EEG and Sleep Effects of Tramadol Suggest Potential Antidepressant Effects with Different Mechanisms of Action
Abstract
1. Introduction
2. Results
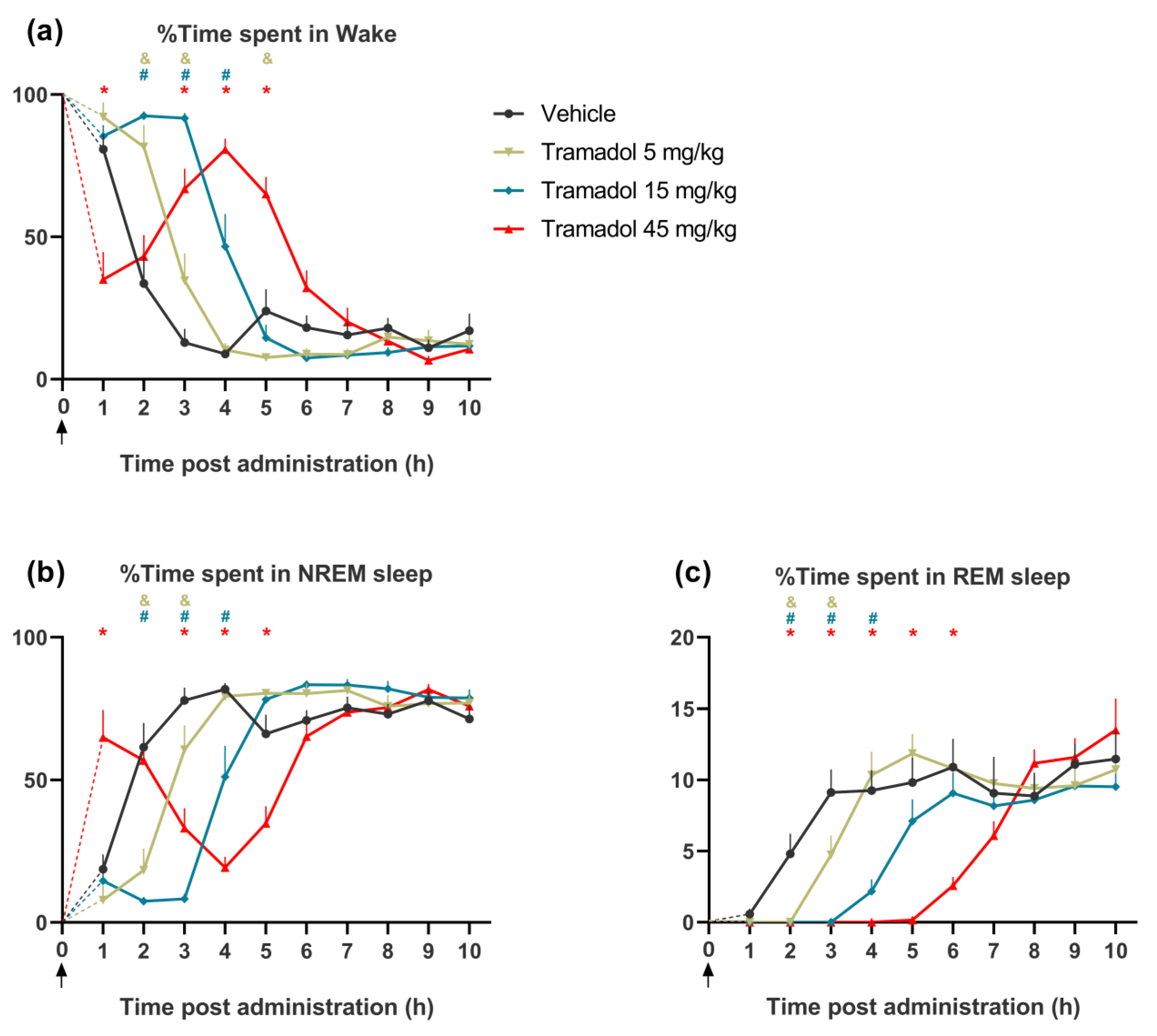
2.1. Effects of Tramadol on the Pattern of Sleep–Wake Cycle
2.1.1. Effects on Wakefulness
2.1.2. Effects on NREM Sleep
2.1.3. Effects on REM Sleep
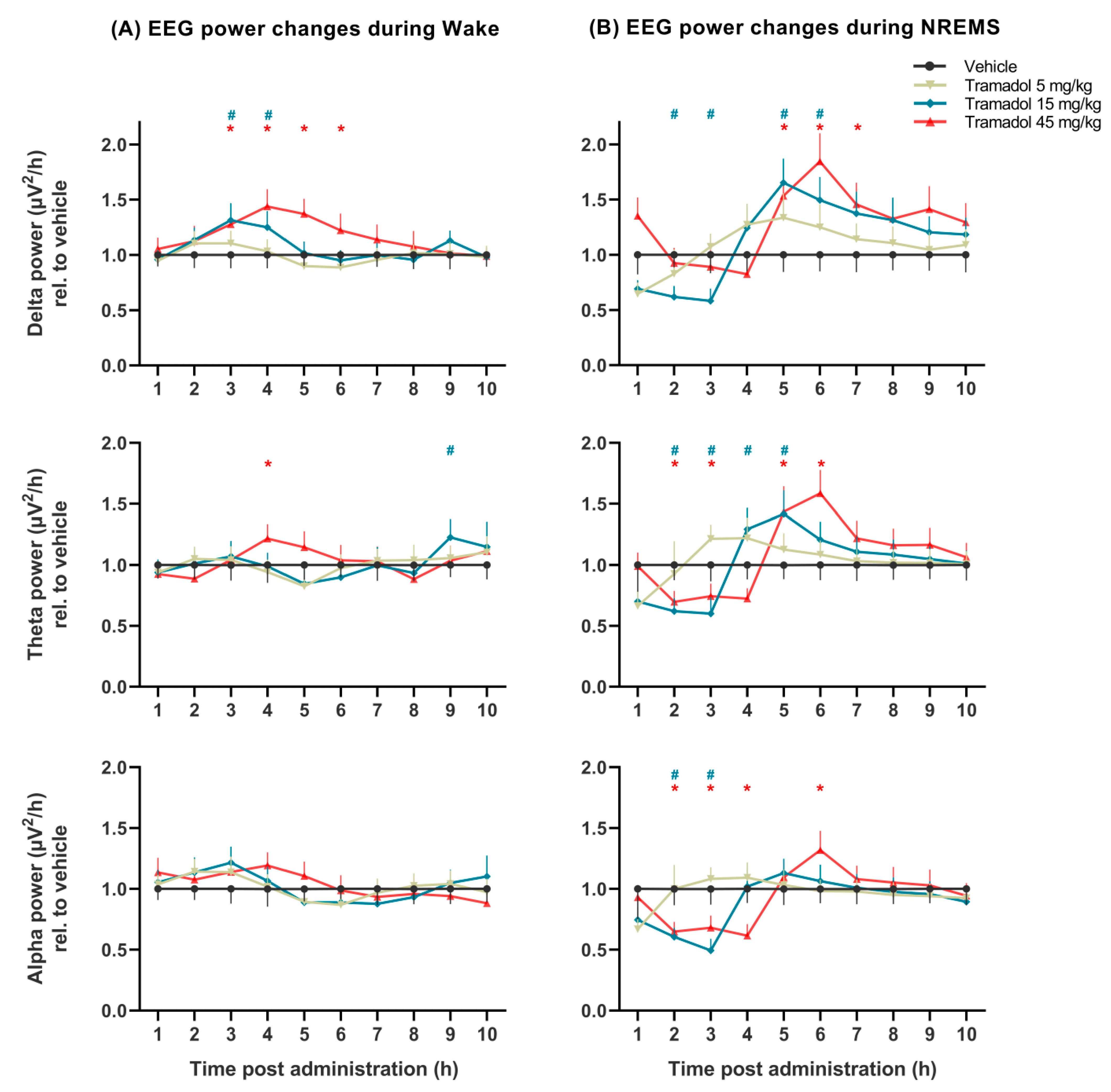
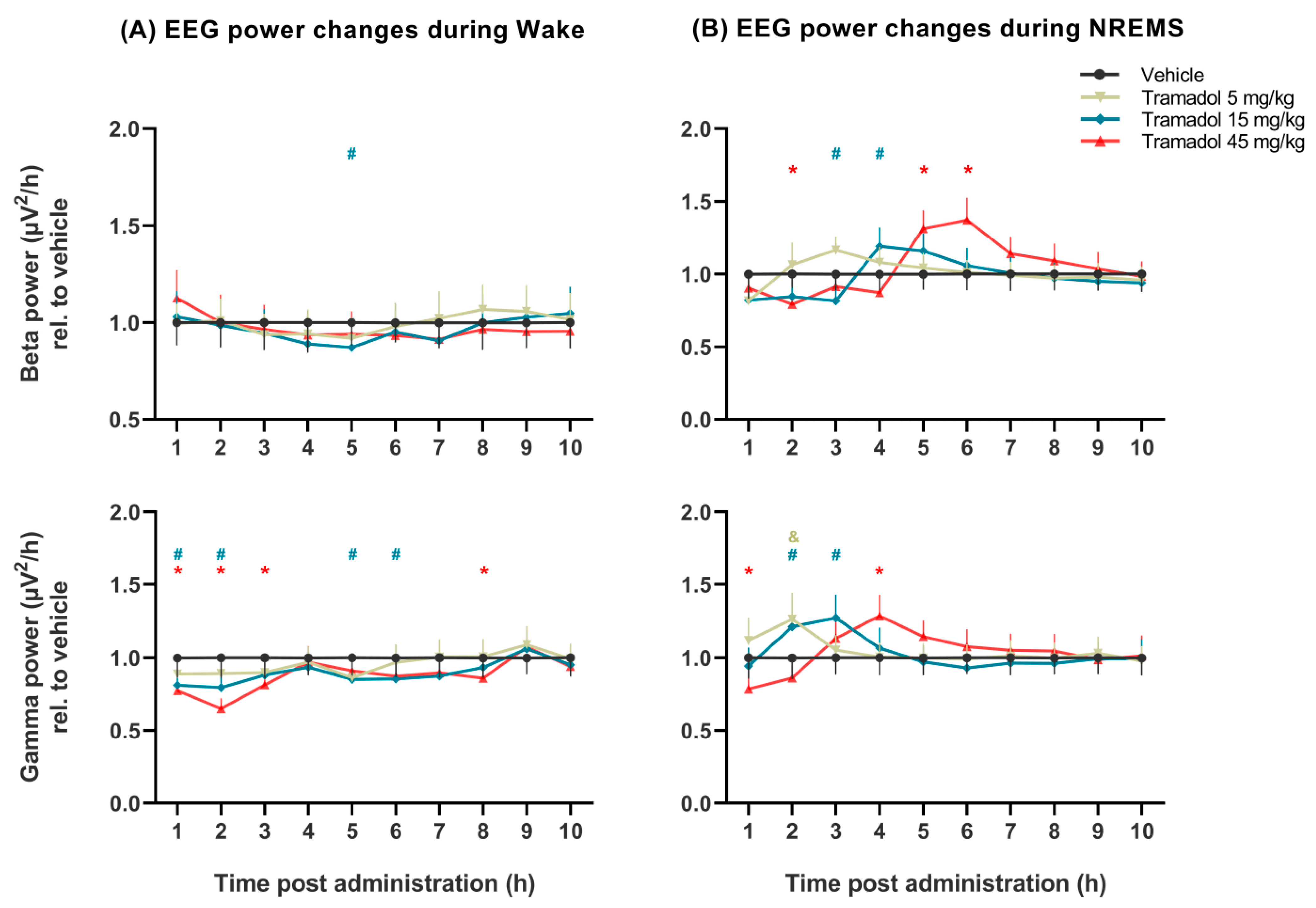
2.2. Effects of Tramadol on qEEG
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgery
4.3. Drugs
4.4. EEG Recording and Analysis
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Bravo, L.; Mico, J.A.; Berrocoso, E. Discovery and development of tramadol for the treatment of pain. Expert Opin. Drug Discov. 2017, 12, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Buschmann, H.; Christoph, T.; Eichenbaum, G.; Englberger, W.; Flores, C.M.; Hertrampf, T.; Kögel, B.; Schiene, K.; Straßburger, W.; et al. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin. Pharmaco. 2012, 13, 1437–1449. [Google Scholar] [CrossRef]
- Vazzana, M.; Andreani, T.; Fangueiro, J.; Faggio, C.; Silva, C.; Santini, A.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Tramadol hydrochloride: Pharmacokinetics, pharmacodynamics, adverse side effects, co-administration of drugs and new drug delivery systems. Biomed. Pharmacother. 2015, 70, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Berrocoso, E.; Sanchez-Blazquez, P.; Garzon, J.; Mico, J.A. Opiates as antidepressants. Curr. Pharm. Des. 2009, 15, 1612–1622. [Google Scholar] [CrossRef]
- Markowitz, J.S.; Patrick, K.S. Venlafaxine-tramadol similarities. Med. Hypotheses 1998, 51, 167–168. [Google Scholar] [CrossRef]
- Barber, J. Examining the use of tramadol hydrochloride as an antidepressant. Exp. Clin. Psychopharmacol. 2011, 19, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, I.; Coubard, S.; Bodard, S.; Chalon, S.; Belzung, C. Effects of 5,7-dihydroxytryptamine lesion of the dorsal raphe nucleus on the antidepressant-like action of tramadol in the unpredictable chronic mild stress in mice. Psychopharmacology 2008, 200, 497–507. [Google Scholar] [CrossRef]
