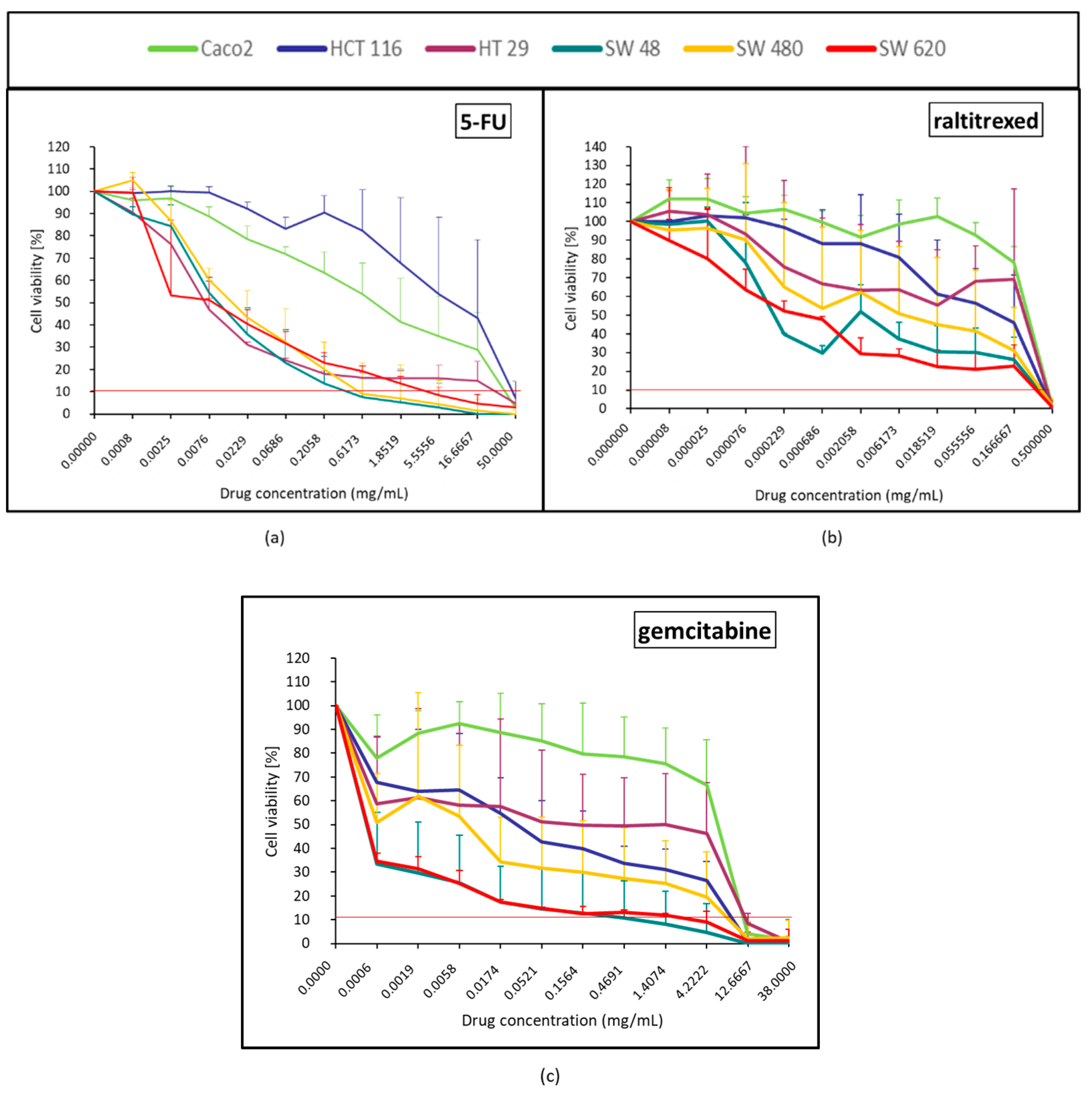
2.1.2. Anti-Metabolites Effect (5-FU, Raltitrexed and Gemcitabine)
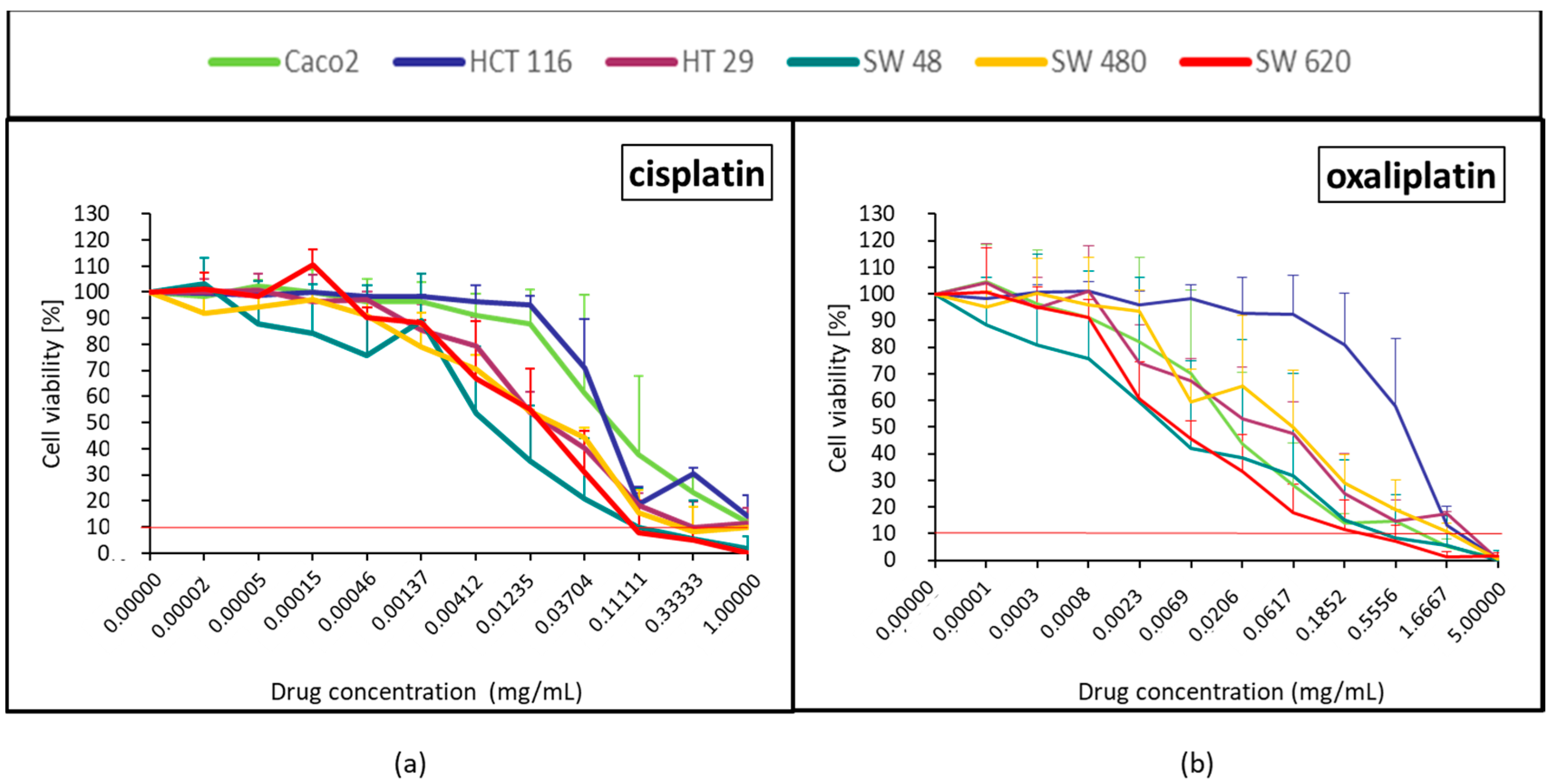
2.1.3. Platinum Derivatives (Cisplatin and Oxaliplatin)
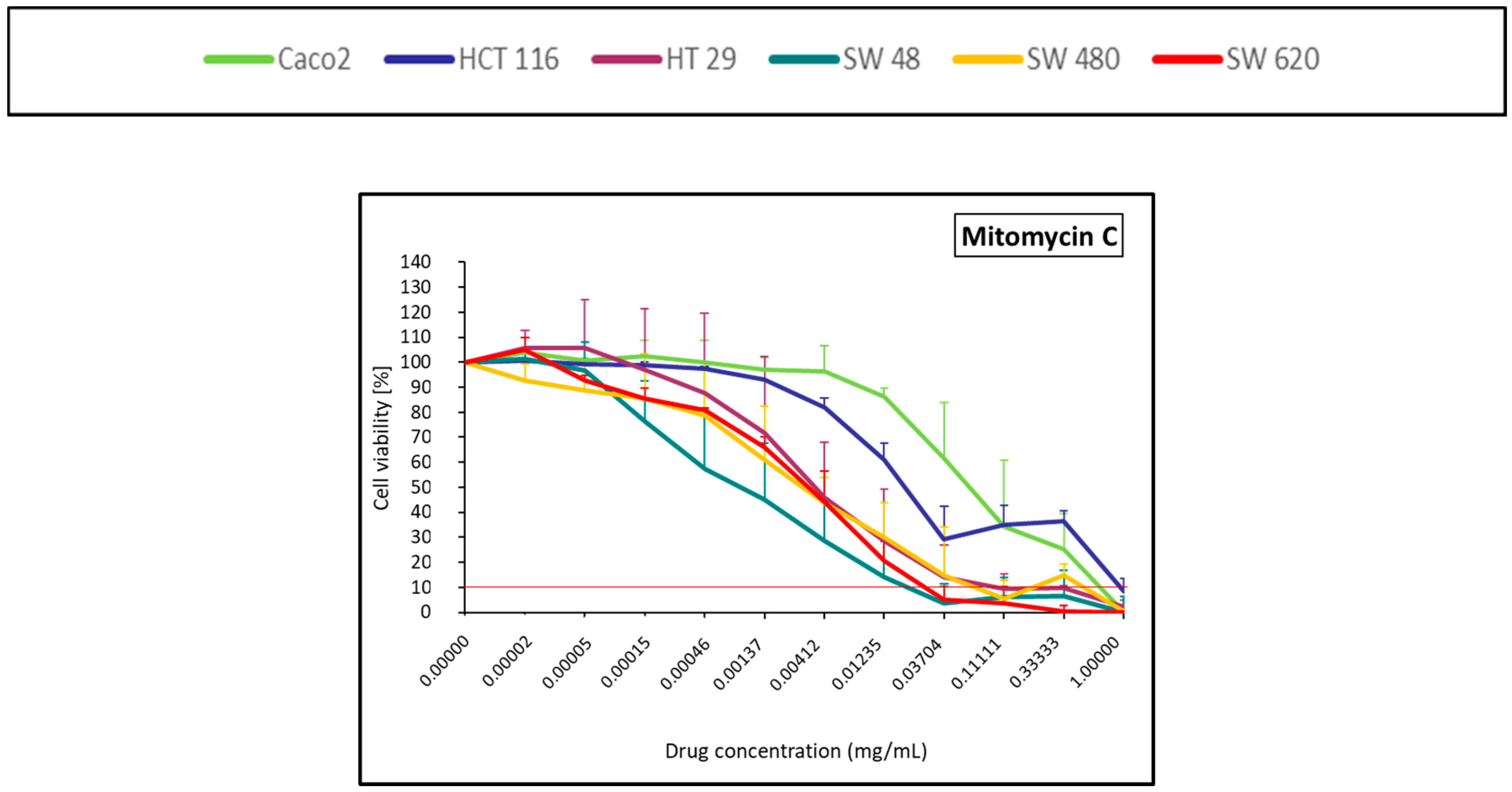
2.1.4. Alkylating Antibiotic (Mitomycin C)
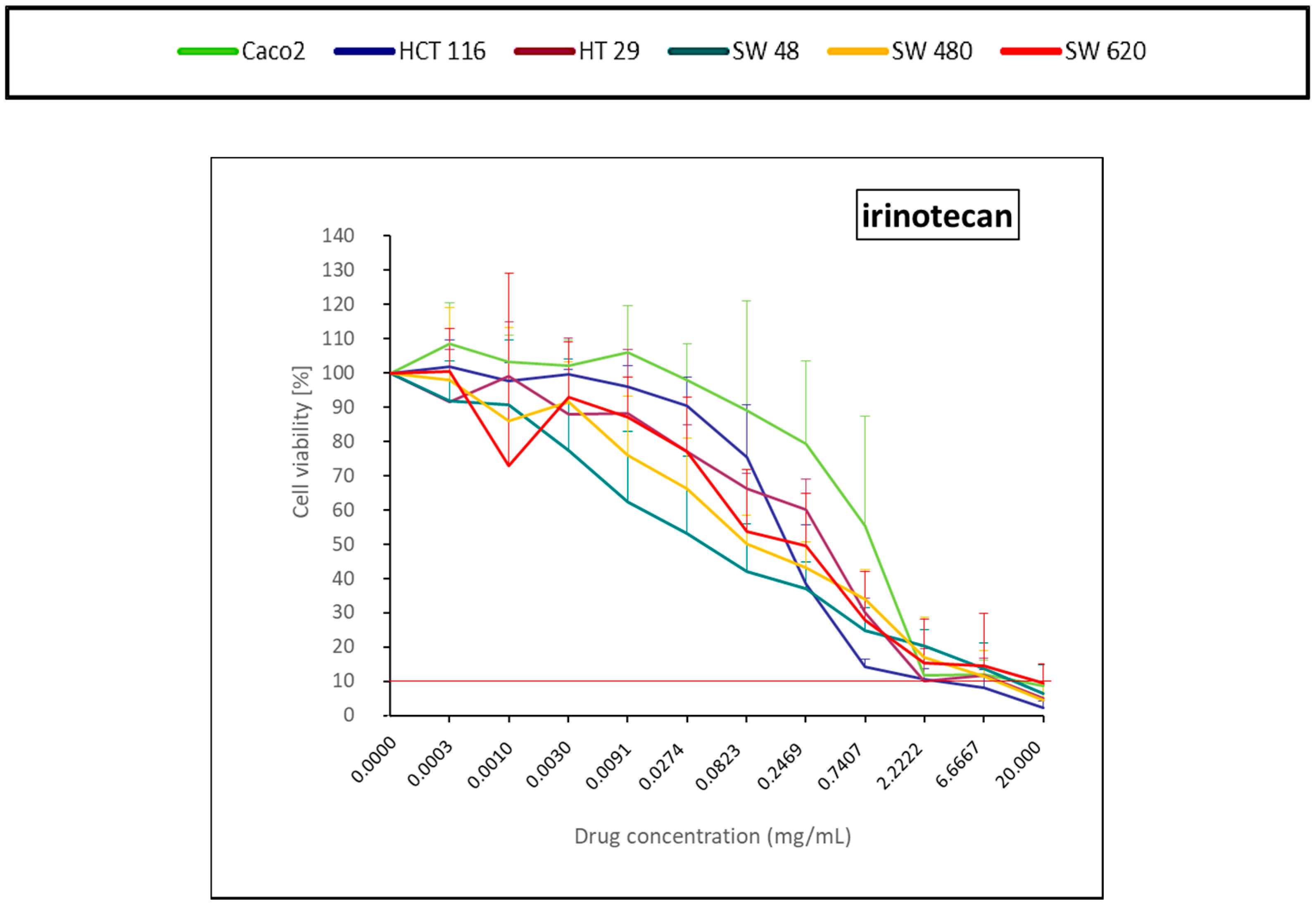
2.1.5. DNA Topoisomerase I Inhibitor (Irinotecan)
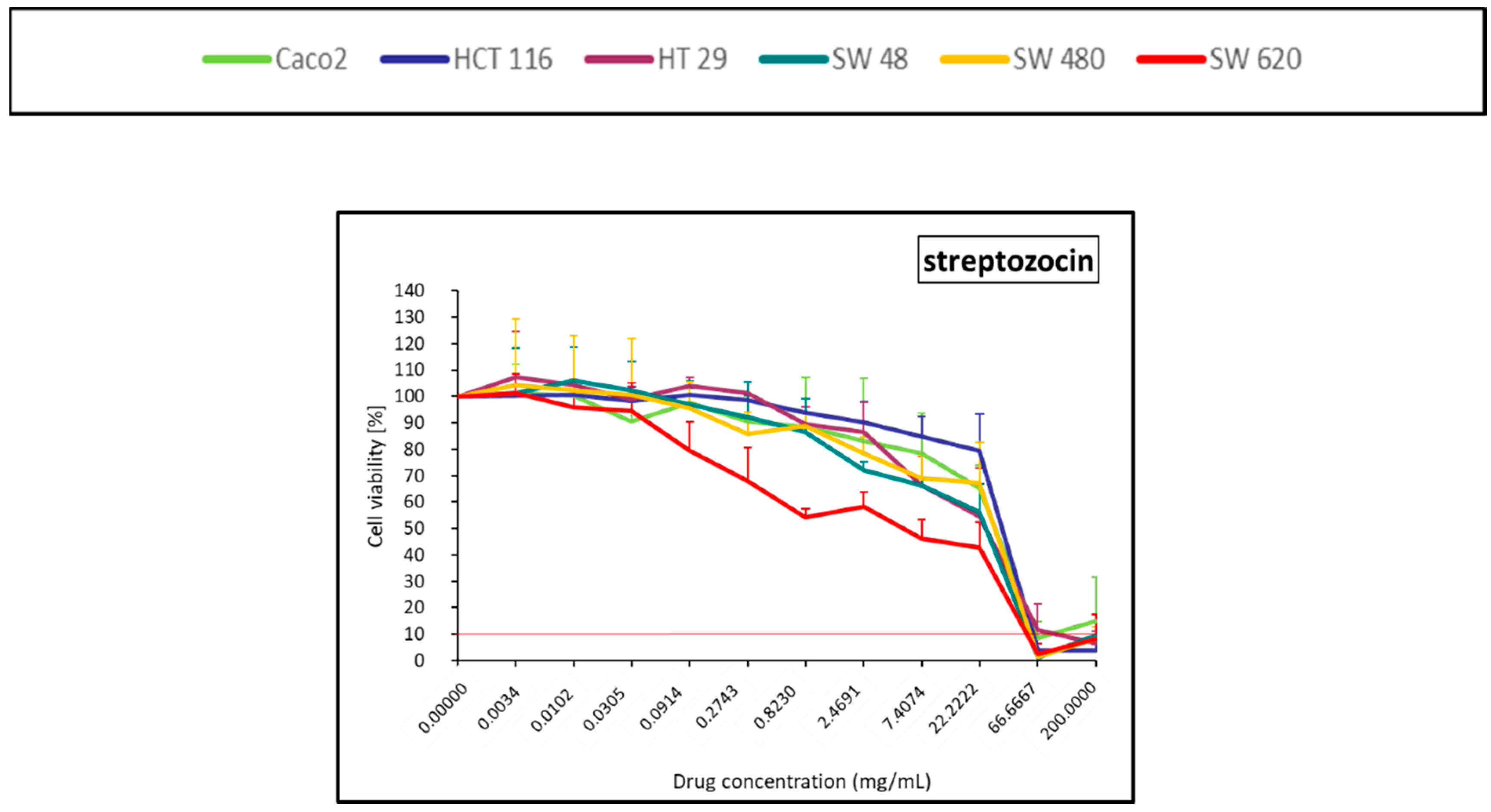
2.1.6. Antitumoral Antibiotic (Streptozocin)
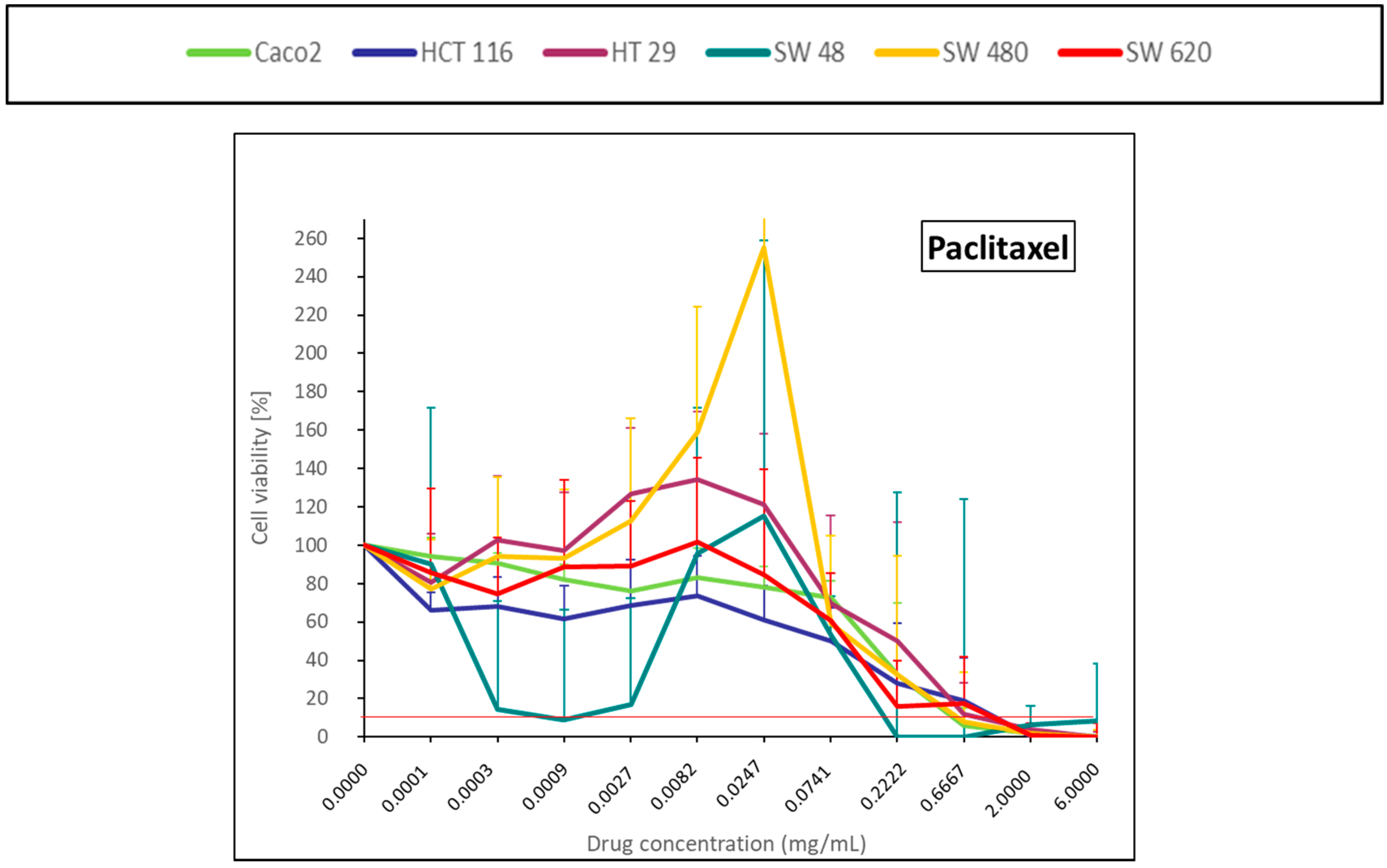
2.1.7. Taxane (Paclitaxel)
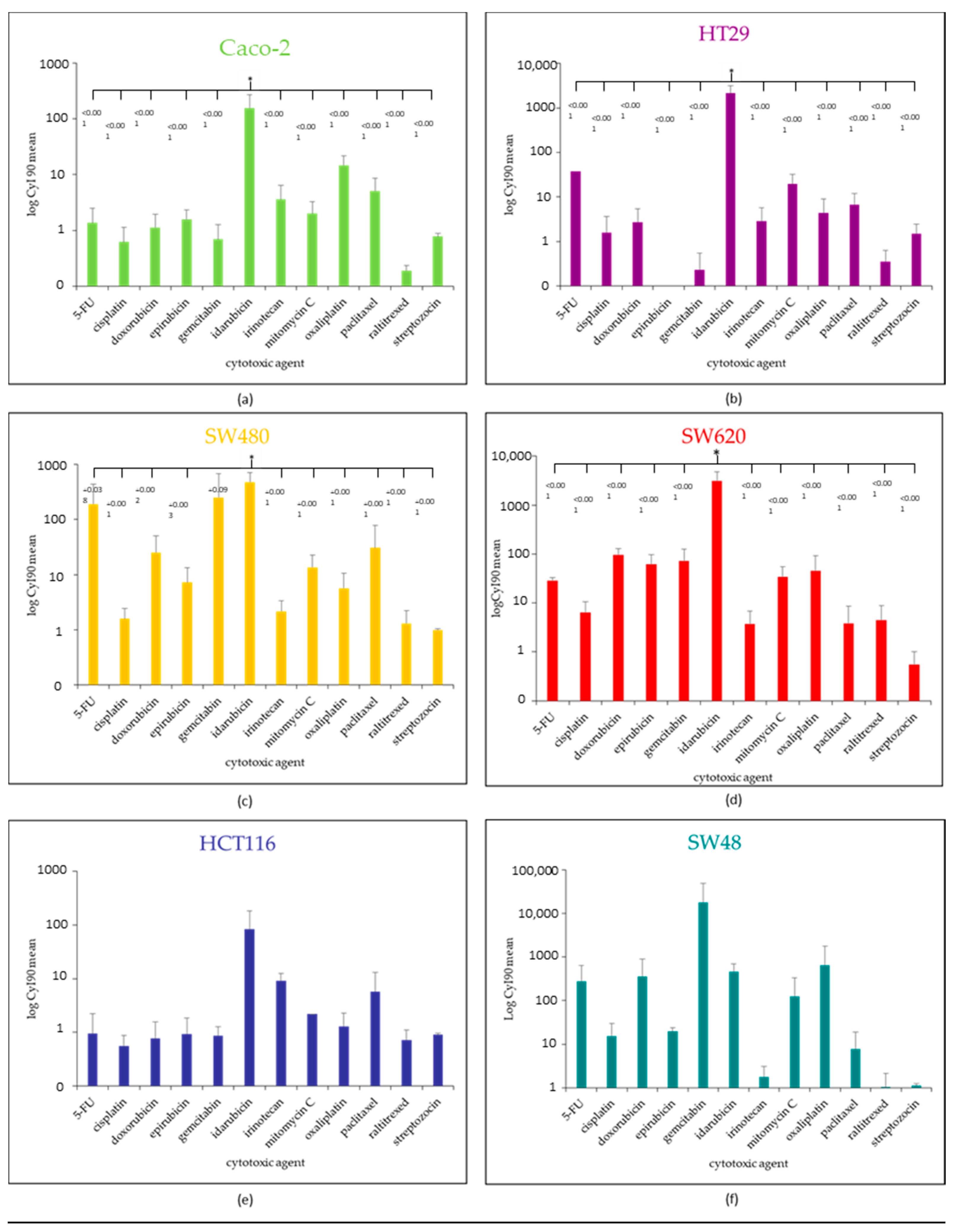
2.2. IC50, IC90 and Cytotoxicity Index (CyI90) for Each CRC Cell Line According the 12 Antitumoral Agents
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Chemotherapeutic Drugs
4.3. Screening Protocol
4.4. Cytotoxicity Assay
4.5. Statistical and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bhutiani, N.; Martin, R.C.G. Transarterial Therapy for Colorectal Liver Metastases. Surg. Clin. N. Am. 2016, 96, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Heinemann, V.; Weikersthal, L.F.; von Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus Cetuximab versus FOLFIRI plus Bevacizumab as First-Line Treatment for Patients with Metastatic Colorectal Cancer (FIRE-3): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients with KRAS Wild-Type Advanced or Metastatic Colorectal Cancer a Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus Bevacizumab versus FOLFIRI plus Bevacizumab as First-Line Treatment of Patients with Metastatic Colorectal Cancer: Updated Overall Survival and Molecular Subgroup Analyses of the Open-Label, Phase 3 TRIBE Study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Assenat, E.; Desseigne, F.; Thezenas, S.; Viret, F.; Mineur, L.; Kramar, A.; Samalin, E.; Portales, F.; Bibeau, F.; Crapez-Lopez, E.; et al. Cetuximab Plus FOLFIRINOX (ERBIRINOX) as First-Line Treatment for Unresectable Metastatic Colorectal Cancer: A Phase II Trial. Oncologist 2011, 16, 1557–1564. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Qiu, H.; Zhang, M.; Sun, L.; Peng, P.; Yu, Q.; Yuan, X. Mechanisms of Resistance to Anti-EGFR Therapy in Colorectal Cancer. Oncotarget 2017, 8, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.; Kriplani, A.; Ozbek, U.; Harpaz, N.; Colombel, J.F.; Itzkowitz, S.; Holcombe, R.F.; Ang, C. Chemotherapy Tolerance and Oncologic Outcomes in Patients With Colorectal Cancer With and Without Inflammatory Bowel Disease. Clin. Colorectal Cancer 2017, 16, e205–e210. [Google Scholar] [CrossRef]
