Budget Impact Analysis of Biosimilar Products in Spain in the Period 2009–2019
Abstract
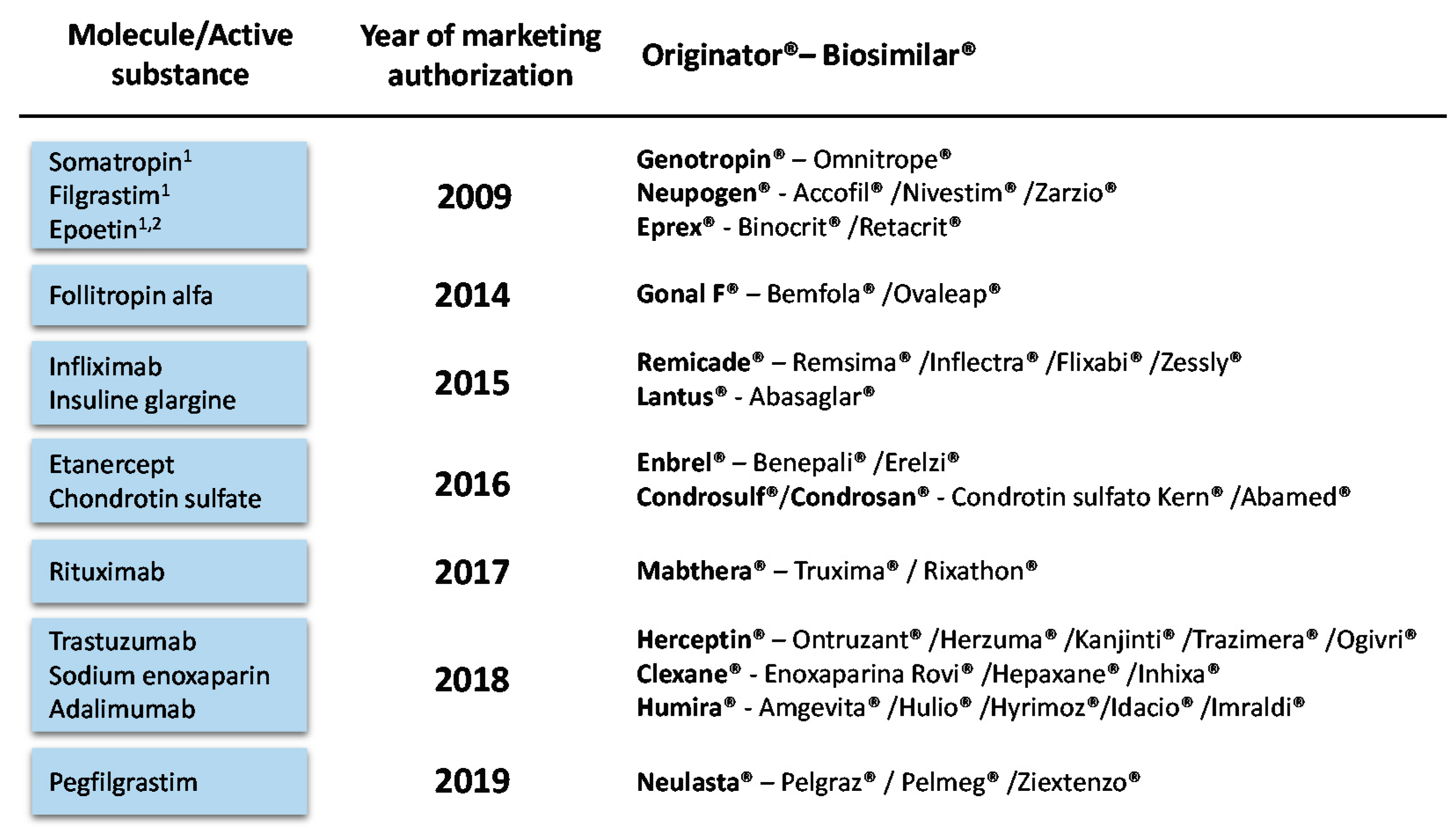
1. Introduction
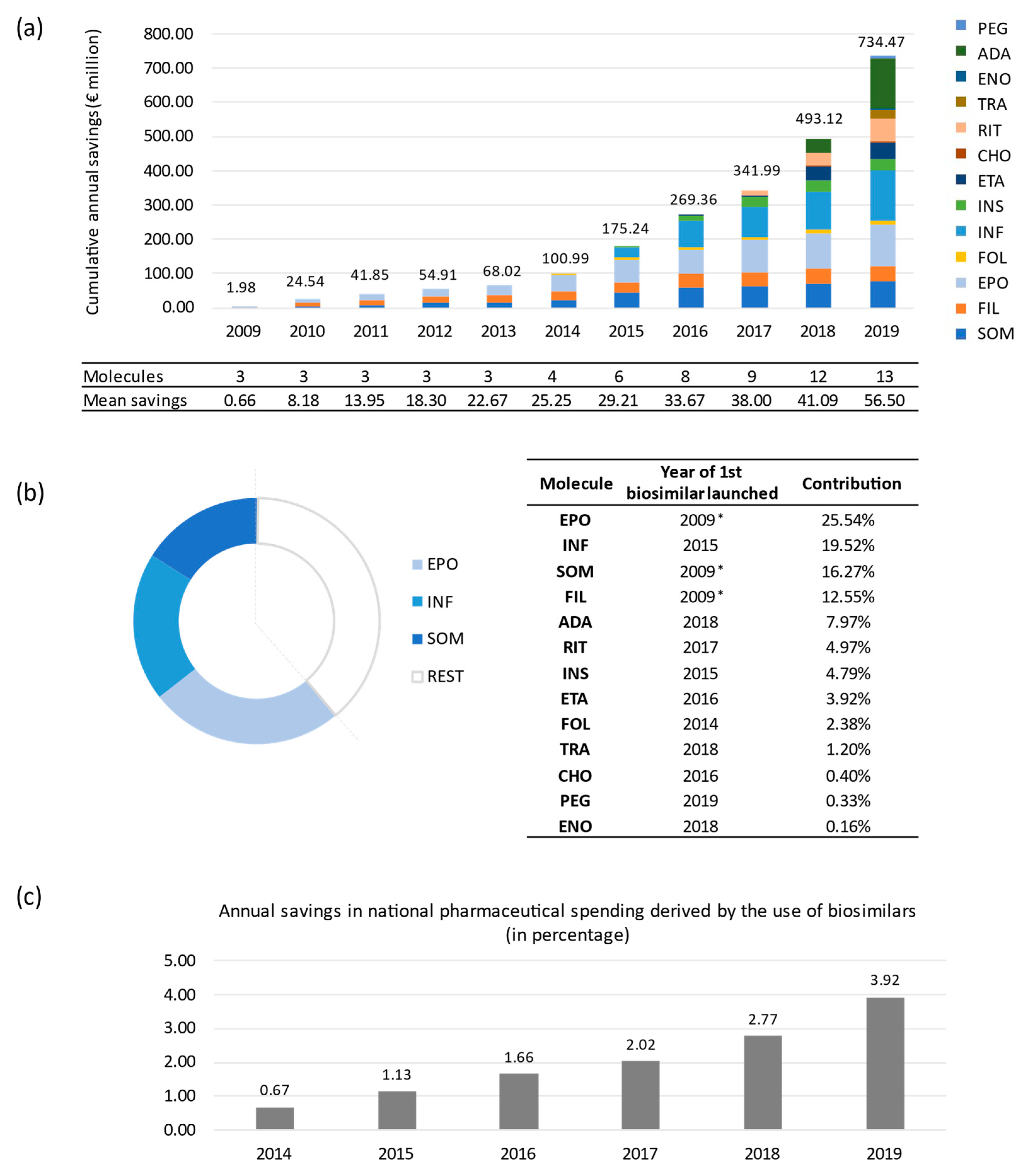
