Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns
Abstract
1. Introduction
2. Results
2.1. Characterization of Zinc Oxide Nanoparticles
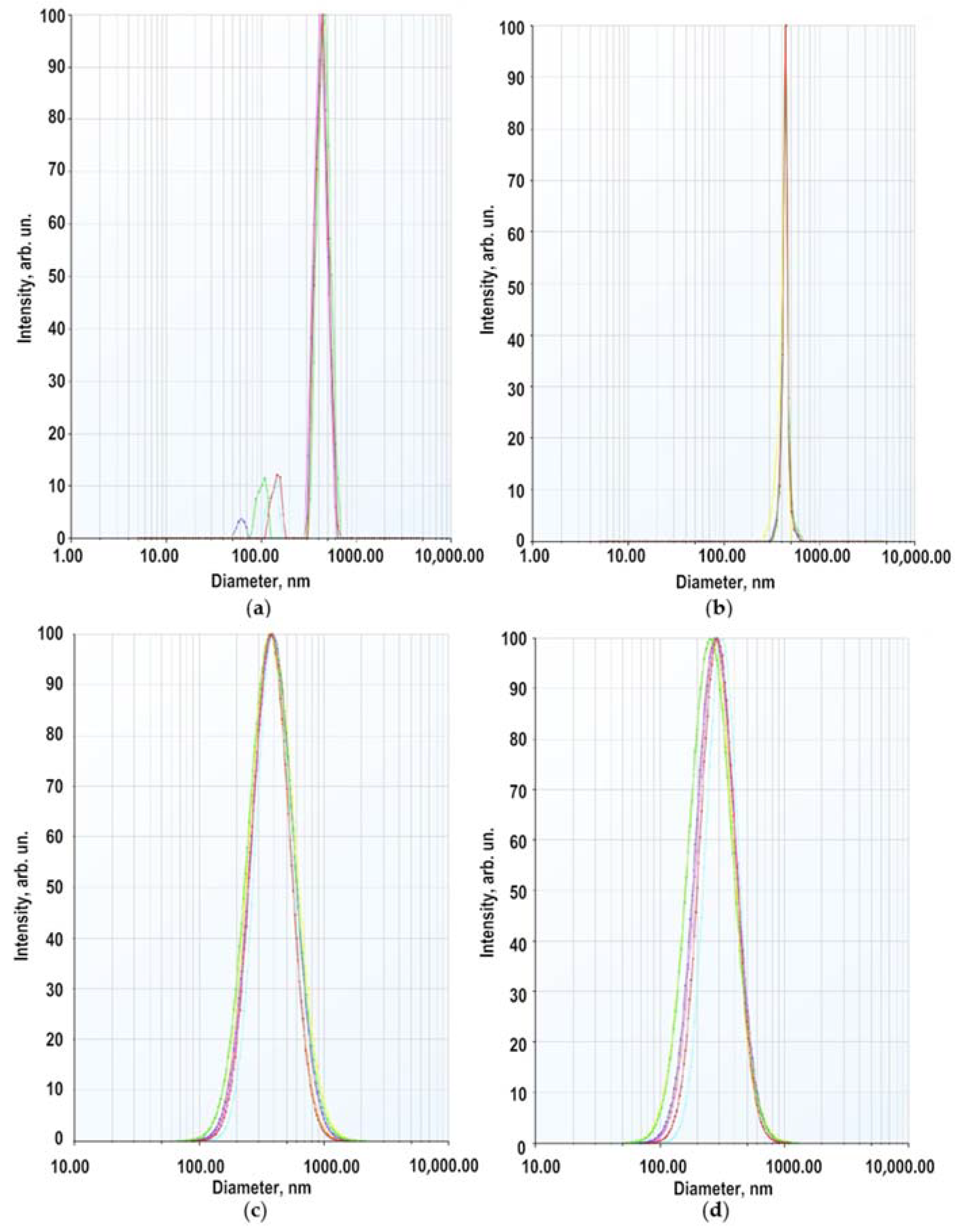
2.2. Properties of Modified ZnO NPs as a Component of Gel-Like Dispersions
2.3. Preparation of Zinc Oxide Nanoparticles with Essential Oils and Triterpenoids
2.4. Composition and Properties of Gels
2.5. Characteristics of Animals’ Health State
2.6. Evaluation of Energy Metabolism by the Activity of Oxidoreductases Lactate Dehydrogenase and Glucose-6-Phosphate Dehydrogenase in the Treatment of Burn Wounds with Gels-like Dispersions with Zinc Oxide Nanoparticles in Rats
2.7. MDA Level Analysis
2.8. The Level of Antioxidant Enzymes (SOD, Catalase)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Zinc Oxide Nanoparticles
4.3. Preparation and Composition of Anti-Burn Gels
4.3.1. Oleogel ZnO NPs-BDP
4.3.2. Hydrogel ZnO NPs-B—Lavender and Hydrogel ZnO NPs-BDP—Lavender
4.3.3. Oleogel ZnO NPs-BDP—Thymol-Lavender and Oleogel ZnO NPs-B—Thymol-Lavender
4.4. Photoluminescence Analysis
4.5. GL Chromatographical Analysis
4.6. Viscosity Estimation
4.7. Specific Area Estimation
4.8. Surface Charge and Dynamic Light Scattering Measurements
4.9. Elemental Analysis
4.10. SEM Analysis
4.11. Biological Activity
4.11.1. Modeling of Thermal Burns in Animals
4.11.2. Biological Activity In Vitro
4.12. Quantum-Chemical Calculations
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef]
- Babu, R.J.; Babu, M. Oxidative stress in major thermal burns: Its implications and significance. Indian J. Burns 2018, 26, 38–43. [Google Scholar] [CrossRef]
- Roshangar, L.; Soleimani Rad, J.; Kheirjou, R.; Reza Ranjkesh, M.; Ferdowsi Khosroshahi, A. Skin Burns: Review of Molecular Mechanisms and Therapeutic Approaches. Wounds 2019, 31, 308–315. [Google Scholar] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Devgan, L.; Bhat, S.; Milner, S.M. The pathogenesis of burn wound conversion. Ann. Plast. Surg. 2007, 59, 109–115. [Google Scholar] [CrossRef]
- Farina, J.A.; Rosique, M.J.; Rosique, R.G. Curbing Inflammation in Burn Patients. Int. J. Inflam. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Miyazaki, H.; Kinoshita, M.; Ono, S.; Seki, S.; Saitoh, D. Burn-Evoked Reactive Oxygen Species Immediately After Injury are Crucial to Restore the Neutrophil Function Against Postburn Infection in Mice. Shock 2015, 44, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, Y.; Wang, L.; Cai, X.; Wang, D.; Wu, K.; Chen, H.; Li, J.; Lei, W. Sustained oxidative stress causes late acute renal failure via duplex regulation on p38 MAPK and Akt phosphorylation in severely burned rats. PLoS ONE 2013, 8, e54593. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003, 189, 75–88. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ying, W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: Regulation and biological consequences. Antioxid. Redox. Signal. 2008, 10, 179–206. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.J. Sources and implications of NADH/NAD(+) redox imbalance in diabetes and its complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, R.; Yan, L.J. Roles of Pyruvate, NADH, and Mitochondrial Complex I in Redox Balance and Imbalance in β Cell Function and Dysfunction. J. Diabetes Res. 2015, 2015, 512618. [Google Scholar] [CrossRef] [PubMed]
