Thymoquinone, as a Novel Therapeutic Candidate of Cancers
Abstract
1. Introduction
2. Properties and Pharmacological Features of TQ
3. TQ and Nanotechnology
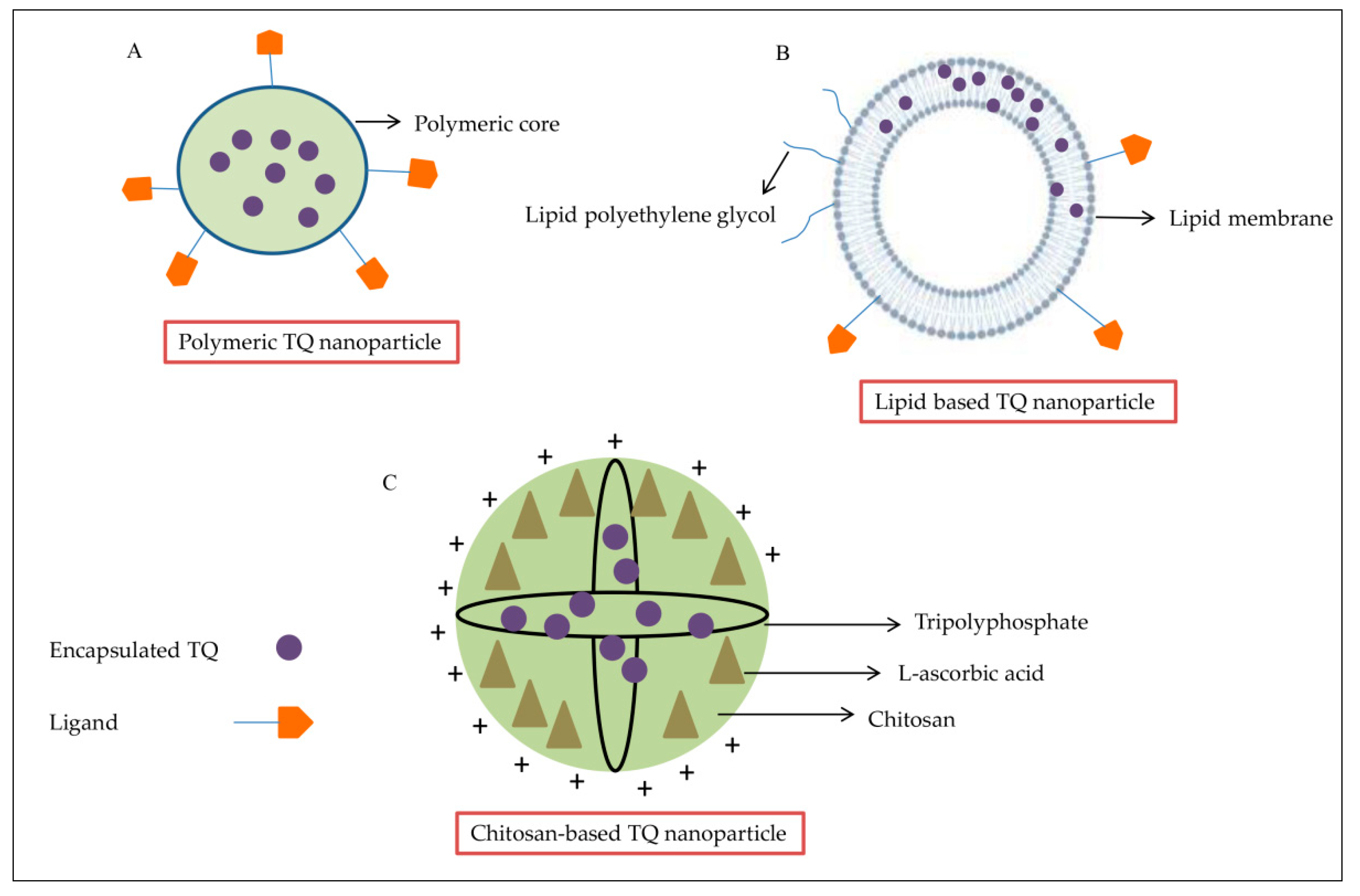
3.1. Polymeric TQ Nanoparticles
3.2. Lipid-Based TQ Nanoparticles
3.3. Chitosan-Based TQ Nanoparticles
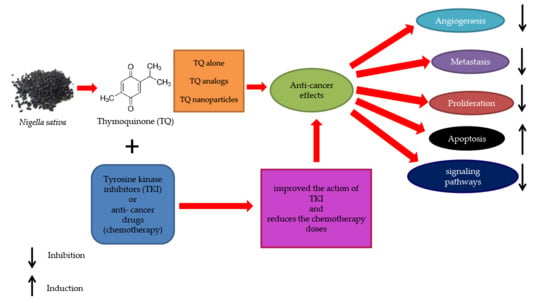
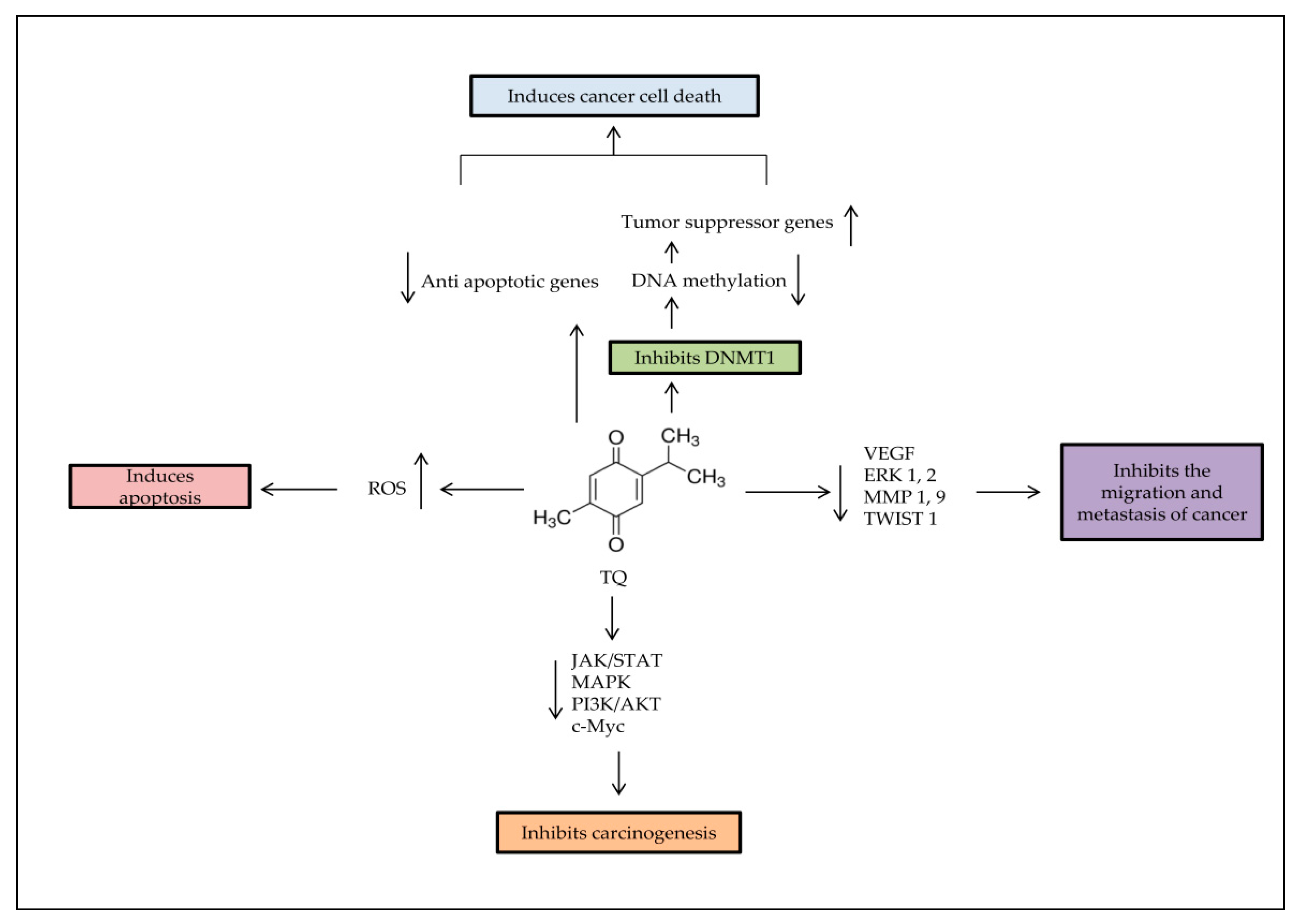
4. Anti-Cancer Effects of TQ
4.1. Breast Cancer
4.2. Lung Cancer
4.3. Gastric Cancer
4.4. Colon Cancer
4.5. Prostate Cancer
4.6. Skin Cancer
4.7. Ovarian Cancer
4.8. Liver Cancer
4.9. Cervical Cancer
4.10. Leukemia
4.11. Head and Neck Cancer
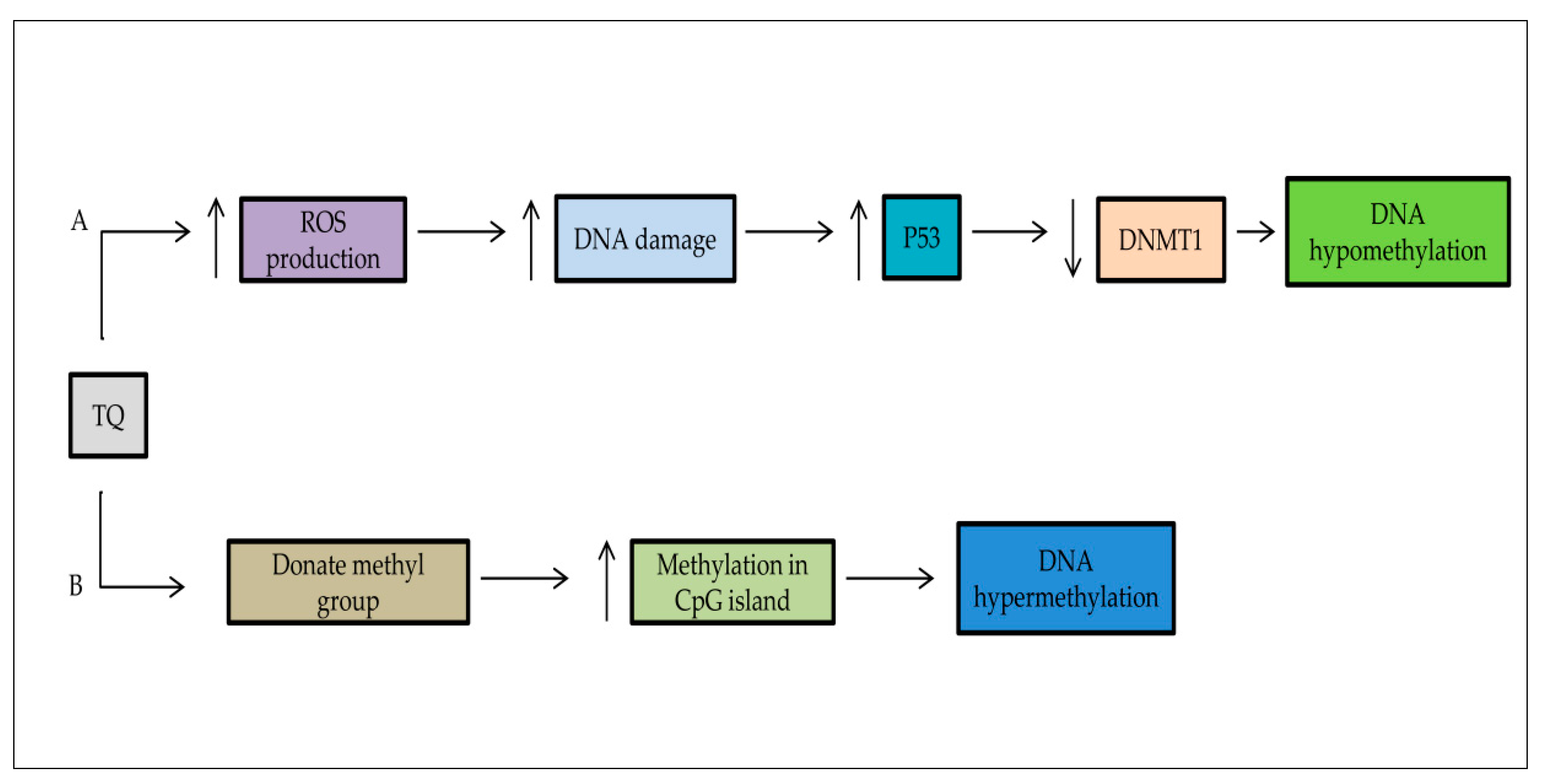
5. Epigenetic Role of TQ
5.1. Histone Acetylation/Deacetylation
5.2. DNA Methylation/Demethylation
5.3. Activating and Deactivating Noncoding RNAs
6. Antioxidant and Anti-Inflammatory Activities of TQ
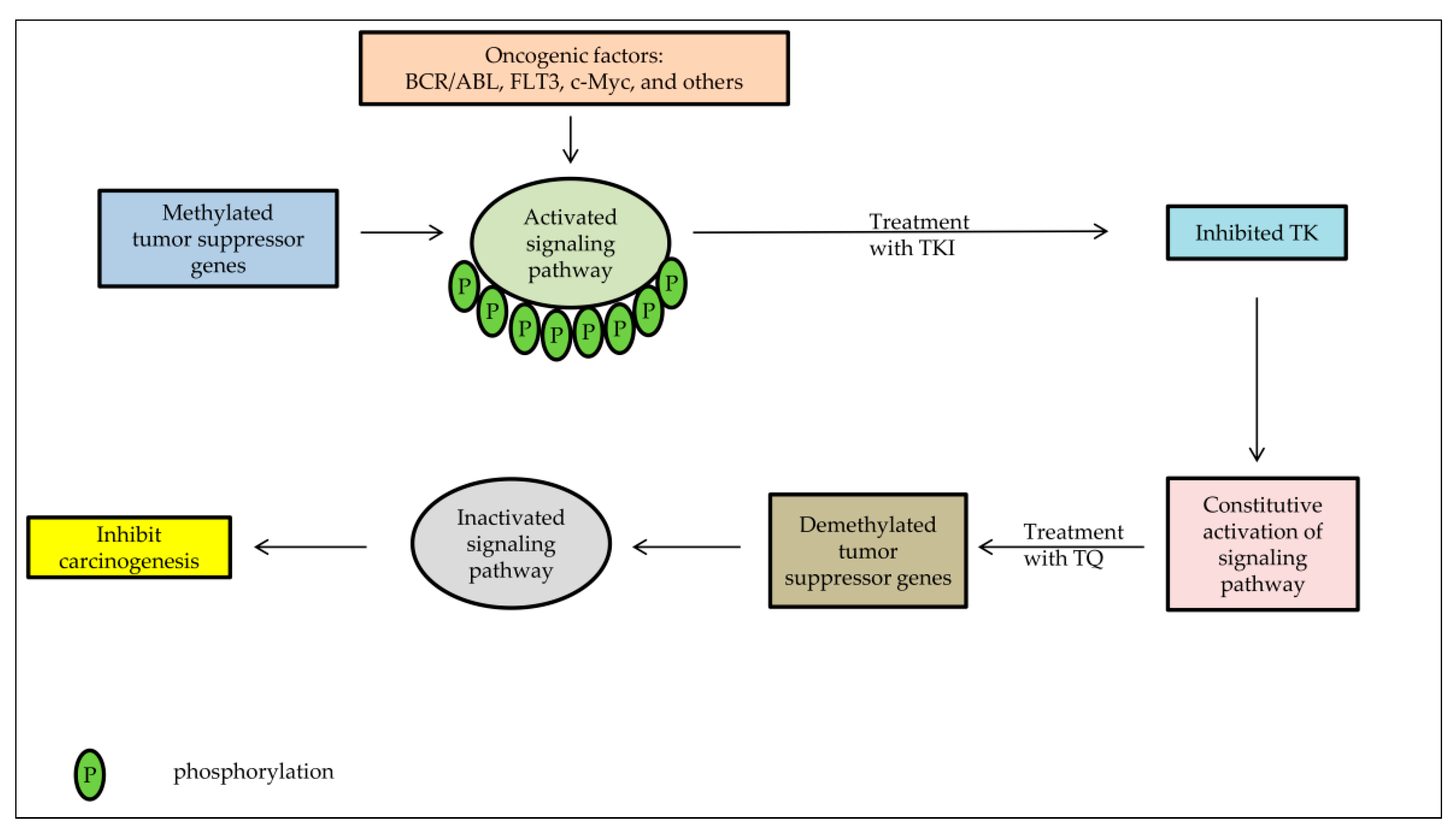
7. Tyrosine Kinase Inhibitors as a Candidate Anti-Cancer Agent Combined with TQ
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGP | α1-acid glycoprotein |
| BPH | Benign prostatic hyperplasia |
| BSA | Bovine serum albumin |
| CAT | Catalase |
| CD | Cyclodextrin |
| COX2 | Cyclooxygenase-2 |
| CS | Chitosan |
| CXCR4 | Chemokine receptor Type 4 |
| DNMT1 | DNA-methyltransferase 1 |
| DOX | Doxorubicin |
| eEF-2K | Eukaryotic elongation Factor 2 kinase |
| EMT | Epithelial-mesenchymal transition |
| EZH2 | Enhancer of zeste homolog 2 |
| GK | Gamma Knife |
| Grb2 | Growth factor receptor-binding protein 2 |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GSK | Glycogen synthase kinase |
| HDAC | Histone deacetylase |
| HSA | Human serum albumin |
| IC50 | 50% inhibitory concentration |
| IFN | Interferon |
| IL | Interleukin |
| I3M | Indirubin-3-monoxime |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase pathway |
| LD50 | Median lethal dose |
| LNPs | Lipid-based nanoparticles |
| LPA | Lysophosphatidic acid |
| MA | Myristic acid |
| MAPK | Mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MMP9 | Matrix metallopeptidase 9 |
| MOMP | Mitochondrial outer membrane permeability |
| mTOR | Mammalian target of rapamycin |
| NF-Kb | Nuclear factor kappa B |
| NK | Natural killer |
| NLCs | Nanostructured lipid carriers |
| PBS | Phosphate-buffered saline |
| PCL | Poly-ε-caprolactone |
| PEG | Polyethylene glycol |
| P-gp | P-glycoprotein |
| PI3K | Phosphoinositide 3-kinases |
| PLGA | Poly-lactide-co-glycolide |
| PPARγ | Peroxisome proliferator-activated receptor-γ |
| PTEN | Phosphatase and tensin homolog |
| PTK | Protein tyrosine kinase |
| PTX | Paclitaxel |
| ROS | Reactive oxygen species |
| SLNs | Solid lipid nanocarriers |
| SOD | Superoxide dismutase |
| STAT | Signal transducer and activator of transcription |
| TF | Transferrin |
| TGF-β1 | Transforming growth factor-beta 1 |
| TKI | Tyrosine kinase inhibitors |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| TQ | Thymoquinone |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TSG | Tumor suppressor genes |
| VCAN | Versican |
| VEGFR | Vascular endothelial growth factor Receptor |
References
- Imran, M.; Rauf, A.; Khan, I.A.; Shahbaz, M.; Qaisrani, T.B.; Fatmawati, S.; Abu-Izneid, T.; Imran, A.; Rahman, K.U.; Gondal, T.A. Thymoquinone: A novel strategy to combat cancer: A review. Biomed. Pharmacother. 2018, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al-Layly, A.; Al-Shawi, H.; Al-Ali, A.; Amir, M.; Mostafa, A. Ultra-high-performance liquid chro-matography-based identification and quantification of thymoquinone in Nigella sativa extract from different geographical re-gions. Pharmacogn. Mag. 2018, 14, 471. [Google Scholar] [CrossRef]
- Awad, A.S.M.; Al Haleem, E.N.A.; El-Bakly, W.M.; Sherief, M.A. Thymoquinone alleviates nonalcoholic fatty liver disease in rats via suppression of oxidative stress, inflammation, apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 381–391. [Google Scholar] [CrossRef]
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114. [Google Scholar] [CrossRef]
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, A.O. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Ecevit, H.; Gunduz, K.; Bilgic, N.; Izmirli, M.; Gogebakan, B. The effect of thymoquinone on BEAS-2B cell viability and TGF-β1 release. AMOR 2017, 3, 15–19. [Google Scholar] [CrossRef][Green Version]