- Breedis, C.; Young, G. The Blood Supply of Neoplasms in the Liver. Am. J. Pathol. 1954, 30, 969–977. [Google Scholar] [PubMed]
- Kan, Z.; Madoff, D.C. Liver Anatomy: Microcirculation of the Liver. Semin. Interv. Radiol. 2008, 25, 77–85. [Google Scholar] [CrossRef]
- Bugyik, E.; Renyi-Vamos, F.; Szabo, V.; Dezso, K.; Ecker, N.; Rokusz, A.; Nagy, P.; Dome, B.; Paku, S. Mechanisms of Vascularization in Murine Models of Primary and Metastatic Tumor Growth. Chin. J. Cancer 2016, 35, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lencioni, R.; de Baere, T.; Soulen, M.C.; Rilling, W.S.; Geschwind, J.-F.H. Lipiodol Transarterial Chemoembolization for Hepatocellular Carcinoma: A Systematic Review of Efficacy and Safety Data. Hepatology 2016, 64, 106–116. [Google Scholar] [CrossRef] [PubMed]
- De Baere, T.; Tselikas, L.; Boige, V.; Ducreux, M.; Malka, D.; Goéré, D.; Benahim, E.; Deschamps, F. Intra-Arterial Therapies for Colorectal Cancer Liver Metastases (Radioembolization Excluded). Bull. Cancer 2016. [Google Scholar] [CrossRef]
- Kemeny, N.; Daly, J.; Reichman, B.; Geller, N.; Botet, J.; Oderman, P. Intrahepatic or Systemic Infusion of Fluorodeoxyuridine in Patients with Liver Metastases from Colorectal Carcinoma. A Randomized Trial. Ann. Intern. Med. 1987, 107, 459–465. [Google Scholar] [CrossRef]
- Ducreux, M.; Ychou, M.; Laplanche, A.; Gamelin, E.; Lasser, P.; Husseini, F.; Quenet, F.; Viret, F.; Jacob, J.-H.; Boige, V.; et al. Hepatic Arterial Oxaliplatin Infusion plus Intravenous Chemotherapy in Colorectal Cancer with Inoperable Hepatic Metastases: A Trial of the Gastrointestinal Group of the Federation Nationale Des Centres de Lutte Contre Le Cancer. J. Clin. Oncol. 2005, 23, 4881–4887. [Google Scholar] [CrossRef] [PubMed]
- Boige, V.; Malka, D.; Elias, D.; Castaing, M.; De Baere, T.; Goere, D.; Dromain, C.; Pocard, M.; Ducreux, M. Hepatic Arterial Infusion of Oxaliplatin and Intravenous LV5FU2 in Unresectable Liver Metastases from Colorectal Cancer after Systemic Chemotherapy Failure. Ann. Surg. Oncol. 2008, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Laforgia, M.; Nardulli, P.; Ferraiuolo, S.; Molinari, P.; Marech, I.; Gadaleta, C.D. Oxaliplatin-Based Intra-Arterial Chemotherapy in Colo-Rectal Cancer Liver Metastases: A Review from Pharmacology to Clinical Application. Cancers 2019, 11, 141. [Google Scholar] [CrossRef]