- Pollak, N.; Dolle, C.; Ziegler, M. The power to reduce: Pyridine nucleotides—Small molecules with a multitude of functions. Biochem. J. 2007, 402, 205–218. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Frew, Q.; Rennekampff, H.-O.; Dziewulski, P.; Moiemen, N.; Zahn, T.; Hartmann, B. Betulin wound gel accelerated healing of superficial partial thickness burns: Results of a randomized, intra-individually controlled, phase III trial with 12-months follow-up. Burns 2019, 45, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.; Vorobyova, O.; Balakireva, A.; Malygina, D.; Solovyeva, A.; Belyaeva, K.; Orekhov, D.; Knyazev, A. The New Pharmaceutical Compositions of Zinc Oxide Nanoparticles and Triterpenoids for the Burn Treatment. Pharmaceuticals 2020, 13, 207. [Google Scholar] [CrossRef]
- Bhunia, A.K.; Samanta, P.K.; Saha, S.; Kamilya, T. ZnO nanoparticle-protein interaction: Corona formation with associated unfolding. Appl. Phys. Lett. 2013, 103, 143701. [Google Scholar] [CrossRef]
- Yin, H.; Chen, R.; Casey, P.S.; Ke, P.C.; Davis, T.P.; Chen, C. Reducing the cytotoxicity of ZnO nanoparticles by a pre-formed protein corona in a supplemented cell culture medium. RSC Adv. 2015, 5, 73963–73973. [Google Scholar] [CrossRef]
- Giau, V.-V.; Park, Y.-H.; Shim, K.-H.; Son, S.-W.; An, S.-S.A. Dynamic changes of protein corona compositions on the surface of zinc oxide nanoparticle in cell culture media. Front. Chem. Sci. Eng. 2019, 13, 90–97. [Google Scholar] [CrossRef]
- Srivastav, A.K.; Dhiman, N.; Khan, H.; Srivastav, A.K.; Yadav, S.K.; Prakash, J.; Arjaria, N.; Singh, D.; Yadav, S.; Patnaik, S.; et al. Impact of surface engineered ZnO nanoparticles on protein corona configuration and their interactions with biological system. J. Pharm. Sci. 2019, 108, 1872–1889. [Google Scholar] [CrossRef]
- Hassanian, M.; Aryapour, H.; Goudarzi, A.; Javan, M.B. Are zinc oxide nanoparticles safe? A structural study on human serum albumin using in vitro and in silico methods. J. Biomol. Struct. Dyn. 2021, 39, 330–335. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In situ localized growth of ordered metal oxide hollow sphere array on microheater platform for sensitive, ultra-fast gas sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Schöttler, S.; Landfester, K.; Mailänder, V. Controlling the stealth effect of nanocarriers through understanding the protein corona. Angew. Chem. Int. Ed. Engl. 2016, 55, 8806–8815. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.; Knyazev, A.; Nikolskiy, V.; Peretyagin, P.; Belyaeva, K.; Nazarova, N.; Liyaskina, E.; Malygina, D.; Revin, V. Wound Healing Composite Materials of Bacterial Cellulose and Zinc Oxide Nanoparticles with Immobilized Betulin Diphosphate. Nanomaterials 2021, 11, 713. [Google Scholar] [CrossRef]
