Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits
Abstract
1. Introduction
2. Results
2.1. Part 1: Biodistribution and Pharmacokinetics
2.1.1. Single-Dose Ocular Bio-Distribution
2.1.2. Single-Dose PK-Profiles for Retinal Tissue and Vitreous Humor
2.1.3. Multiple Dose Bio Distribution
2.1.4. Blood-Plasma PK
2.2. Part 2: Toxicity and Local Tolerability
3. Discussion
4. Materials and Methods
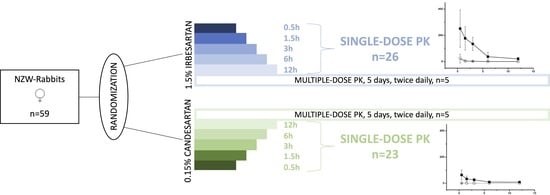
4.1. Test Animals
4.2. Study Drug Compounds
4.3. Experimental Paradigm
4.3.1. Part 1: Bio Distribution and Pharmacokinetics
Blood Sampling
Determination of Drug Concentrations
4.3.2. Part 2: Local Tolerability
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirabito Colafella, K.M.; Bovée, D.M.; Danser, A.H.J. The renin-angiotensin-aldosterone system and its therapeutic targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef] [PubMed]
- Singhania, N.; Bansal, S.; Mohandas, S.; Nimmatoori, D.P.; Ejaz, A.A.; Singhania, G. Role of renin-angiotensin-aldosterone system inhibitors in heart failure and chronic kidney disease. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Matchar, D.B.; McCrory, D.C.; Orlando, L.A.; Patel, M.R.; Patel, U.D.; Patwardhan, M.B.; Powers, B.; Samsa, G.P.; Gray, R.N. Systematic review: Comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann. Intern. Med. 2008, 148, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Reichhart, N.; Figura, A.; Skosyrski, S.; Strauß, O. Control of the retinal local RAS by the RPE: An interface to systemic RAS activity. Exp. Eye Res. 2019, 189, 107838. [Google Scholar] [CrossRef] [PubMed]
- Phipps, J.A.; Dixon, M.A.; Jobling, A.I.; Wang, A.Y.; Greferath, U.; Vessey, K.A.; Fletcher, E.L. The renin-angiotensin system and the retinal neurovascular unit: A role in vascular regulation and disease. Exp. Eye Res. 2019, 187, 107753. [Google Scholar] [CrossRef] [PubMed]
- Holappa, M.; Vapaatalo, H.; Vaajanen, A. Local ocular renin-angiotensin-aldosterone system: Any connection with intraocular pressure? A comprehensive review. Ann. Med. 2020, 52, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, T.; Takahashi, A.; Sato, E.; Yokota, H.; Izumi, N.; Yoshida, A. Effect of systemic administration of valsartan, an angiotensin II type 1 receptor blocker, on retinal circulation in healthy humans. Eye 2009, 23, 1491–1492. [Google Scholar] [CrossRef] [PubMed]
- Sjolie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Chaturvedi, N.; Group, D.P.S. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008, 372, 1385–1393. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Porta, M.; Klein, R.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Sjolie, A.K.; Group, D.P.S. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet 2008, 372, 1394–1402. [Google Scholar] [CrossRef]
- Mauer, M.; Zinman, B.; Gardiner, R.; Suissa, S.; Sinaiko, A.; Strand, T.; Drummond, K.; Donnelly, S.; Goodyer, P.; Gubler, M.C.; et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 40–51. [Google Scholar] [CrossRef]
- Sjolie, A.K.; Dodson, P.; Hobbs, F.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int. J. Clin. Pract. 2011, 65, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, F.; Zhang, Y.; Zhao, S.H.; Zhao, W.J.; Yan, S.L.; Wang, Y.G. Effects of RAS inhibitors on diabetic retinopathy: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015, 3, 263–274. [Google Scholar] [CrossRef]
- Oktaviono, Y.H.; Kusumawardhani, N. Hyperkalemia Associated with Angiotensin Converting Enzyme Inhibitor or Angiotensin Receptor Blockers in Chronic Kidney Disease. Acta Med. Indones 2020, 52, 74–79. [Google Scholar] [PubMed]
- Li, E.C.; Heran, B.S.; Wright, J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014, 2014, Cd009096. [Google Scholar] [CrossRef]