- Szkutnik-Fiedler, D.; Kus, K.; Balcerkiewicz, M.; Grześkowiak, E.; Nowakowska, E.; Burda, K.; Ratajczak, P.; Sadowski, C. Concomitant use of tramadol and venlafaxine–evaluation of antidepressant-like activity and other behavioral effects in rats. Pharmacol. Rep. 2012, 64, 1350–1358. [Google Scholar] [CrossRef]
- Zhang, T.T.; Xue, R.; Wang, X.; Zhao, S.W.; An, L.; Li, Y.F.; Zhang, Y.Z.; Li, S. Network-based drug repositioning: A novel strategy for discovering potential antidepressants and their mode of action. Eur. Neuropsychopharmacol. 2018, 28, 1137–1150. [Google Scholar] [CrossRef]
- Pulley, J.M.; Rhoads, J.P.; Jerome, R.N.; Challa, A.P.; Erreger, K.B.; Joly, M.M.; Lavieri, R.R.; Perry, K.E.; Zaleski, N.M.; Shirey-Rice, J.K.; et al. Using what we already have: Uncovering new drug repurposing strategies in existing omics data. Annu. Rev. Pharmacol. 2020, 60, 333–352. [Google Scholar] [CrossRef]
- Bumpus, J.A. Low-Dose Tramadol as an Off-Label Antidepressant: A Data Mining Analysis from the Patients’ Perspective. ACS Pharmacol. Transl. Sci. 2020, 3, 1293–1303. [Google Scholar] [CrossRef]
- Steiger, A.; Pawlowski, M. Depression and Sleep. Int. J. Mol. Sci. 2019, 20, 607. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Walecka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM sleep dysregulation in depression: State of the art. Sleep Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef]
- Riemann, D.; Krone, L.B.; Wulff, K.; Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology 2019, 45, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, N.; de la Salle, S.; Ibrahim, M.H.; Blier, P.; Knott, V. Leveraging Machine Learning Approaches for Predicting Antidepressant Treatment Response Using Electroencephalography (EEG) and Clinical Data. Front. Psychiatry 2019, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Steiger, A.; Mayumi, K. Wake and sleep EEG provide biomarkers in depression. J. Psychiatr. Res. 2010, 44.4, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Vas, S.; Katai, Z.; Kostyalik, D.; Pap, D.; Molnar, E.; Petschner, P.; Kalmar, L.; Bagdy, G. Differential adaptation of REM sleep latency, intermediate stage and theta power effects of escitalopram after chronic treatment. J. Neural Transm. 2013, 120, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Walder, B.; Tramer, M.R.; Blois, R. The effects of two single doses of tramadol on sleep: A randomized, cross-over trial in healthy volunteers. Eur. J. Anaesth. 2001, 18, 36–42. [Google Scholar] [CrossRef]