- Swierz, M.J.; Storman, D.; Riemsma, R.P.; Wolff, R.; Mitus, J.W.; Pedziwiatr, M.; Kleijnen, J.; Bala, M.M. Transarterial (Chemo) Embolisation versus No Intervention or Placebo for Liver Metastases. Cochrane Database Syst. Rev. 2020, 2020, CD009498. [Google Scholar] [CrossRef]
- Mocellin, S.; Pilati, P.; Lise, M.; Nitti, D. Meta-Analysis of Hepatic Arterial Infusion for Unresectable Liver Metastases from Colorectal Cancer: The End of an Era? J. Clin. Oncol. 2007, 25, 5649–5654. [Google Scholar] [CrossRef]
- Mocellin, S.; Pasquali, S.; Nitti, D. Fluoropyrimidine-HAI (Hepatic Arterial Infusion) versus Systemic Chemotherapy (SCT) for Unresectable Liver Metastases from Colorectal Cancer. Cochrane Database Syst. Rev. 2009, CD007823. [Google Scholar] [CrossRef]
- Pwint, T.P.; Midgley, R.; Kerr, D.J. Regional Hepatic Chemotherapies in the Treatment of Colorectal Cancer Metastases to the Liver. Semin. Oncol. 2010, 37, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, F.; Rao, P.; Teriitehau, C.; Hakime, A.; Malka, D.; Boige, V.; Ducreux, M.; Elias, D.; Goere, D.; de Baere, T. Percutaneous Femoral Implantation of an Arterial Port Catheter for Intraarterial Chemotherapy: Feasibility and Predictive Factors of Long-Term Functionality. J. Vasc. Interv. Radiol. JVIR 2010, 21, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, F.; Elias, D.; Goere, D.; Malka, D.; Ducreux, M.; Boige, V.; Auperin, A.; de Baere, T. Intra-Arterial Hepatic Chemotherapy: A Comparison of Percutaneous Versus Surgical Implantation of Port-Catheters. Cardiovasc. Interv. Radiol. 2011, 34, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Tsitskari, M.; Filippiadis, D.; Kostantos, C.; Palialexis, K.; Zavridis, P.; Kelekis, N.; Brountzos, E. The Role of Interventional Oncology in the Treatment of Colorectal Cancer Liver Metastases. Ann. Gastroenterol. 2019, 32, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of Hepatocellular Carcinoma with Drug Eluting Beads: Efficacy and Doxorubicin Pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef]
- Boulin, M.; Guiu, S.; Chauffert, B.; Aho, S.; Cercueil, J.-P.; Ghiringhelli, F.; Krause, D.; Fagnoni, P.; Hillon, P.; Bedenne, L.; et al. Screening of Anticancer Drugs for Chemoembolization of Hepatocellular Carcinoma. Anti-Cancer Drugs 2011, 22, 741–748. [Google Scholar] [CrossRef]