- Chawla, G.; Bansal, A.K. A comparative assessment of solubility advantage from glassy and crystalline forms of a water-insoluble drug. Eur. J. Pharm. Sci. 2007, 32, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, H.H.; Stefánsson, E.; Gudmundsdóttir, E.; Eysteinsson, T.; Thorsteinsdóttir, M.; Loftsson, T. Cyclodextrin formulation of dorzolamide and its distribution in the eye after topical administration. J. Control. Release 2005, 102, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Stefansson, E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Sripetch, S.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: Thermodynamic considerations. Int. J. Pharm. 2021, 597, 120332. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Soler, L.; Olafsdottir, O.B.; Garhofer, G.; Jansook, P.; Kristinsdottir, I.M.; Tan, A.; Loftsson, T.; Stefansson, E. Angiotensin Receptor Blockers in cyclodextrin nanoparticle eye drops: Ocular pharmacokinetics and pharmacologic effect on intraocular pressure. Acta Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Loftsson, T.; Masson, M. Cyclodextrins in topical drug formulations: Theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Loftsson, T.; Stefánsson, E. Cyclodextrins in eye drop formulations: Enhanced topical delivery of corticosteroids to the eye. Acta Ophthalmol. Scand. 2002, 80, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Johannsdottir, S.; Kristinsson, J.K.; Fulop, Z.; Asgrimsdottir, G.; Stefansson, E.; Loftsson, T. Formulations and toxicologic in vivo studies of aqueous cyclosporin A eye drops with cyclodextrin nanoparticles. Int. J. Pharm. 2017, 529, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Morrison, P.W.; Connon, C.J.; Khutoryanskiy, V.V. Cyclodextrin-mediated enhancement of riboflavin solubility and corneal permeability. Mol. Pharm. 2013, 10, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Burnier, M. Angiotensin II type 1 receptor blockers. Circulation 2001, 103, 904–912. [Google Scholar] [CrossRef] [PubMed]
- van Rodijnen, W.F.; van Lambalgen, T.A.; van Teijlingen, M.E.; Tangelder, G.J.; Ter Wee, P.M. Comparison of the AT1-receptor blockers candesartan, irbesartan and losartan for inhibiting renal microvascular constriction. J. Renin Angiotensin Aldosterone Syst. 2001, 2, S204–S210. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdottir, D.; Stefansson, E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: Aqueous dexamethasone eye drops. J. Pharm. Pharmacol. 2007, 59, 629–635. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Konráethsdóttir, F.; Loftsson, T.; Stefánsson, E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007, 85, 598–602. [Google Scholar] [CrossRef]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Kristinsdottir, I.M.; Asgrimsdottir, G.M.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: The effect of benzalkonium chloride on topical dexamethasone penetration into the eye in vivo. J. Drug Deliv. Sci. Technol. 2018, 48, 125–127. [Google Scholar] [CrossRef]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Kristinsdottir, I.M.; Fulop, Z.; Asgrimsdottir, G.M.; Thorsteindsottir, M.; Eiriksson, F.F.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: Dexamethasone concentrations in various eye tissues after topical administration for up to 15 days to rabbits. J. Drug Deliv. Sci. Technol. 2018, 45, 449–454. [Google Scholar] [CrossRef]
- Himawan, E.; Ekstrom, P.; Buzgo, M.; Gaillard, P.; Stefansson, E.; Marigo, V.; Loftsson, T.; Paquet-Durand, F. Drug delivery to retinal photoreceptors. Drug Discov. Today 2019, 24, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.L.; Aachmann, F.L.; Wimmer, R.; Stella, V.J.; Kjølner, U.M. Phase solubility and structure of the inclusion complexes of prednisolone and 6 alpha-methyl prednisolone with various cyclodextrins. J. Pharm. Sci. 2005, 94, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Gawad, A.E.H.; Soliman, O.A.; El-Dahan, M.S.; Al-Zuhairy, S.A.S. Improvement of the Ocular Bioavailability of Econazole Nitrate upon Complexation with Cyclodextrins. AAPS PharmSciTech 2017, 18, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.F.S.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxid. Med. Cell. Longev. 2018, 2018, 5260976. [Google Scholar] [CrossRef]
- Munro, I.C.; Newberne, P.M.; Young, V.R.; Bär, A. Safety assessment of γ-cyclodextrin. Regul. Toxicol. Pharmacol. 2004, 39 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef]