- Katai, Z.; Adori, C.; Kitka, T.; Vas, S.; Kalmar, L.; Kostyalik, D.; Tothfalusi, L.; Palkovits, M.; Bagdy, G. Acute escitalopram treatment inhibits REM sleep rebound and activation of MCH-expressing neurons in the lateral hypothalamus after long term selective REM sleep deprivation. Psychopharmacology 2013, 228, 439–449. [Google Scholar] [CrossRef][Green Version]
- Argyropoulos, S.V.; Wilson, S.J. Sleep disturbances in depression and the effects of antidepressants. Int. Rev. Psychiatry 2005, 17, 237–245. [Google Scholar] [CrossRef]
- Bacque-Cazenave, J.; Bharatiya, R.; Barriere, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdere, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed]
- Ursin, R. Serotonin and sleep. Sleep Med. Rev. 2002, 6, 55–67. [Google Scholar] [CrossRef]
- Lu, J.; Sherman, D.; Devor, M.; Saper, C.B. A putative flip–flop switch for control of REM sleep. Nature 2006, 441, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Bamigbade, T.A.; Davidson, C.; Langford, R.M.; Stamford, J.A. Actions of tramadol, its enantiomers and principal metabolite, O-desmethyltramadol, on serotonin (5-HT) efflux and uptake in the rat dorsal raphe nucleus. Br. J. Anaesth. 1997, 79, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Halfpenny, D.M.; Callado, L.F.; Hopwood, S.E.; Bamigbade, T.A.; Langford, R.M.; Stamford, J.A. Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Br. J. Anaesth. 1999, 83, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Bloms-Funke, P.; Dremencov, E.; Cremers, T.I.; Tzschentke, T.M. Tramadol increases extracellular levels of serotonin and noradrenaline as measured by in vivo microdialysis in the ventral hippocampus of freely-moving rats. Neurosci. Lett. 2011, 490, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, M.J.; Dulawa, S.C. Identifying fast-onset antidepressants using rodent models. Mol. Psychiatry 2017, 22, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Opal, M.D.; Klenotich, S.C.; Morais, M.; Bessa, J.; Winkle, J.; Doukas, D.; Kay, L.J.; Sousa, N.; Dulawa, S.M. Serotonin 2C receptor antagonists induce fast-onset antidepressant effects. Mol. Psychiatry 2013, 19, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Ahnaou, A.; Huysmans, H.; Biermans, R.; Manyakov, N.V.; Drinkenburg, W. Ketamine: Differential neurophysiological dynamics in functional networks in the rat brain. Transl. Psychiatry 2017, 7, e1237. [Google Scholar] [CrossRef]