- Su, J.; Chen, J.; Li, L.; Li, B.; Shi, L.; Zhang, H.; Ding, X. Preparation of Natural Borne-ol/2-Hydroxypropyl-β-cyclodextrin Inclusion Complex and Its Effect on the Absorption of Tetramethylpyrazine Phosphate in Mouse. Chem. Pharm. Bull. 2012, 60, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, N.B.; Malygina, D.S.; Klabukova, I.N.; Belov, D.V.; Vasin, V.A.; Petrov, P.S.; Knyazev, A.V.; Markin, A.V. Betulin-3,28-diphosphate. Physico-Chemical Properties and In Vitro Biological Activity Experiments. Molecules 2018, 23, 1175. [Google Scholar] [CrossRef]
- Rupasinghe, R.-A.-T.P. Dissolution and Aggregation of Zinc Oxide Nanoparticles at Circumneutral pH; a Study of Size Effects in the Presence and Absence of Citric Acid. Master’s Thesis, University of Iowa, Iowa, IA, USA, 2011. [Google Scholar]
- Danciu, C.; Pinzaru, I.; Coricovac, D.; Andrica, F.; Sizemore, I.; Dehelean, C.; Baderca, F.; Lazureanu, V.; Soica, C.; Mioc, M.; et al. Betulin silver nanoparticles qualify as efficient antimelanoma agents in in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2019, 134, 1–19. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.-V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The Cytotoxic Effects of Betulin-Conjugated Gold Nanoparticles as Stable Formulations in Normal and Melanoma Cells. Front. Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef]
- Murthy, H.N.; Manohar, S.H.; Naik, P.M.; Lee, E.J.; Paek, K.Y. Physicochemical characteristics and antioxidant activity of Lavandula bipinnata seed oil. Int. Food Res. J. 2014, 21, 1473–1476. [Google Scholar]
- European Pharmacopoeia 5.0. Lavender Oil; 01/2005:1338; Council of Europe: Strasbourg, France, 2005; pp. 1894–1895. [Google Scholar]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Gennis, R.B. Biomembranes: Molecular Structure and Function, 1st ed.; Springer: New York, NY, USA, 1989; pp. 1–533. [Google Scholar]
- Melnikova, N.B.; Malygina, D.S.; Solovyeva, O.N.; Zhiltsova, O.E.; Vasin, V.A.; Petrov, P.S.; Klabukova, I.N. Betulin-3,28-diphosphate salt complexes with amines and their antioxidant activity. Int. J. Pharm. Pharm. Sci. 2018, 10, 87–95. [Google Scholar] [CrossRef][Green Version]
- Kumar Jangir, L.; Kumari, Y.; Kumar, A.; Kumar, M.; Awasthi, K. Investigation of luminescence and structural properties of ZnO nanoparticles, synthesized with different precursors. Mater. Chem. Front. 2017, 1, 1413–1421. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, C. Oily Thixotropic Nasal Spray. Patent EP 1374856A1, 2 January 2004. [Google Scholar]
- Vonbehren, D.; Lynch, M.G.; Miranda, A.C. Cosmetic Composition Containing Microcrystalline Cellulose. Patent EP 1539098B1, 10 August 2011. [Google Scholar]