- Klaus, R.; Jin, C.; Maier-Salamon, A.; Jäger, W.; Knopf, C.; Zeitlinger, M.; Richter-Müksch, S.; Schmidl, D.; Schmetterer, L.; Garhöfer, G. An Exploratory Microdialysis Study to Assess the Ocular Pharmacokinetics of Ciprofloxacin Eye Drops in Rabbits. J. Ocul. Pharmacol. Ther. 2016, 32, 390–395. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Mondelo-Garcia, C.; Gonzalez-Barcia, M.; Zarra-Ferro, I.; Otero-Espinar, F.J.; Ruibal-Morell, A.; Aguiar-Fernandez, P.; Fernandez-Ferreiro, A. Ocular Biodistribution Studies using Molecular Imaging. Pharmaceutics 2019, 11, 237. [Google Scholar] [CrossRef]
- Bauer, N.J.; Motamedi, M.; Wicksted, J.P.; March, W.F.; Webers, C.A.; Hendrikse, F. Non-invasive assessment of ocular pharmacokinetics using Confocal Raman Spectroscopy. J. Ocul. Pharmacol. Ther. 1999, 15, 123–134. [Google Scholar] [CrossRef]
- Jansook, P.; Muankaew, C.; Stefansson, E.; Loftsson, T. Development of eye drops containing antihypertensive drugs: Formulation of aqueous irbesartan/γCD eye drops. Pharm. Dev. Technol. 2015, 20, 626–632. [Google Scholar] [CrossRef]
- Tan, A.; Gui, X.; Wong, M.; Deng, H.; Gu, G.; Fanaras, C.; Fanaras, J.C. Simultaneous quantification of candesartan and irbesartan in rabbit eye tissues by liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2020, 34, e4808. [Google Scholar] [CrossRef]
- Draize, J.; Woodard, G.; Calvery, H. Method for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Cochet, P.; Bonnet, R. L’ésthésie cornéenne. Clin. Ophthalmol. 1960, 4, 12–40. [Google Scholar]

| Study Drug-Tissue | Cmax SE (ng/g) | Tmax SE (h) | Cmax CE (ng/g) | Tmax CE (h) |
|---|---|---|---|---|
| Irbesartan-RT | 251 ± 143 | 0.5 | 23 ± 34 | 0.5 |
| Irbesartan-VH | 14 ± 16 | 0.5 | <5 | 0.5 |
| Irbesartan-AH | 121 ± 69 | 3 | <5 | 0.5 |
| Irbesartan-CT | 3663 ± 988 | 1.5 | 49 ± 85 | 1.5 |
| Candesartan-RT | 63 ± 39 | 0.5 | <2 | 0.5 |
| Candesartan-VH | <2 | 0.5 | <2 | N/A |
| Candesartan-AH | 30 ± 14 | 3 | <2 | N/A |
| Candesartan-CT | 3504 ± 801 | 1.5 | 2 ± 4 | 0.5 |
| Study Drug | T0.5 | T1.5 | T3.0 | T6.0 | T12.0 |
|---|---|---|---|---|---|
| 1.5% irbesartan | |||||
| RT-Study eye (ng/g) | 251 ± 143 | 177 ± 89 | 134 ± 51 | 37 ± 34 | 21 ± 2 |
| RT-Control eye (ng/g) | 23 ± 34 | <5 | <5 | <5 | <5 |
| VH-Study eye (ng/g) | 14 ± 16 | <5 | <5 | <5 | <5 |
| VH-Control eye (ng/g) | <5 | <5 | <5 | <5 | <5 |
| 0.15% candesartan | |||||
| RT-Study eye (ng/g) | 63 ± 39 | 33 ± 22 | 27 ± 9 | 9 ± 4 | 9 ± 3 |
| RT-Control eye (ng/g) | <2 | <2 | <2 | <2 | <2 |
| VH-Study eye (ng/g) | <2 | <2 | <2 | <2 | <2 |
| VH-Control eye (ng/g) | <2 | <2 | <2 | <2 | <2 |
| 1.5% Irbesartan | 0.15% Candesartan | |||
|---|---|---|---|---|
| Tissue Samples | SE (ng/g) | CE (ng/g) | SE (ng/g) | CE (ng/g) |
| RT | 338 ± 124 | 7 ± 8 | 36 ± 10 | <2 |
| VH | 13 ± 5 | <5 | <2 | <2 |
| AH | 231 ± 68 | <5 | 70 ± 22 | <2 |
| CT | 9027 ± 2156 | 39 ± 30 | 7468 ± 908 | <2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallab, M.; Schuetzenberger, K.; Hommer, N.; Schäfer, B.J.; Schmidl, D.; Bergmeister, H.; Zeitlinger, M.; Tan, A.; Jansook, P.; Loftsson, T.; et al. Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits. Pharmaceuticals 2021, 14, 480. https://doi.org/10.3390/ph14050480
Kallab M, Schuetzenberger K, Hommer N, Schäfer BJ, Schmidl D, Bergmeister H, Zeitlinger M, Tan A, Jansook P, Loftsson T, et al. Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits. Pharmaceuticals. 2021; 14(5):480. https://doi.org/10.3390/ph14050480
Chicago/Turabian StyleKallab, Martin, Kornelia Schuetzenberger, Nikolaus Hommer, Bhavapriya Jasmin Schäfer, Doreen Schmidl, Helga Bergmeister, Markus Zeitlinger, Aimin Tan, Phatsawee Jansook, Thorsteinn Loftsson, and et al. 2021. "Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits" Pharmaceuticals 14, no. 5: 480. https://doi.org/10.3390/ph14050480
APA StyleKallab, M., Schuetzenberger, K., Hommer, N., Schäfer, B. J., Schmidl, D., Bergmeister, H., Zeitlinger, M., Tan, A., Jansook, P., Loftsson, T., Stefansson, E., & Garhöfer, G. (2021). Bio-Distribution and Pharmacokinetics of Topically Administered γ-Cyclodextrin Based Eye Drops in Rabbits. Pharmaceuticals, 14(5), 480. https://doi.org/10.3390/ph14050480