- Favelier, S.; Boulin, M.; Hamza, S.; Cercueil, J.-P.; Cherblanc, V.; Lepage, C.; Hillon, P.; Chauffert, B.; Krausé, D.; Guiu, B. Lipiodol Trans-Arterial Chemoembolization of Hepatocellular Carcinoma with Idarubicin: First Experience. Cardiovasc. Interv. Radiol. 2013, 36, 1039–1046. [Google Scholar] [CrossRef]
- Boulin, M.; Hillon, P.; Cercueil, J.P.; Bonnetain, F.; Dabakuyo, S.; Minello, A.; Jouve, J.L.; Lepage, C.; Bardou, M.; Wendremaire, M.; et al. Idarubicin-Loaded Beads for Chemoembolisation of Hepatocellular Carcinoma: Results of the IDASPHERE Phase I Trial. Aliment. Pharmacol. Ther. 2014, 39, 1301–1313. [Google Scholar] [CrossRef]
- Guiu, B.; Schmitt, A.; Reinhardt, S.; Fohlen, A.; Pohl, T.; Wendremaire, M.; Denys, A.; Blümmel, J.; Boulin, M. Idarubicin-Loaded ONCOZENE Drug-Eluting Embolic Agents for Chemoembolization of Hepatocellular Carcinoma: In Vitro Loading and Release and In Vivo Pharmacokinetics. J. Vasc. Interv. Radiol. JVIR 2015, 26, 262–270. [Google Scholar] [CrossRef]
- Guiu, B.; Chevallier, P.; Assenat, E.; Barbier, E.; Merle, P.; Bouvier, A.; Dumortier, J.; Nguyen-Khac, E.; Gugenheim, J.; Rode, A.; et al. Idarubicin-Loaded Beads for Chemoembolization of Hepatocellular Carcinoma: The IDASPHERE II Single-Arm Phase II Trial. Radiology 2019, 291, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Boulin, M.; Schmitt, A.; Delhom, E.; Cercueil, J.-P.; Wendremaire, M.; Imbs, D.-C.; Fohlen, A.; Panaro, F.; Herrero, A.; Denys, A.; et al. Improved Stability of Lipiodol-Drug Emulsion for Transarterial Chemoembolisation of Hepatocellular Carcinoma Results in Improved Pharmacokinetic Profile: Proof of Concept Using Idarubicin. Eur. Radiol. 2016, 26, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Jouve, J.-L.; Schmitt, A.; Minello, A.; Bonnetain, F.; Cassinotto, C.; Piron, L.; Cercueil, J.-P.; Loffroy, R.; Latournerie, M.; et al. Intra-Arterial Idarubicin_lipiodol without Embolisation in Hepatocellular Carcinoma: The LIDA-B Phase I Trial. J. Hepatol. 2018, 68, 1163–1171. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical Outcome from Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes: Secondary Analysis of NSABP C-07/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Trinh, A.; Trumpi, K.; De Sousa E Melo, F.; Wang, X.; De Jong, J.H.; Fessler, E.; Kuppen, P.J.K.; Reimers, M.S.; Swets, M.; Koopman, M.; et al. Practical and Robust Identification of Molecular Subtypes in Colorectal Cancer by Immunohistochemistry. Clin. Cancer Res. 2017, 23, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Sveen, A.; Bruun, J.; Eide, P.W.; Eilertsen, I.A.; Ramirez, L.; Murumagi, A.; Arjama, M.; Danielsen, S.A.; Kryeziu, K.; Elez, E.; et al. Colorectal Cancer Consensus Molecular Subtypes Translated to Preclinical Models Uncover Potentially Targetable Cancer Cell Dependencies. Clin. Cancer Res. 2018, 24, 794–806. [Google Scholar] [CrossRef]