- Khan, A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.D.; Tania, M.; Wei, C.; Mei, Z.; Fu, S.; Cheng, J.; Xu, J.; Fu, J. Thymoquinone inhibits cancer metastasis by downreg-ulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget 2015, 6, 19580. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Shen, N.; Yan, F.; Zhao, N.; Dou, L.; Wu, L.-C.; Seiler, C.L.; Yu, L.; Yang, K.; Bachanova, V.; et al. Thymoquinone exerts potent growth-suppressive activity on leukemia through DNA hypermethylation reversal in leukemia cells. Oncotarget 2017, 8, 34453–34467. [Google Scholar] [CrossRef] [PubMed]
- Barkat, M.D.; Ahmad, J.; Khan, M.A.; Beg, S.; Ahmad, F.J. Insights into the targeting potential of thymoquinone for therapeu-tic intervention against triple-negative breast cancer. Curr. Drug Targets 2018, 19, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Thymoquinone-induced antitumor and apoptosis in human lung adenocarcinoma cells. J. Cell. Physiol. 2019, 234, 10421–10431. [Google Scholar] [CrossRef] [PubMed]
- Martinovich, G.G.; Martinovich, I.V.; Vcherashniaya, A.V.; Shadyro, O.I.; Cherenkevich, S.N. Thymoquinone, a biologically active component of Nigella sativa, induces mitochondrial production of reactive oxygen species and programmed death of tumor cells. Biophys. 2016, 61, 963–970. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Chen, M.-C.; Day, C.H.; Lin, Y.-M.; Li, S.-Y.; Tu, C.-C.; Padma, V.V.; Shih, H.-N.; Kuo, W.-W.; Huang, C.-Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activa-tion. World J. Gastroenterol. 2017, 23, 1171. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.M.M.; Sheikh, B.Y.; Salim, A.L.Z.; Mohan, S.; Khan, A.; Kamalidehghan, B.; Ahmadipour, F.; I Abdelwahab, S. Thymoquinone induces apoptosis and increase ROS in ovarian cancer cell line. Cell. Mol. Boil. 2016, 62, 97–101. [Google Scholar]
- Sarman, H.; Bayram, R.; Benek, S.B. Anticancer drugs with chemotherapeutic interactions with thymoquinone in osteosar-coma cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1263–1270. [Google Scholar]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Sarker, S.; Ghosh, A.; Gupta, P.; Das, S.; Ahir, M.; Bhattacharya, S.; Chattopadhyay, S.; Ghosh, S.; Adhikary, A. Transferrin-decorated thymoquinone-loaded PEG-PLGA nanoparticles exhibit anticarcinogenic effect in non-small cell lung carcinoma via the modulation of miR-34a and miR-16. Biomater. Sci. 2019, 7, 4325–4344. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef]
- Salmani, J.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thy-moquinone; probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef]
- Nagi, M.N.; Almakki, H.A. Thymoquinone supplementation induces quinone reductase and glutathione transferase in mice liver: Possible role in protection against chemical carcinogenesis and toxicity. Phytotherapy Res. 2009, 23, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alzohairy, M.A.; Khan, M.A.; Aly, S.M. Therapeutic implications of black seed and its constituent thymo-quinone in the prevention of cancer through inactivation and activation of molecular pathways. Evid. Based Complement Alternat. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A.; Ginawi, O.T.; El-Hadiyah, T.; El-Khatib, A.S.; Al-Shabanah, O.A.; Al-Sawaf, H.A. Effects of volatile oil con-stituents of Nigella sativa on carbon tetrachloride-induced hepatotoxicity in mice: Evidence for antioxidant effects of thymo-quinone. Res. Commun. Mol. Pathol. Pharmacol. 2001, 110, 239–252. [Google Scholar]
- Kanter, M. Thymoquinone attenuates lung injury induced by chronic toluene exposure in rats. Toxicol. Ind. Heal. 2010, 27, 387–395. [Google Scholar] [CrossRef]
- Jain, A.; Pooladanda, V.; Bulbake, U.; Doppalapudi, S.; Rafeeqi, T.A.; Godugu, C.; Khan, W. Liposphere mediated topical delivery of thymoquinone in the treatment of psoriasis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2251–2262. [Google Scholar] [CrossRef]
- Lupidi, G.; Scire, A.; Camaioni, E.; Khalife, K.H.; De Sanctis, G.; Tanfani, F.; Damiani, E. Thymoquinone, a potential thera-peutic agent of Nigella sativa, binds to site I of human serum albumin. Phytomedicine 2010, 17, 714–720. [Google Scholar] [CrossRef]