- Bogathy, E.; Papp, N.; Tothfalusi, L.; Vas, S.; Bagdy, G. Additive effect of 5-HT2C and CB1 receptor blockade on the regulation of sleep-wake cycle. BMC Neurosci. 2019, 20, 14. [Google Scholar] [CrossRef]
- Peprah, P.; Agyemang-Duah, W.; Appiah-Brempong, E.; Akwasi, A.G.; Morgan, A.K. “With tramadol, I ride like a Jaguar”: A qualitative study of motivations for non-medical purpose tramadol use among commercial vehicle operators in Kumasi, Ghana. Subst. Abuse Treat. Prev. Policy 2020, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef]
- Mrdalj, J.; Pallesen, S.; Milde, A.M.; Jellestad, F.K.; Murison, R.; Ursin, R.; Bjorvatn, B.; Gronli, J. Early and later life stress alter brain activity and sleep in rats. PLoS ONE 2013, 8, e69923. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Vas, S.; Bogathy, E.; Katai, Z.; Kostyalik, D.; Bagdy, G. Acute and chronic escitalopram alter EEG gamma oscillations differently: Relevance to therapeutic effects. Eur. J. Pharm. Sci. 2018, 121, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wichniak, A.; Wierzbicka, A.; Jernajczyk, W. Sleep as a biomarker for depression. Int. Rev. Psychiatry 2013, 25, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Kantor, S.; Jakus, R.; Bodizs, R.; Halasz, P.; Bagdy, G. Acute and long-term effects of the 5-HT2 receptor antagonist ritanserin on EEG power spectra, motor activity, and sleep: Changes at the light–dark phase shift. Brain Res. 2002, 943, 105–111. [Google Scholar] [CrossRef]
- Ogata, J.; Minami, K.; Uezono, Y.; Okamoto, T.; Shiraishi, M.; Shigematsu, A.; Ueta, Y. The inhibitory effects of tramadol on 5-hydroxytryptamine type 2C receptors expressed in Xenopus oocytes. Anesth. Analg. 2004, 98, 1401–1406. [Google Scholar] [CrossRef]
- Horishita, T.; Minami, K.; Uezono, Y.; Shiraishi, M.; Ogata, J.; Okamoto, T.; Shigematsu, A. The tramadol metabolite, O-desmethyl tramadol, inhibits 5-hydroxytryptamine type 2C receptors expressed in Xenopus Oocytes. Pharmacology 2006, 77, 93–99. [Google Scholar] [CrossRef]