- Kornmann, M.; Fakler, H.; Butzer, U.; Beger, H.G.; Link, K.H. Oxaliplatin Exerts Potent in Vitro Cytotoxicity in Colorectal and Pancreatic Cancer Cell Lines and Liver Metastases. Anticancer Res. 2000, 20, 3259–3264. [Google Scholar]
- Kornmann, M.; Butzer, U.; Blatter, J.; Beger, H.G.; Link, K.H. Pre-Clinical Evaluation of the Activity of Gemcitabine as a Basis for Regional Chemotherapy of Pancreatic and Colorectal Cancer. Eur. J. Surg. Oncol. 2000, 26, 583–587. [Google Scholar] [CrossRef]
- Hofmann, C.; Buttenschoen, K.; Straeter, J.; Henne-Bruns, D.; Kornmann, M. Pre-Clinical Evaluation of the Activity of Irinotecan as a Basis for Regional Chemotherapy. Anticancer Res. 2005, 25, 795–804. [Google Scholar]
- Matsumoto, K.; Nagahara, T.; Okano, J.-I.; Murawaki, Y. The Growth Inhibition of Hepatocellular and Cholangiocellular Carcinoma Cells by Gemcitabine and the Roles of Extracellular Signal-Regulated and Checkpoint Kinases. Oncol. Rep. 2008, 20, 863–872. [Google Scholar] [PubMed]
- Garrido, C.; Chauffert, B.; Pinard, D.; Tibaut, F.; Genne, P.; Assem, M.; Dimanche-Boitrel, M.T. Circumvention of Confluence-Dependent Resistance in a Human Multi-Drug-Resistant Colon-Cancer Cell Line. Int. J. Cancer 1995, 61, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Roovers, D.J.; van Vliet, M.; Bloem, A.C.; Lokhorst, H.M. Idarubicin Overcomes P-Glycoprotein-Related Multidrug Resistance: Comparison with Doxorubicin and Daunorubicin in Human Multiple Myeloma Cell Lines. Leuk. Res. 1999, 23, 539–548. [Google Scholar] [CrossRef]
- Den Dunnen, J.T.; Antonarakis, S.E. Mutation Nomenclature Extensions and Suggestions to Describe Complex Mutations: A Discussion. Hum. Mutat. 2000, 15, 7–12. [Google Scholar] [CrossRef]
- Hill, A.V. A New Mathematical Treatment of Changes of Ionic Concentration in Muscle and Nerve under the Action of Electric Currents, with a Theory as to Their Mode of Excitation. J. Physiol. 1910, 40, 190–224. [Google Scholar] [CrossRef]
| Cell Line | Caco-2 | HCT116 | HT29 | SW48 | SW480 | SW620 | |
|---|---|---|---|---|---|---|---|
| Drug | |||||||