- Espada, J.; Carrasco, E.; Calvo-Sánchez, M.I.; Fernández-Martos, S.; Montoya, J.J. Stimulation of Stem Cell Niches and Tissue Regeneration in Mouse Skin by Switchable Protoporphyrin IX-Dependent Photogeneration of Reactive Oxygen Species in Situ. J. Vis. Exp. 2020, 159, e60859. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a Gatekeeper of Immune Function. Nutrients 2017, 25, 1286. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, B.; Ding, N.; Ding, W.; Wang, L.; Yu, M.; Zhang, Q. Fast synthesize ZnO quantum dots via ultrasonic method. Ultrason. Sonochem. 2015, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-P450 aldehyde oxidizing enzymes: The aldehyde dehydrogenase superfamily. Expert Opin. Drug. Metab. Toxicol. 2008, 4, 697–720. [Google Scholar] [CrossRef]
- Kim, N.; Park, Y.; Lee, B.I.; Park, M.-H.; Shin, S.-H.; Byeon, J.-J.; Choi, J.; Shin, Y.G. Quantification of a Novel Aldehyde Dehydrogenase Inhibitor in Rat Using Liquid Chromatography-quadrupole Time-of-flight Mass Spectrometric Method and Its Application to Pharmacokinetic Modeling in Rat. Yakhak Hoeji 2019, 63, 75–81. [Google Scholar] [CrossRef]
- Yue, L.; Huang, Z.-M.; Fong, S.; Leong, S.; Jakowatz, J.G.; Charruyer-Reinwald, A.; Wei, M.; Ghadially, R. Targeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanoma. Melanoma Res. 2015, 25, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Koppaka, V.; Thompson, D.C.; Chen, Y.; Ellermann, M.; Nicolaou, K.C.; Juvonen, R.O.; Petersen, D.; Deitrich, R.A.; Hurley, T.D.; Vasiliou, V. Aldehyde dehydrogenase inhibitors: A comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012, 64, 520–539. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.; Al-Quraishy, S.; Wahab, R. Anticoccidial and antioxidant activities of zinc oxide nanoparticles on Eimeria papillata-induced infection in the jejunum. Int. J. Nanomed. 2015, 10, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.M.; Song, Z.; Moghimi, H.R.; Roberts, M.S. Relative Penetration of Zinc Oxide and Zinc Ions into Human Skin after Application of Different Zinc Oxide Formulations. ACS Nano 2016, 10, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Caldis-Coutris, N.; Gawaziuk, J.P.; Logsetty, S. Zinc Supplementation in Burn Patients. J. Burn Care Res. 2012, 33, 678–682. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Sabui, S.; Santra, A.; Holloway, P.H. Photoluminescence of ZnO quantum dots produced by a sol-gel process. Opt. Mater. 2008, 30, 1233–1239. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, B.-H.; Jeong, H.-M.; Kim, S.-W.; Xu, B. Quantum dot light emitting diodes using size-controlled ZnO NPs. Curr. Appl. Phys. 2018, 18, 681–685. [Google Scholar] [CrossRef]
- GOST 30418-96. Vegetable Oils. Method for Determination of Fatty Acid Content. Available online: https://docs.cntd.ru/document/1200023035 (accessed on 1 January 1998).