- Feinberg, I.; Campbell, I.G. Ketamine administration during waking increases delta EEG intensity in rat sleep. Neuropsychopharmacology 1993, 9, 41–48. [Google Scholar] [CrossRef]
- Duncan, W.C., Jr.; Zarate, C.A., Jr. Ketamine, sleep, and depression: Current status and new questions. Curr. Psychiatry Rep. 2013, 15, 394. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Ogata, J.; Uezono, Y. What is the main mechanism of tramadol? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 999–1007. [Google Scholar] [CrossRef]
- Yang, C.; Li, W.Y.; Yu, H.Y.; Gao, Z.Q.; Liu, X.L.; Zhou, Z.Q.; Yang, J.J. Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J. Biomed. Biotechnol. 2012, 2012, 175619. [Google Scholar] [CrossRef]
- Ostadhadi, S.; Norouzi-Javidan, A.; Chamanara, M.; Akbarian, R.; Imran-Khan, M.; Ghasemi, M.; Dehpour, A.R. Involvement of NMDA receptors in the antidepressant-like effect of tramadol in the mouse forced swimming test. Brain Res. Bull. 2017, 134, 136–141. [Google Scholar] [CrossRef]
- Dolsen, M.R.; Cheng, P.; Arnedt, J.T.; Swanson, L.; Casement, M.D.; Kim, H.S.; Goldschmied, J.R.; Hoffmann, R.F.; Armitage, R.; Deldin, P.J. Neurophysiological correlates of suicidal ideation in major depressive disorder: Hyperarousal during sleep. J. Affect. Disord. 2017, 212, 160–166. [Google Scholar] [CrossRef]
- Jaimchariyatam, N.; Rodriguez, C.L.; Budur, K. Prevalence and correlates of alpha-delta sleep in major depressive disorders. Innov. Clin. Neurosci. 2011, 8, 35. [Google Scholar] [PubMed]
- da Rocha, A.P.; Mizzaci, C.C.; Nunes Pinto, A.C.P.; da Silva Vieira, A.G.; Civile, V.; Trevisani, V.F.M. Tramadol for management of fibromyalgia pain and symptoms: Systematic review. Int. J. Clin. Pract. 2019, 74, e13455. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Klerman, E.B.; Adler, G.K.; Kopell, N.J. Thalamic mechanisms underlying alpha-delta sleep with implications for fibromyalgia. J. Neurophysiol. 2015, 114, 1923–1930. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl. Psychiatry 2018, 8, 177. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Watson, B.O. In vivo electrophysiological recordings of the effects of antidepressant drugs. Exp. Brain Res. 2019, 237, 1593–1614. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef]