- El-Najjar, N.; Ketola, R.A.; Nissilä, T.; Mauriala, T.; Antopolsky, M.; Jänis, J.; Gali-Muhtasib, H.; Urtti, A.; Vuorela, H. Impact of protein binding on the analytical detectability and anticancer activity of thymoquinone. J. Chem. Biol. 2011, 4, 97–107. [Google Scholar] [CrossRef]
- Zaher, N.H.; Rashed, E.R.; El-Ghazaly, M.A. Semi-synthetic thymoquinone analogs: New prototypes as potential antihyper-lipidemics in irradiated rats. Future Med. Chem. 2017, 9, 1483–1493. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Ullah, I.; Mbye, H.; Richardson, A.; Horrocks, P.; Li, W.-W. The synthesis and evaluation of thymo-quinone analogues as anti-ovarian cancer and antimalarial agents. Bioorg. Med. Chem. Lett. 2018, 28, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, U.; Padhye, S.; Špirtović-Halilović, S.; Osmanović, A.; Veljović, E.; Roca, S.; Novakovic, I.; Mandic, B.; Turel, I.; Kljun, J.; et al. Synthesis, biological evaluation and docking studies of benzoxazoles derived from thymoquinone. Molecules 2018, 23, 3297. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ahir, M.; Patra, P.; Mukherjee, S.; Ghosh, S.; Mazumdar, M.; Chattopadhyay, S.; Das, T.; Chattopadhyay, D.; Adhikary, A. PEGylated-thymoquinone-nanoparticle mediated retardation of breast cancer cell migration by deregulation of cytoskeletal actin polymerization through miR-34a. Biomaterials 2015, 51, 91–107. [Google Scholar] [CrossRef]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manouchehri; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef]
- Ganea, G.M.; Fakayode, S.O.; Losso, J.N.; Van Nostrum, C.F.; Sabliov, C.M.; Warner, I.M. Delivery of phytochemical thy-moquinone using molecular micelle modified poly (D, L lactide-co-glycolide)(PLGA) nanoparticles. Nanotechnology 2010, 21, 285104. [Google Scholar] [CrossRef]
- Sun, H.; Guo, B.; Cheng, R.; Meng, F.; Liu, H.; Zhong, Z. Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 2009, 30, 6358–6366. [Google Scholar] [CrossRef]
- Shah, M.; Choi, M.H.; Ullah, N.; Kim, M.O.; Yoon, S.C. Synthesis and characterization of PHV-block-mPEG diblock copoly-mer and its formation of amphiphilic nanoparticles for drug delivery. J. Nanosci. Nanotechnol. 2011, 11, 5702–5710. [Google Scholar] [CrossRef]
- Mona, M.A.; Mottaleb, A. Biodegradable thymoquinone nanoparticles for higher therapeutic efficiency in murine colorectal cancer. Int. J. Pharm. Pharm. Res. 2016, 7, 436–450. [Google Scholar]
- Del Valle, E. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Abu-Dahab, R.; Odeh, F.; Ismail, S.I.; Azzam, H.; Al Bawab, A. Preparation, characterization and antiproliferative activity of thymoquinone-β-cyclodextrin self-assembling nanoparticles. Pharmazie 2013, 68, 939–944. [Google Scholar] [PubMed]
- Wang, T.; Luo, Y. Biological fate of ingested lipid-based nanoparticles: Current understanding and future directions. Nanoscale 2019, 11, 11048–11063. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal Drug Delivery Systems for Ocular Disease—Recent Advances and Future Prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Rajput, S.; Puvvada, N.; Kumar, B.N.; Sarkar, S.; Konar, S.; Bharti, R.; Dey, G.; Mazumdar, A.; Pathak, A.; Fisher, P.B. Overcoming Akt induced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multila-mellar gold niosomes. Mol. Pharm. 2015, 12, 4214–4225. [Google Scholar] [CrossRef]
- Üner, M. Characterization and Imaging of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. Handb. Nanoparticles 2016, 117–141. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, I.; Akhter, S.; Jain, G.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J. Nanocarrier based formulation of Thy-moquinone improves oral delivery: Stability assessment, in vitro and in vivo studies. Colloids Surf. B 2013, 102, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Surekha, R.; Aishwarya, V.; Sumathi, T. Thymoquinone loaded solid lipid nanoparticle: Formulation, characterization and in-vitro cell viability assay. Int. J. Pharm. Bio. Sci. 2014, 6, 449–464. [Google Scholar]