| Topoisomerase II inhibitor doxorubicin | 0.22 ± 0.28 | 0.01 ± 0.01 | 0.001 ± 0.001 | 0.0007 ± 0.0006 | 0.0006 ± 0.0003 | 0.0008 ± 0.0007 | |
| Topoisomerase II inhibitor epirubicin | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.001 ± 0.001 | 0.0007 ± 0.0001 | 0.0003 ± 0.0002 | 0.0005 ± 0.0002 | |
| Topoisomerase II inhibitor idarubicin | 0.0004 ± 0.0001 | 0.00005 ± 0.00006 | 0.00003 ± 0.00002 | 0.00002 ± 0.00001 | 0.00007 ± 0.00009 | 0.00003 ± 0.00002 | |
| Anti-metabolites 5-FU | 0.47 ± 0.08 | 213.75 ± 345.11 | 0.007 ± 0.001 | 0.01 ± 0.005 | 0.02 ± 0.01 | 0.01 ± 0.009 | |
| Anti-metabolites raltitrexed | 0.25 ± 0.07 | 0.08 ± 0.08 | 0.12 ± 0.22 | 0.10 ± 0.17 | 0.05 ± 0.08 | 0.0002 ± 0.0002 | |
| Anti-metabolites gemcitabin | 3.03 ± 2.21 | 0.09 ± 0.15 | 0.79 ± 1.36 | 0.0004 ± 0.0006 | 0.05 ± 0.09 | 0.0001 ± 0.00005 | |
| Platinum derivatives cisplatin | 0.09 ± 0.10 | 0.14 ± 0.03 | 0.01 ± 0.005 | 0.01 ± 0.01 | 0.01 ± 0.003 | 0.01 ± 0.007 | |
| Platinum derivatives oxaliplatin | 0.02 ± 0.01 | 0.61 ± 00.39 | 0.04 ± 0.04 | 0.04 ± 0.06 | 0.07 ± 0.06 | 0.008 ± 0.005 | |
| Alkylating antibiotic mitomycin C | 0.08 ± 0.08 | 0.02 ± 0.01 | 0.005 ± 0.007 | 0.001 ± 0.0008 | 0.004 ± 0.005 | 0.002 ± 0.0007 | |
| Topoisomerase I inhibitor irinotecan | 0.95 ± 0.83 | 0.20 ± 0.13 | 0.20 ± 0.05 | 0.07 ± 0.09 | 0.12 ± 0.10 | 0.18 ± 0.10 | |
| Antitumoral antibiotic streptozocin | 22.93 ± 10.86 | 24.58 ± 1.33 | 19.31 ± 10.15 | 13.88 ± 3.21 | 18.09 ± 4.77 | 2.65 ± 1.12 | |
| Taxane paclitaxel | 0.10 ± 0.03 | 0.05 ± 0.09 | 0.25 ± 0.19 | 0.0006 ± 0.0009 | 0.16 ± 0.22 | 0.05 ± 0.05 | |
| Cell Line | Caco-2 | HCT116 | HT29 | SW48 | SW480 | SW620 | |
|---|---|---|---|---|---|---|---|
| Drug | |||||||
| Topoisomerase II inhibitor doxorubicin | 2.68 ± 2.02 | 5.40 ± 4.77 | 1.47 ± 1.47 | 0.07 ± 0.10 | 0.13 ± 0.08 | 0.02 ± 0.009 | |
| Topoisomerase II inhibitor epirubicin | 1.55 ± 0.93 | 4.16 ± 4.07 | na | 0.10 ± 0.02 | 0.43 ± 0.36 | 0.04 ± 0.03 | |
| Topoisomerase II inhibitor idarubicin | 0.009 ± 0.007 | 0.04 ± 0.04 | 0.0005 ± 0.0003 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.0003 ± 0.0001 | |
| Anti-metabolites 5-FU | 138.28 ± 193.78 | 82,843.1 ± 103,130.2 | 1.31 ± na | 2.97 ± 4.74 | 1.92 ± 2.75 | 1.77 ± 0.27 | |
| Anti-metabolites raltitrexed | 260.88 ± 36.32 | 221.28 ± 12.01 | 173.89 ± 91.41 | 181.32 ± 20.44 | 202.82 ± 8.82 | 538.65 ± 437.50 | |
| Anti-metabolites gemcitabin | 202.35 ± 284.002 | 55.87 ± 37.11 | 9832.8 ± 13,788.9 | 3.87 ± 4.36 | 77.70 ± 110.10 | 0.74 ± 0.40 | |