- Akhmetshina, D.; Zakharov, A.; Vinokurova, D.; Nasretdinov, A.; Valeeva, G.; Khazipov, R. The serotonin reuptake inhibitor citalopram suppresses activity in the neonatal rat barrel cortex in vivo. Brain Res. Bull. 2016, 124, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mendez, P.; Pazienti, A.; Szabo, G.; Bacci, A. Direct alteration of a specific inhibitory circuit of the hippocampus by antidepressants. J. Neurosci. 2012, 32, 16616–16628. [Google Scholar] [CrossRef]
- Puig, M.V.; Watakabe, A.; Ushimaru, M.; Yamamori, T.; Kawaguchi, Y. Serotonin Modulates Fast-Spiking Interneuron and Synchronous Activity in the Rat Prefrontal Cortex through 5-HT1A and 5-HT2A Receptors. J. Neurosci. 2010, 30, 2211–2222. [Google Scholar] [CrossRef]
- Shin, Y.W.; O’Donnell, B.F.; Youn, S.; Kwon, J.S. Gamma oscillation in schizophrenia. Psychiatry Investig. 2011, 8, 288–296. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Demiralp, T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005, 116, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Le Van Quyen, M.; Muller, L.E., 2nd; Telenczuk, B.; Halgren, E.; Cash, S.; Hatsopoulos, N.G.; Dehghani, N.; Destexhe, A. High-frequency oscillations in human and monkey neocortex during the wake-sleep cycle. Proc. Natl. Acad. Sci. USA 2016, 113, 9363–9368. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, B. State-dependent increase of cortical gamma activity during REM sleep after selective blockade of NR2B subunit containing NMDA receptors. Sleep 2012, 35, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Koncz, S.; Kostyalik, D.; Kitka, T.; Petschner, P.; Vas, S.; Bagdy, G. Acute 5-HT2C Receptor Antagonist SB-242084 Treatment Affects EEG Gamma Band Activity Similarly to Chronic Escitalopram. Front. Pharmacol. 2020, 10, 1636. [Google Scholar] [CrossRef]
- Spencer, C. The efficacy of intramuscular tramadol as a rapid-onset antidepressant. Aust. N. Z. J. Psychiatry 2000, 34, 1032–1033. [Google Scholar] [CrossRef] [PubMed]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L.; Jacoby, H.I.; Selve, N. Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J. Pharmacol. Exp. Ther. 1993, 267, 331–340. [Google Scholar]