- Badawy, M.E.; Rabea, E.I. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant patho-gens and their applications in crop protection. Int. J. Carbohydr. Chem. 2011, 2011. [Google Scholar] [CrossRef]
- Cheba, B.A. Chitin and chitosan: Marine biopolymers with unique properties and versatile applications. Glob. J. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Othman, N.; Masarudin, M.J.; Kuen, C.Y.; Dasuan, N.A.; Abdullah, L.C. Synthesis and optimization of chitosan nanoparti-cles loaded with L-ascorbic acid and thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymo-quinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomed. 2012, 7, 5705. [Google Scholar] [CrossRef]
- Talib, W.H. Regressions of Breast Carcinoma Syngraft Following Treatment with Piperine in Combination with Thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef]
- Dastjerdi, M.N.; Mehdiabady, E.M.; Iranpour, F.G.; Bahramian, H. Effect of thymoquinone on P53 gene expression and con-sequence apoptosis in breast cancer cell line. Int. J. Prev. Med. 2016, 7. [Google Scholar] [CrossRef]
- Yıldırım, I.H.; Azzawri, A.A.; Duran, T. Thymoquinone induces apoptosis via targeting the Bax/BAD and Bcl-2 pathway in breast cancer cells. Dicle Tıp Derg. 2019, 46, 411–417. [Google Scholar] [CrossRef]
- Fatfat, M.; Fakhoury, I.; Habli, Z.; Mismar, R.; Gali-Muhtasib, H. Thymoquinone enhances the anticancer activity of doxoru-bicin against adult T-cell leukemia in vitro and in vivo through ROS-dependent mechanisms. Life Sci. 2019, 232, 116628. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Hsu, A.; Hui, K.M.; Tan, B.K.; Sethi, G. Abstract 4123: Thymoquinone inhibits bone metastasis in a breast cancer mouse model by modulating CXCR4/CXCL12 signaling axis. Tumor Biology 2016, 76, 4123. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate an-giogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322. [Google Scholar] [CrossRef] [PubMed]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in tri-ple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thy-moquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumor Biol. 2016, 37, 4467–4477. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, W.; Sun, C.; Liu, Q.; Wang, J.; Wang, M. miR‑145‑5p inhibits epithelial‑mesenchymal transition via the JNK signaling pathway by targeting MAP3K1 in non‑small cell lung cancer cells. Oncol. Lett. 2017, 14, 6923–6928. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kuang, X.-R.; Lv, P.-T.; Yan, X.-X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2014, 36, 259–269. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; A Fahmy, U.; Alruwaili, N.K.; Awan, Z.A.; Caruso, G.; Alfaleh, M.A.; Alaofi, A.L.; Arif, F.O.; Ahmed, A.A. Thymoquinone-Loaded Soy-Phospholipid-Based Phytosomes Exhibit Anticancer Potential against Human Lung Cancer Cells. Pharmaceutics 2020, 12, 761. [Google Scholar] [CrossRef]
- Hussein, S.A.; Abdel-Aal, S.A.; Amin, A.; Khalaf, H.A. Caspase-3, Bcl-2, p53, CYP1A1 and COX-2 as a potential target in chemoprevention of Benzo (a) pyrene-induced lung carcinogenesis in mice: Role of thymoquinone. Nat. Sci. 2016, 4, 430–441. [Google Scholar]
- Dera, A.A.; Rajagopalan, P.; Al Fayi, M.; Ahmed, I.; Chandramoorthy, H.C. Indirubin-3-monoxime and thymoquinone ex-hibit synergistic efficacy as therapeutic combination in in-vitro and in-vivo models of Lung cancer. Arch. Pharm. Res. 2020, 43, 655–665. [Google Scholar] [CrossRef]
- Singh, S.K.; Mishra, M.K.; Lillard, J.W.; Singh, R. Thymoquinone enhanced the tumoricidal activity of NK Cells against Lung Cancer. Am. Assoc. Immnol. 2018, 200, 124.5. [Google Scholar]
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tu-mor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46. [Google Scholar] [CrossRef]
- Pazhouhi, M.; Sariri, R.; Rabzia, A.; Khazaei, M. Thymoquinone synergistically potentiates temozolomide cytotoxicity through the inhibition of autophagy in U87MG cell line. Iran. J. Basic. Med. Sci. 2016, 19, 890–898. [Google Scholar]
- Bitarafan, S.; Yari, M.; Broumand, M.A.; Ghaderian, S.M.H.; Rahimi, M.; Mirfakhraie, R.; Azizi, F.; Omrani, M.D. Association of Increased Levels of lncRNA H19 in PBMCs with Risk of Coronary Artery Disease. Cell J. 2018, 20, 564–568. [Google Scholar]