| Platinum derivatives cisplatin | 2.81 ± 2.47 | 2.53 ± 2.03 | 3.34 ± 4.26 | 00.50 ± 0.79 | 0.87 ± 0.68 | 0.20 ± 0.11 | |
| Platinum derivatives oxaliplatin | 0.40 ± 0.23 | 5.57 ± 3.59 | 2.24 ± 1.64 | 0.83 ± 1.20 | 1.34 ± 0.79 | 0.26 ± 0.26 | |
| Alkylating antibiotic mitomycin C | 0.80 ± 0.76 | 0.45 ± Na | 0.08 ± 0.08 | 0.04 ± 0.05 | 0.13 ± 0.14 | 0.04 ± 0.03 | |
| Topoisomerase I inhibitor irinotecan | 8.91 ± 7.10 | 2.39 ± 0.86 | 13.64 ± 10.02 | 15.34 ± 11.25 | 11.28 ± 5.57 | 19.77 ± 27.26 | |
| Antitumoral antibiotic streptozocin | 20.70 ± 0.54 | 0.88 ± 0.57 | 20.03 ± 10.35 | 10.31 ± 10.33 | 0.66 ± 0.64 | 0.20 ± 0.15 | |
| Taxane paclitaxel | 1.99 ± 1.72 | 3.02 ± 2.60 | 1.73 ± 1.79 | 585.43 ± 1011.63 | 1.56 ± 1.98 | 41.65 ± 50.47 | |
| Cell Line | CaCo2 | HCT116 | HT29 | SW48 | SW480 | SW620 | |
|---|---|---|---|---|---|---|---|
| Drug | |||||||
| Topoisomerase II inhibitor doxorubicin | 1.13 ± 0.84 | 0.76 ± 0.79 | 2.72 ± 2.72 | 359.57 ± 556.29 | 25.30 ± 25.53 | 96.25 ± 31.85 | |
| Topoisomerase II inhibitor epirubicin | 1.58 ± 0.74 | 0.92 ± 0.90 | na ± na | 19.90 ± 4.08 | 7.25 ± 6.19 | 61.78 ± 35.10 | |
| Topoisomerase II inhibitor idarubicin | 156.97 ± 114.03 | 83.32 ± 99.27 | 2170.84 ± 993.71 | 468.19 ± 223.29 | 479.79 ± 235.56 | 3168.35 ± 1567.32 | |
| Anti-metabolites 5-FU | 1.37 ± 1.14 | 0.94 ± 1.26 | 37.92 ± na | 276.55 ± 368.76 | 191.74 ± 250.78 | 28.53 ± 4.47 | |
| Anti-metabolites raltitrexed | 0.19 ± 0.04 | 0.72 ± 0.37 | 0.36 ± 0.27 | 1.01 ± 1.14 | 1.31 ± 0.93 | 4.45 ± 4.48 | |
| Anti-metabolites gemcitabin | 0.70 ± 0.56 | 0.86 ± 0.42 | 0.23 ± 0.32 | 18,126.37 ± 31,381.13 | 252.01 ± 435.20 | 71.45 ± 56.09 | |
| Platinum derivatives cisplatin | 0.62 ± 0.50 | 0.56 ± 0.31 | 1.59 ± 2.02 | 15.46 ± 14.33 | 1.59 ± 0.86 | 6.45 ± 4.15 | |
| Platinum derivatives oxaliplatin | 14.78 ± 6.91 | 1.30 ± 1.00 | 4.36 ± 4.62 | 658.91 ± 1124.13 | 5.67 ± 5.01 | 45.61 ± 47.78 | |
| Alkylating antibiotic mitomycin C | 2.00 ± 1.24 | 2.18 ± na | 19.86 ± 12.45 | 125.52 ± 203.90 | 13.60 ± 9.02 | 34.54 ± 20.12 | |
| Topoisomerase I inhibitor irinotecan | 3.60 ± 2.84 | 9.12 ± 3.31 | 2.83 ± 2.98 | 1.78 ± 1.31 | 2.15 ± 1.20 | 3.68 ± 3.18 | |
| Antitumoral antibiotic streptozocin | 0.77 ± 0.11 | 0.91 ± 0.05 | 1.49 ± 1.00 | 1.11 ± 0.13 | 0.99 ± 0.04 | 0.55 ± 0.45 | |
| Taxane paclitaxel | 5.12 ± 3.54 | 5.77 ± 7.37 | 6.76 ± 5.18 | 7.68 ± 11.24 | 30.66 ± 48.10 | 3.84 ± 4.66 | |
| CRC Cell Line Designation | Caco-2 | HCT116 | HT-29 | SW48 | SW480 | SW620 |