- Pakhomova, A.E.; Pakhomova, E.E.; Pakhomova, J.V.; Yavorsky, E.M. Method Experimental Modeling of Thermal Combustion at Laboratory Animals. U.S. Patent RU 2,582,458 C1, 24 December 2014. [Google Scholar]
- Pakhomova, A.E.; Pakhomova, J.V.; Ovsyanko, E.V.; Zhurakovsky, I.P.; Karabintseva, N.O.; Pakhomova, E.E. Preclinical research of repalen ointment at treatment of thermal combustions in experiment. J. Sib. Med. Sci. 2015, 3, 98. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Sirota, T.V. A new approach to studying the autoxidation of adrenaline: Possibility of the determination of superoxide dismutase activity and the antioxidant properties of various preparations by polarography. Biomed. Khi. 2012, 58, 77–87. [Google Scholar] [CrossRef][Green Version]
- Packer, L. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Sibgatullina, G.V.; Khartendinova, L.R.; Gumerova, E.A.; Akulov, A.N.; Kostyukova, Y.A.; Nikonorova, N.A.; Rumyantseva, N.I. Methods for Determining the Redox Status of Cultured Plant. Cells; Kazan (Privolzhsky) Federal University: Kazan, Russia, 2011; pp. 18–20. [Google Scholar]
- Kochetov, G.A. Practical Guide to Enzymology, 2nd ed.; Severin, S.E., Ed.; High School: Moscow, Russia, 1980; p. 272. [Google Scholar]
- Solov’eva, A.G.; Zimin, Y.V. A new way to assess the dynamics of blood metabolism in patients with thermal trauma. Mod. Technol. Med. 2012, 2, 116–117. [Google Scholar]
- Guru, S.C.; Shetty, K.T. Methodological aspects of aldehyde dehydrogenase assay by spectrophotometric technique. Alcohol 1990, 7, 397–401. [Google Scholar] [CrossRef]
- Dawson, J.M.; Heatlic, P.L. Lowry method of protein quantification evidence for photosensitivity. Anal. Biochem. 1984, 140, 391–393. [Google Scholar] [CrossRef]
- Solovyev, M.E.; Solovyev, M.M. Computer Chemistry; SOLON-Press: Moscow, Russia, 2005; pp. 1–207. [Google Scholar]







| ZnO NPs-Lavender Oil | ZnO NPs-BDP [17] | |||||
|---|---|---|---|---|---|---|
| Peak | 101 | 002 | 100 | 101 | 002 | 100 |
| β‘, 2θ | 0.595 | 0.476 | 0.595 | 0.714 | 0.476 | 0.714 |
| β, rad | 0.0103 | 0.0083 | 0.0103 | 0.0125 | 0.0083 | 0.0125 |
| 2θ, ° | 31.68 | 34.34 | 36.42 | 31.76 | 34.36 | 36.22 |
| Cosθ | 0.959 | 0.955 | 0.950 | 0.959 | 0.954 | 0.950 |
| D, nm | 17.3 | 17.5 | 14.0 | 11.6 | 17.5 | 11.7 |
| № | ZnO NPs, % | Medium | Zeta Potential, mV |
|---|---|---|---|
| 1 | 0.00625 | 1.25 × 10−4 M citric acid in ethanol: water (1:1) | −6.47 ± 1.10 |
| 2 | 0.00625 | 1.25 × 10−4 M BDP in ethanol | −1.49 ± 1.81 |
| 3 | 0.025 | 5 × 10−4 M sodium salt of BDP in ethanol: water (1:1) | −7.09 ± 0.25 |
| 4 | 0.025 | 5 × 10−4 M BDP in ethanol | −6.51 ± 0.60 |
| 5 | 0.025 | ethanol: water (1:1) | +15.90 |
| 6 | 0.025 1 | 1 × 10−3 M BDP in ethanol | −41.20 |
| ν,cm−1 | -OH 3200–3600 | -C-O 1000–1100 | =CH2 800–890 |
|---|---|---|---|