- Khalife, R.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Thymoquinone from Nigella sativa Seeds Promotes the Antitumor Activity of Noncytotoxic Doses of Topotecan in Human Colorectal Cancer Cells in Vitro. Planta Med. 2016, 82, 312–321. [Google Scholar] [CrossRef]
- Dirican, A.; Atmaca, H.; Bozkurt, E.; Erten, C.; Karaca, B.; Uslu, R. Novel combination of docetaxel and thymoquinone in-duces synergistic cytotoxicity and apoptosis in DU-145 human prostate cancer cells by modulating PI3K–AKT pathway. Clin. Transl. Oncol. 2015, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Wei, C.; Cheng, J.; Khan, A.; Fu, S.; Yang, L.; Tania, M.; Zhang, X.; Xiao, X.; Zhang, X.; et al. MicroRNA-34a targets epithelial to mesenchymal transition-inducing transcription factors (EMT-TFs) and inhibits breast cancer cell migration and invasion. Oncotarget 2017, 8, 21362–21379. [Google Scholar] [CrossRef]
- Feng, L.-M.; Wang, X.-F.; Huang, Q.-X. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017, 42, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hu, X.; Li, J.; Wu, D.; Lan, Q.; Wang, Q.; Tian, S.; Dong, W. Enhancing conventional chemotherapy drug cispla-tin-induced anti-tumor effects on human gastric cancer cells both in vitro and in vivo by thymoquinone targeting PTEN gene. Oncotarget 2017, 8, 85926. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Lee, N.-H.; Hsu, H.-H.; Ho, T.-J.; Tu, C.-C.; Hsieh, D.J.-Y.; Lin, Y.-M.; Chen, L.-M.; Kuo, W.-W.; Huang, C.-Y. Thymoquinone Induces Caspase-Independent, Autophagic Cell Death in CPT-11-Resistant LoVo Colon Cancer via Mitochondrial Dysfunction and Activation of JNK and p38. J. Agric. Food Chem. 2015, 63, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, T.; Ndreshkjana, B.; Muenzner, J.K.; Reiter, C.; Hofmeister, E.; Mederer, S.; Fatfat, M.; El-Baba, C.; Gali-Muhtasib, H.; Schneider-Stock, R. Synthesis of novel hybrids of thymoquinone and artemisinin with high activity and selec-tivity against colon cancer. ChemMedChem 2017, 12, 226–234. [Google Scholar] [CrossRef]
- Chen, M.-C.; Lee, N.-H.; Hsu, H.; Ho, T.-J.; Tu, C.; Chen, R.-J.; Lin, Y.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2016, 32, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, Y.; Yang, Y. Thymoquinone chemosensitizes colon cancer cells through inhibition of NF-κB. Oncol. Lett. 2016, 12, 2840–2845. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Refaat, B.A.; El-Shemi, A.G.; Kensara, O.A.; Ahmad, J.; Idris, S. Thymoquinone potentiates chemoprotec-tive effect of Vitamin D3 against colon cancer: A pre-clinical finding. Am. J. Transl. Res. 2017, 9, 774. [Google Scholar] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA A Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Kou, B.; Liu, W.; Zhao, W.; Duan, P.; Yang, Y.; Yi, Q.; Guo, F.; Li, J.; Zhou, J.; Kou, Q. Thymoquinone inhibits epitheli-al-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol. Rep. 2017, 38, 3592–3598. [Google Scholar] [PubMed]
- Singh, S.K.; Lillard Jr, J.W.; Singh, R. Thymoquinone regulates cytochrome P450 genes involved in prostate cancer disparity. Cancer Epidemiol. Biomark. Prev. 2018. [Google Scholar] [CrossRef]
- Singh, S.K.; Apata, T.; Gordetsky, J.B.; Singh, R. Docetaxel Combined with Thymoquinone Induces Apoptosis in Prostate Cancer Cells via Inhibition of the PI3K/AKT Signaling Pathway. Cancers 2019, 11, 1390. [Google Scholar] [CrossRef]
- Al-Trad, B.; Al-Zoubi, M.; Qar, J.; Al-Batayneh, K.; Hussien, E.; Muhaidat, R.; Aljabali, A.; Alkhateeb, H.; Al Omari, G. In-hibitory effect of thymoquinone on testosterone-induced benign prostatic hyperplasia in Wistar rats. Phytother. Res. 2017, 31, 1910–1915. [Google Scholar] [CrossRef]
- Ranjbari, A.; Heidarian, E.; Ghatreh-Samani, K. Effects of Thymoquinone on IL-6 Gene Expression and Some Cellular Signaling Pathways in Prostate Cancer PC3 Cells. Jundishapur J. Nat. Pharm. Prod. 2017, 12. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, D.-H.; Ha, E.; Choi, S.M.; Choi, J.-S.; Chun, K.-S.; Joo, S.H. Thymoquinone induces apoptosis of human epi-dermoid carcinoma A431 cells through ROS-mediated suppression of STAT3. Chem. Biol. Interact. 2019, 312, 108799. [Google Scholar] [CrossRef]