- Berrocoso, E.; Ikeda, K.; Sora, I.; Uhl, G.R.; Sanchez-Blazquez, P.; Mico, J.A. Active behaviours produced by antidepressants and opioids in the mouse tail suspension test. Int. J. Neuropsychopharmacol. 2013, 16, 151–162. [Google Scholar] [CrossRef]
- Tetsunaga, T.; Tetsunaga, T.; Tanaka, M.; Ozaki, T. Efficacy of tramadol-acetaminophen tablets in low back pain patients with depression. J. Orthop. Sci. 2015, 20, 281–286. [Google Scholar] [CrossRef]
- Kantor, S.; Jakus, R.; Balogh, B.; Benko, A.; Bagdy, G. Increased wakefulness, motor activity and decreased theta activity after blockade of the 5-HT2B receptor by the subtype-selective antagonist SB-215505. Br. J. Pharmacol. 2004, 142, 1332–1342. [Google Scholar] [CrossRef]
- Jesse, C.R.; Bortolatto, C.F.; Savegnago, L.; Rocha, J.B.; Nogueira, C.W. Involvement of l-arginine–nitric oxide–cyclic guanosine monophosphate pathway in the antidepressant-like effect of tramadol in the rat forced swimming test. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Corrales, M.O.; Berrocoso, E.; Gibert-Rahola, J.; Mico, J.A. Antidepressant-like effects of tramadol and other central analgesics with activity on monoamines reuptake, in helpless rats. Life Sci. 2002, 72, 143–152. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koncz, S.; Papp, N.; Menczelesz, N.; Pothorszki, D.; Bagdy, G. EEG and Sleep Effects of Tramadol Suggest Potential Antidepressant Effects with Different Mechanisms of Action. Pharmaceuticals 2021, 14, 431. https://doi.org/10.3390/ph14050431
Koncz S, Papp N, Menczelesz N, Pothorszki D, Bagdy G. EEG and Sleep Effects of Tramadol Suggest Potential Antidepressant Effects with Different Mechanisms of Action. Pharmaceuticals. 2021; 14(5):431. https://doi.org/10.3390/ph14050431
Chicago/Turabian StyleKoncz, Szabolcs, Noémi Papp, Noémi Menczelesz, Dóra Pothorszki, and György Bagdy. 2021. "EEG and Sleep Effects of Tramadol Suggest Potential Antidepressant Effects with Different Mechanisms of Action" Pharmaceuticals 14, no. 5: 431. https://doi.org/10.3390/ph14050431
APA StyleKoncz, S., Papp, N., Menczelesz, N., Pothorszki, D., & Bagdy, G. (2021). EEG and Sleep Effects of Tramadol Suggest Potential Antidepressant Effects with Different Mechanisms of Action. Pharmaceuticals, 14(5), 431. https://doi.org/10.3390/ph14050431