| Betulin | 3380 | 1028 | 881 |
| Thymol | 3280 | 1091 | 806 |
| Betulin: Thymol mixture (1:1) | 3380, 3280, 3550 | 1044 | 804 |
| Number | Dispersion | ZnO NPs | B | BDP | Surfactant | Excipients | Medium |
|---|---|---|---|---|---|---|---|
| 1 | Oleo ZnO NPs-BDP [17] | 5.0 | 10.0 | 1.0 | - | Ascorbic acid 0.1 α-tocopherol acetate 0.1 | Sunflower oil up to 100 |
| 2 | Oleo ZnO NPs-BDP—lavender | 6.0 | 12.0 | 1.0 | GS—1.0 | Thymol 2.0 Lavender oil 2.0 | Sunflower oil up to 100 |
| 3 | Oleo ZnO NPs-B—lavender | 6.0 | 13.0 | - | GS—1.0 | Thymol 2.0 Lavender oil 2.0 | Sunflower oil up to 100 |
| 4 | Hydro ZnO NPs-B | 4.7 | 6.0 | - | PEG30—3.7 GS—2.7 | HEC—2.1 Lavender oil 2.0 | distilled water up to 100 |
| 5 | Hydro ZnO NPs-BDP | 4.7 | 5.0 | 1.0 | PEG30—3.7 GS—2.7 | HEC—2.1 Lavender oil 2.0 | distilled water up to 100 |
| Dispersion | D, nm | ZnO, % | ||
|---|---|---|---|---|
| Added | Founded | |||
| Complexonometry | AAS | |||
| Oleo ZnO NPs-BDP—thymol-lavender | 15.74 ± 0.93 | 6.0 | 5.98 ± 0.67 | 5.87 ± 0.31 |
| Oleo ZnO NPs-B—thymol-lavender | 16.42 ± 2.23 | 6.0 | 5.91 ± 0.91 | 5.85 ± 0.83 |
| Hydro ZnO NPs-B—lavender | 16.90 ± 4.12 | 4.7 | 4.71 ± 0.13 | 4.68 ± 0.43 |
| Hydro ZnO NPs-BDP—lavender | 15.89 ± 4.33 | 4.7 | 4.65 ± 0.20 | 4.61 ± 0.35 |
| Group | Wound Area, cm2 | ||
|---|---|---|---|
| 0 Day | 10 Day | 21 Day | |
| Burnt untreated | 21.746 ± 0.612 | 20.127 ± 0.230 | 16.184 ± 0.971 |
| Oleo ZnO NPs-BDP | 21.599 ± 0.628 | 19.897 ± 0.313 | 12.825 ± 0.311 |
| Oleo ZnO NPs-BDP—thymol-lavender | 22.620 ± 0.199 | 18.384 ± 1.321 | 10.028 ± 0.224 |
| Oleo ZnO NPs-B—thymol-lavender | 23.714 ± 0.392 | 19.329 ± 0.968 | 11.519 ± 0.120 |
| Hydro ZnO NPs-B—lavender | 22.783 ± 0.198 | 18.635 ± 0.457 | 11.689 ± 0.672 |
| Hydro ZnO NPs-BDP—lavender | 21.459 ± 0231 | 18.058 ± 0.391 | 10.818 ± 0.973 |
| τ, Day | G6PDH Activity, % of Control 1 | ||
|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | |
| 3 | 79.52 ± 2.17 | 100.93 ± 3.51 | 146.01 ± 1.68 |
| 7 | 90.14 ± 1.57 | 117.72 ± 1.96 | 152.80 ± 2.86 |
| 10 | 104.02 ± 1.92 | 128.02 ± 3.37 | 165.21 ± 3.63 |
| 21 | N/a | 138.12 ± 3.60 | 187.48 ± 4.22 |
| Enzyme | τ, Day | LDH Activity, % of Control 1 | ||
|---|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | ||
| LDHdirect | 3 | 73.25 ± 1.61 | 89.15 ± 0.98 | 107.49 ± 2.31 |
| 7 | 101.32 ± 0.82 | 115.06 ± 3.18 | 118.39 ± 0.60 | |
| 10 | 134.14 ± 1.58 | 141.43 ± 1.44 | 125.08 ± 2.11 | |
| 21 | N/a | 120.93 ± 1.62 | 134.28 ± 0.61 | |
| LDHreverse | 3 | 87.23 ± 0.92 | 150.59 ± 2.03 | 172.10 ± 1.83 |
| 7 | 92.38 ± 1.96 | 120.19 ± 1.85 | 153.72 ± 0.29 | |
| 10 | 102.01 ± 1.36 | 125.82 ± 0.45 | 107.42 ± 1.99 | |
| 21 | N/a | 127.18 ± 2.27 | 100.93 ± 0.82 | |
| τ, Day | GR Activity, % of Control 1 | ||
|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | |
| 3 | 58.23 ± 0.94 | 80.73 ± 1.80 | 101.23 ± 5.25 |