- Jeong, H.; Yu, S.; Kim, S.J. Inhibitory effects on melanogenesis by thymoquinone are mediated through the β‑catenin pathway in B16F10 mouse melanoma cells. Int. J. Oncol. 2019, 56, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Luo, W. Exploration of pro-apoptotic effect of Thymoquinone on oral squamous cell carcinoma cells through PI3K/Akt signaling pathway. Cell. Mol. Biol. 2019, 65, 61–64. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Koçyiğit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tüzgen, S. Thymoquinone Induces Apoptosis in B16-F10 Melanoma Cell Through Inhibition of p-STAT3 and Inhibits Tumor Growth in a Murine Intracerebral Melanoma Model. World Neurosurg. 2018, 114, e182–e190. [Google Scholar] [CrossRef]
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Khan, I.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone Enhances the Effect of Gamma Knife in B16-F10 Melanoma Through Inhibition of Phosphorylated STAT3. World Neurosurg. 2019, 128, e570–e581. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Jayaraman, M.; Radhakrishnan, R.; Gomathinayagam, R.; Yan, M.; Song, Y.S.; Isidoro, C.; Dhanasekaran, D.N. Differential effects of thymoquinone on lysophosphatidic acid-induced oncogenic pathways in ovarian cancer cells. J. Tradit. Complement. Med. 2020, 10, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, J.; Cai, W.; Pan, Y.; Li, R.; Li, B. The Effect of Thymoquinone on Apoptosis of SK-OV-3 Ovarian Cancer Cell by Regulation of Bcl-2 and Bax. Int. J. Gynecol. Cancer 2017, 27, 1596–1601. [Google Scholar] [CrossRef]
- İnce, I.; Yıldırım, Y.; Güler, G.; Medine, E.I.; Ballıca, G.; Kuşdemir, B.C.; Göker, E. Synthesis and characterization of folic ac-id-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J. Radioanal. Nucl. Chem. 2020, 324, 1–15. [Google Scholar] [CrossRef]
- Johnson-Ajinwo, O.R.; Richardson, A.; Li, W.-W. Synthesis and evaluation of thymoquinone analogues as anti-ovarian can-cer agents. MDPI 2019, 22, 42. [Google Scholar]
- Alhassani, M.Y.; Zohny, S.F.; Sheikh, R.A.; Hassan, M.A.; Kalantan, A.A.; Hosawi, S.; Alhosin, M. Thymoquinone exerts anti-tumor activities on human hepatocellular carcinoma cells: Role of angiogenesis-related genes VCAN, Grb2 and EZH2. Eur. J. Cell Sci. 2019, 10–16. [Google Scholar] [CrossRef]
- Meral, I.; Pala, M.; Akbas, F.; Ustunova, S.; Yildiz, C.; Demirel, M. Effects of thymoquinone on liver miRNAs and oxidative stress in Ehrlich acid mouse solid tumor model. Biotech. Histochem. 2018, 93, 301–308. [Google Scholar] [CrossRef]
- Helmy, S.A.; El-Mesery, M.; El-Karef, A.; Eissa, L.A.; El Gayar, A.M. Thymoquinone upregulates TRAIL/TRAILR2 expression and attenuates hepatocellular carcinoma in vivo model. Life Sci. 2019, 233, 116673. [Google Scholar] [CrossRef] [PubMed]
- Haron, A.S.; Syed Alwi, S.S.; Saiful Yazan, L.; Abd Razak, R.; Ong, Y.S.; Zakarial Ansar, F.H.; Roshini Alexander, H. Cyto-toxic effect of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) on liver cancer cell integrated with hepatitis B genome, Hep3B. Evid. Based Complement Alternat. Med. 2018. [Google Scholar] [CrossRef]
- Bashir, A.O.; El-Mesery, M.E.; Anwar, R.; Eissa, L.A. Thymoquinone potentiates miR-16 and miR-375 expressions in hepato-cellular carcinoma. Life Sci. 2020, 117794. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37. [Google Scholar] [CrossRef]
- Li, J.; Khan, A.; Wei, C.; Cheng, J.; Chen, H.; Yang, L.; Ijaz, I.; Fu, J. Thymoquinone Inhibits the Migration and Invasive Characteristics of Cervical Cancer Cells SiHa and CaSki In Vitro by Targeting Epithelial to Mesenchymal Transition Associated Transcription Factors Twist1 and Zeb1. Molecules 2017, 22, 2105. [Google Scholar] [CrossRef]
- Ng, W.K.; Saiful Yazan, L.; Yap, L.H.; Wan, N.H.; Wan, A.G.; How, C.W.; Abdullah, R. Thymoquinone-loaded nanostruc-tured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). BioMed Res. Int. 2015. [Google Scholar] [CrossRef]
- Houssein, M.; Fatfat, M.; Habli, Z.; Ghazal, N.; Mokdad, S.; Khalife, H.; Khalil, M.; Gali-Muhtasib, H. Thymoquinone syner-gizes with arsenic and interferon alpha to target human T-cell leukemia/lymphoma. Life Sci. 2020, 117639. [Google Scholar] [CrossRef]