|---|---|---|---|---|---|---|
| (ATCC Number) | (HTB-37) | (CCL-247) | (HTB-38) | (CCL-231) | (CCL-228) | (CCL-227) |
| Image (Day 1; X20) |  |  |  |  |  |  |
| Disease | colorectal carcinoma | colorectal carcinoma | colorectal adenocarcinoma | colorectal adenocarcinoma | colorectal adenocarcinoma | colorectal adenocarcinoma |
| Primary tissue | colon | colon | colon | colon | colon | colon |
| Tumor localization | colon | ascending colon | colon | transverse colon | descending colon | descending colon |
| Duke’stype | n/a | D | C | C | B | C |
| Grade | n/a | n/a | n/a | IV | n/a | n/a |
| Metastatic site | lymph node | |||||
| Patient age (yo) | 72 | 48 | 44 | 83 | 50 | 51 |
| Patient gender | male | male | female | female | male | male |
| Ethnicity | caucasian | na | caucasian | caucasian | caucasian | caucasian |
| Genes expressed | EGF | CEA | CEA | CEA | EGF | CEA |
| TP53 | E204X | wt | R273H | wt | R273H;P309S | R273H;P309S |
| K-ras | wt | G13D | wt | wt | G12V | G12V |
| B-raf | wt | wt | V600E | wt | wt | wt |
| PTEN | wt | wt | Wt | wt | wt | wt |
| PIK3CA | wt | H1047R | P449T | wt | wt | wt |
| CIN | x | X | x | X | ||
| MSS/MSI | MSS | MSI | MSS | MSI | MSS | MSS |
| CMS | 4 | 4 | 3 | 1 | 4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fohlen, A.; Bordji, K.; Assenat, E.; Gongora, C.; Bazille, C.; Boulonnais, J.; Naveau, M.; Breuil, C.; Pérès, E.A.; Bernaudin, M.; et al. Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals 2021, 14, 639. https://doi.org/10.3390/ph14070639
Fohlen A, Bordji K, Assenat E, Gongora C, Bazille C, Boulonnais J, Naveau M, Breuil C, Pérès EA, Bernaudin M, et al. Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals. 2021; 14(7):639. https://doi.org/10.3390/ph14070639
Chicago/Turabian StyleFohlen, Audrey, Karim Bordji, Eric Assenat, Céline Gongora, Céline Bazille, Jérémy Boulonnais, Mikaël Naveau, Cécile Breuil, Elodie A. Pérès, Myriam Bernaudin, and et al. 2021. "Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time" Pharmaceuticals 14, no. 7: 639. https://doi.org/10.3390/ph14070639
APA StyleFohlen, A., Bordji, K., Assenat, E., Gongora, C., Bazille, C., Boulonnais, J., Naveau, M., Breuil, C., Pérès, E. A., Bernaudin, M., & Guiu, B. (2021). Anticancer Drugs for Intra-Arterial Treatment of Colorectal Cancer Liver Metastases: In-Vitro Screening after Short Exposure Time. Pharmaceuticals, 14(7), 639. https://doi.org/10.3390/ph14070639