| 7 | 67.05 ± 0.58 | 87.04 ± 5.71 | 104.09 ± 2.65 |
| 10 | 73.77 ± 1.43 | 91.62 ± 3.03 | 106.38 ± 1.02 |
| 21 | 120.27 ± 2.95 | 121.87 ± 3.01 | |
| τ, Day | MDA Level, % of Control | |||
|---|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | ||
| MDApl | 3 | 240.32 ± 6.22 | 231.34 ± 3.72 | 169.82 ± 2.99 |
| 7 | 180.48 ± 3.83 | 152.21 ± 8.60 | 143.73 ± 3.92 | |
| 10 | 150.62 ± 4.09 | 124.83 ± 5.34 | 121.82 ± 7.28 | |
| 21 | N/a | 112.04 ± 5.22 | 85.48 ± 5.27 | |
| MDAer | 3 | 152.62 ± 5.38 | 153.81 ± 4.94 | 126.13 ± 8.17 |
| 7 | 147.24 ± 4.24 | 122.25 ± 6.37 | 113.67 ± 7.51 | |
| 10 | 140.72 ± 8.12 | 103.78 ± 4.32 | 96.14 ± 8.11 | |
| 21 | N/a | 86.42 ± 4.92 | 84.66 ± 4.57 | |
| τ, Day | SOD Activity, % of Control 1 | ||
|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | |
| 3 | 61.54 ± 0.82 | 83.63 ± 3.61 | 102.26 ± 6.20 |
| 7 | 48.27 ± 0.62 | 102.24 ± 4.61 | 121.73 ± 7.72 |
| 10 | 54.21 ± 2.01 | 107.71 ± 1.73 | 123.28 ± 2.67 |
| 21 | 113.24 ± 5.26 | 126.91 ± 7.19 | |
| τ, Day | Catalase Activity, % of Control 1 | ||
|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | |
| 3 | 43.82 ± 3.10 | 52.92 ± 2.87 | 56.28 ± 2.82 |
| 7 | 52.37 ± 0.82 | 61.78 ± 3.16 | 75.60 ± 7.28 |
| 10 | 61.72 ± 1.78 | 83.48 ± 3.72 | 92.03 ± 4.82 |
| 21 | - | 118.34 ± 2.02 | 120.82 ± 5.22 |
| τ, Day | AlDH Activity, % of Control 1 | ||
|---|---|---|---|
| Burnt (Untreated) | Oleo ZnO NPs-BDP—Thymol-Lavender | Hydro ZnO NPs-BDP—Lavender | |
| 3 | 51.21 ± 0.88 | 146.56 ± 2.92 | 149.82 ± 4.62 |
| 7 | 50.76 ± 2.82 | 142.53 ± 6.82 | 132.48 ± 4.51 |
| 10 | 56.37 ± 2.37 | 134.82 ± 3.37 | 121.32 ± 6.70 |
| 21 | 106.82 ± 5.40 | 95.06 ± 2.42 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, N.; Balakireva, A.; Orekhov, D.; Kamorin, D.; Didenko, N.; Malygina, D.; Knyazev, A.; Novopoltsev, D.; Solovyeva, A. Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns. Pharmaceuticals 2021, 14, 492. https://doi.org/10.3390/ph14060492
Melnikova N, Balakireva A, Orekhov D, Kamorin D, Didenko N, Malygina D, Knyazev A, Novopoltsev D, Solovyeva A. Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns. Pharmaceuticals. 2021; 14(6):492. https://doi.org/10.3390/ph14060492
Chicago/Turabian StyleMelnikova, Nina, Alyona Balakireva, Dmitry Orekhov, Denis Kamorin, Natalia Didenko, Darina Malygina, Alexander Knyazev, Denis Novopoltsev, and Anna Solovyeva. 2021. "Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns" Pharmaceuticals 14, no. 6: 492. https://doi.org/10.3390/ph14060492
APA StyleMelnikova, N., Balakireva, A., Orekhov, D., Kamorin, D., Didenko, N., Malygina, D., Knyazev, A., Novopoltsev, D., & Solovyeva, A. (2021). Zinc Oxide Nanoparticles Protected with Terpenoids as a Substance in Redox Imbalance Normalization in Burns. Pharmaceuticals, 14(6), 492. https://doi.org/10.3390/ph14060492