- Musalli, M.G.; Hassan, M.A.; Sheikh, R.A.; Kalantan, A.A.; Halwani, M.A.; Zeyadi, M.; Hosawi, S.; Alhosin, M. Thymo-quinone induces cell proliferation inhibition and apoptosis in acute myeloid leukemia cells: Role of apoptosis-related WT1 and BCL2 genes. Eur. J. Cell Sci. 2019, 1, 2–9. [Google Scholar] [CrossRef]
- Alaufi, O.M.; Noorwali, A.; Zahran, F.; Al-Abd, A.M.; Al-Attas, S. Cytotoxicity of thymoquinone alone or in combination with cisplatin (CDDP) against oral squamous cell carcinoma in vitro. Sci. Rep. 2017, 7, 13131. [Google Scholar] [CrossRef]
- Kotowski, U.; Heiduschka, G.; Kadletz, L.; Fahim, T.; Seemann, R.; Schmid, R.; Schneider, S.; Mitterbauer, A.; Thurnher, D. Effect of thymoquinone on head and neck squamous cell carcinoma cells in vitro: Synergism with radiation. Oncol. Lett. 2017, 14, 1147–1151. [Google Scholar] [CrossRef]
- Khan, A.; Tania, M.; Fu, J. Epigenetic role of thymoquinone: Impact on cellular mechanism and cancer therapeutics. Drug Discov. Today 2019, 24, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Yeh, S.-J.; Lin, C.-U.; Li, C.-W.; Chen, B.-S. Systems Biology Approaches to Investigate Genetic and Epigenetic Molecular Pro-gression Mechanisms for Identifying Gene Expression Signatures in Papillary Thyroid Cancer. Int. J. Mol. Sci. 2019, 20, 2536. [Google Scholar] [CrossRef]
- Parbin, S.; Shilpi, A.; Kar, S.; Pradhan, N.; Sengupta, D.; Deb, M.; Rath, S.K.; Patra, S.K. Insights into the molecular interac-tions of thymoquinone with histone deacetylase: Evaluation of the therapeutic intervention potential against breast cancer. Mol. Biosyst. 2016, 12, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H. Thymoquinone promotes pancreatic cancer cell death and reduc-tion of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.P.; Zayasbazan Burgos, D.; Yuan, T.; Seeram, N.; Rebar, R.; Follmer, R.; Heart, E.A. Thymoquinone, a bioactive com-ponent of Nigella sativa, normalizes insulin secretion from pancreatic β-cells under glucose overload via regulation of malo-nyl-CoA. Am. J. Physiol. 2016, 310, E394–E404. [Google Scholar]
- Gonfloni, S.; Iannizzotto, V.; Maiani, E.; Bellusci, G.; Ciccone, S.; Diederich, M. P53 and Sirt1: Routes of metabolism and ge-nome stability. Biochem. Pharmacol. 2014, 92, 149–156. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Hong, D.; Nam, S.H.; Kim, J.M.; Paik, Y.H.; Joh, J.W.; Kwon, C.D.; Park, J.B.; Choi, G.-S.; Jang, K.Y. SIRT1 regulates oncogenesis via a mutant p53-dependent pathway in hepatocellular carcinoma. J. Hepatol. 2015, 62, 121–130. [Google Scholar] [CrossRef]
- Karandrea, S.; Yin, H.; Liang, X.; Slitt, A.L.; Heart, E.A. Thymoquinone ameliorates diabetic phenotype in Diet-Induced Obe-sity mice via activation of SIRT-1-dependent pathways. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, Y.; Liu, D.; Zhang, Z.; Gao, K.; Zhang, W.; Tang, H. Thymoquinone Attenuates Myocardial Ischemia/Reperfusion Injury Through Activation of SIRT1 Signaling. Cell. Physiol. Biochem. 2018, 47, 1193–1206. [Google Scholar] [CrossRef]
- Alhosin, M. Thymoquinone is a novel potential inhibitor of SIRT1 in cancers with p53 mutation: Role in the reactivation of tumor suppressor p73. World Acad. Sci. J. 2020, 2, 1. [Google Scholar] [CrossRef]
- Khan, M.D.; Zheng, M.; Fu, J. Epigenetic modification of oncogenes or tumor suppressor genes by thymoquinone in triple negative breast cancer. AACR 2019. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Tao, Y.W.; Gao, S.; Li, P.; Zheng, J.-M.; Zhang, S.-E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.-H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, srep42995. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Maysarah, N.M.; El-Sherbiny, M.; Al-Gayyar, M.M. Renal protective effects of thymoquinone against so-dium nitrite-induced chronic toxicity in rats: Impact on inflammation and apoptosis. Life Sci. 2017, 180, 1–8. [Google Scholar] [CrossRef]
- Amin, B.; Hosseinzadeh, H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: An overview on the anal-gesic and anti-inflammatory effects. Planta Med. 2016, 82, 8–16. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Sayed, A.A.; Abdeen, A.; Aleya, L.; Ali, D.; Alkahtane, A.A.; Alarifi, S.; Alkahtani, S. Piperine enhances the antioxidant and anti-inflammatory activities of thymoquinone against microcystin-LR-induced hepatotoxicity and neu-rotoxicity in mice. Oxid. Med. Cell Longev. 2019. [Google Scholar] [CrossRef]
- Amartey, J.; Gapper, S.; Hussein, N.; Morris, K.; Withycombe, C.E. Nigella sativa Extract and Thymoquinone Regulate In-flammatory Cytokine and TET-2 Expression in Endothelial Cells. Artery Res. 2019, 25, 157–163. [Google Scholar] [CrossRef]
- Fan, Z.; Duan, J.; Wang, L.; Xiao, S.; Li, L.; Yan, X.; Yao, W.; Wu, L.; Zhang, S.; Zhang, Y.; et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/β-catenin signaling. Cancer Lett. 2019, 450, 132–143. [Google Scholar] [CrossRef]
- Jiao, Q.; Bi, L.; Ren, Y.; Song, S.; Wang, Q.; Wang, Y.-S. Advances in studies of tyrosine kinase inhibitors and their acquired resistance. Mol. Cancer 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.M.; Silveira, C.D.; Rezende, A.S.; Silva, R.O.; Crema, V.O. Tyrosine kinase inhibitor TKI-258 inhibits cell motility in oral squamous cell carcinoma in vitro. J. Oral Pathol. Med. 2017, 46, 484–488. [Google Scholar] [CrossRef]
- Tong, Y.; Huang, C.; Zhang, J. A novel EGFR-TKI inhibitor (cAMP-H3BO3complex) combined with thermal therapy is a promising strategy to improve lung cancer treatment outcomes. Oncotarget 2017, 8, 56327. [Google Scholar] [CrossRef][Green Version]
- Botting, G.M.; Rastogi, I.; Chhabra, G.; Nlend, M.; Puri, N. Mechanism of Resistance and Novel Targets Mediating Resistance to EGFR and c-Met Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PLoS ONE 2015, 10, e0136155. [Google Scholar] [CrossRef] [PubMed]
- Namba, K.; Shien, K.; Yoshioka, T.; Torigoe, H.; Sato, H.; Yamamoto, H.; Soh, J.; Tsukuda, K.; Toyooka, S. Abstract 3156: Multiple acquired resistance mechanisms against third generationEGFR-TKI osimeritinib in non-smal cell lung cancer cells. Exp. Mol. Ther. 2017, 77, 3156. [Google Scholar] [CrossRef]
- Chen, M.; Zang, M.; Guo, X. MicroRNA-106a-5p functions as an oncogene via regulating PTEN in breast cancer cells. Int. J. Clin. Exp. Med. 2019, 12, 8044–8056. [Google Scholar]
- Ni, J.; Zhou, L.-L.; Ding, L.; Zhao, X.; Cao, H.; Fan, F.; Li, H.; Lou, R.; Du, Y.; Dong, S.; et al. PPARγ agonist efatutazone and gefitinib synergistically inhibit the proliferation of EGFR-TKI-resistant lung adenocarcinoma cells via the PPARγ/PTEN/Akt pathway. Exp. Cell Res. 2017, 361, 246–256. [Google Scholar] [CrossRef] [PubMed]
| Type | Disease Models | References |
|---|---|---|
| sulfur-containing TQ-analogs | radiation-induced dyslipidemia in rats | [30] |
| nitrogen-substituted TQ analogues | human ovarian cancer cell lines | [31] |
| 3-aminothymoquinone | antifungal effect against Candida albicans, Saccharomyces cerevisiae and Aspergillus brasiliensis | [32] |
| Name of Drug | Action of Drug | References |
|---|---|---|
| Cisplatin | Induction of DNA damage through Pt-mediated DNA crosslinking (Alkylating-like mechanism) | [71] |
| Temozolomide (TMZ) | DNA damage through alkylation and cell cycle arrest at G2/M phase | [72] |
| Tamoxifen (TAM) | Anti-estrogens (compete with estrogen to bind with estrogen receptor) | [73] |
| Topotecan (TP) | Topoisomerase-I inhibitor | [74] |
| Paclitaxel (Pac) | Interfere in mitotic spindle formation through stabilization of microtubule assembly | [64] |
| Docetaxel | Microtubule disrupting agent | [75] |
| miR-34a | MicroRNA | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. https://doi.org/10.3390/ph14040369
Almajali B, Al-Jamal HAN, Taib WRW, Ismail I, Johan MF, Doolaanea AA, Ibrahim WN. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals. 2021; 14(4):369. https://doi.org/10.3390/ph14040369
Chicago/Turabian StyleAlmajali, Belal, Hamid Ali Nagi Al-Jamal, Wan Rohani Wan Taib, Imilia Ismail, Muhammad Farid Johan, Abd Almonem Doolaanea, and Wisam Nabeel Ibrahim. 2021. "Thymoquinone, as a Novel Therapeutic Candidate of Cancers" Pharmaceuticals 14, no. 4: 369. https://doi.org/10.3390/ph14040369
APA StyleAlmajali, B., Al-Jamal, H. A. N., Taib, W. R. W., Ismail, I., Johan, M. F., Doolaanea, A. A., & Ibrahim, W. N. (2021). Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals, 14(4), 369. https://doi.org/10.3390/ph14040369