2. Results
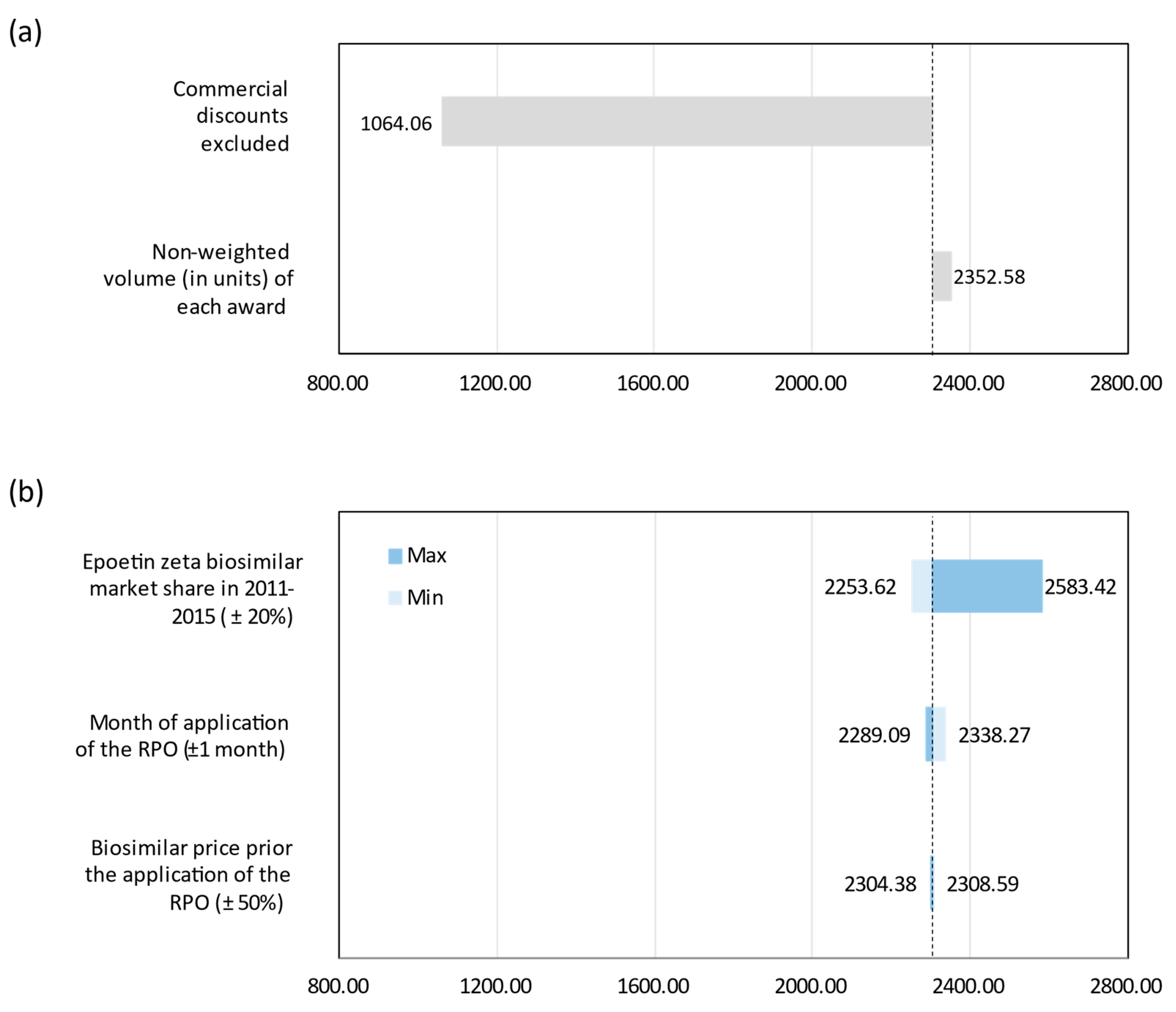
Sensitivity Analysis
3. Discussion
Limitations
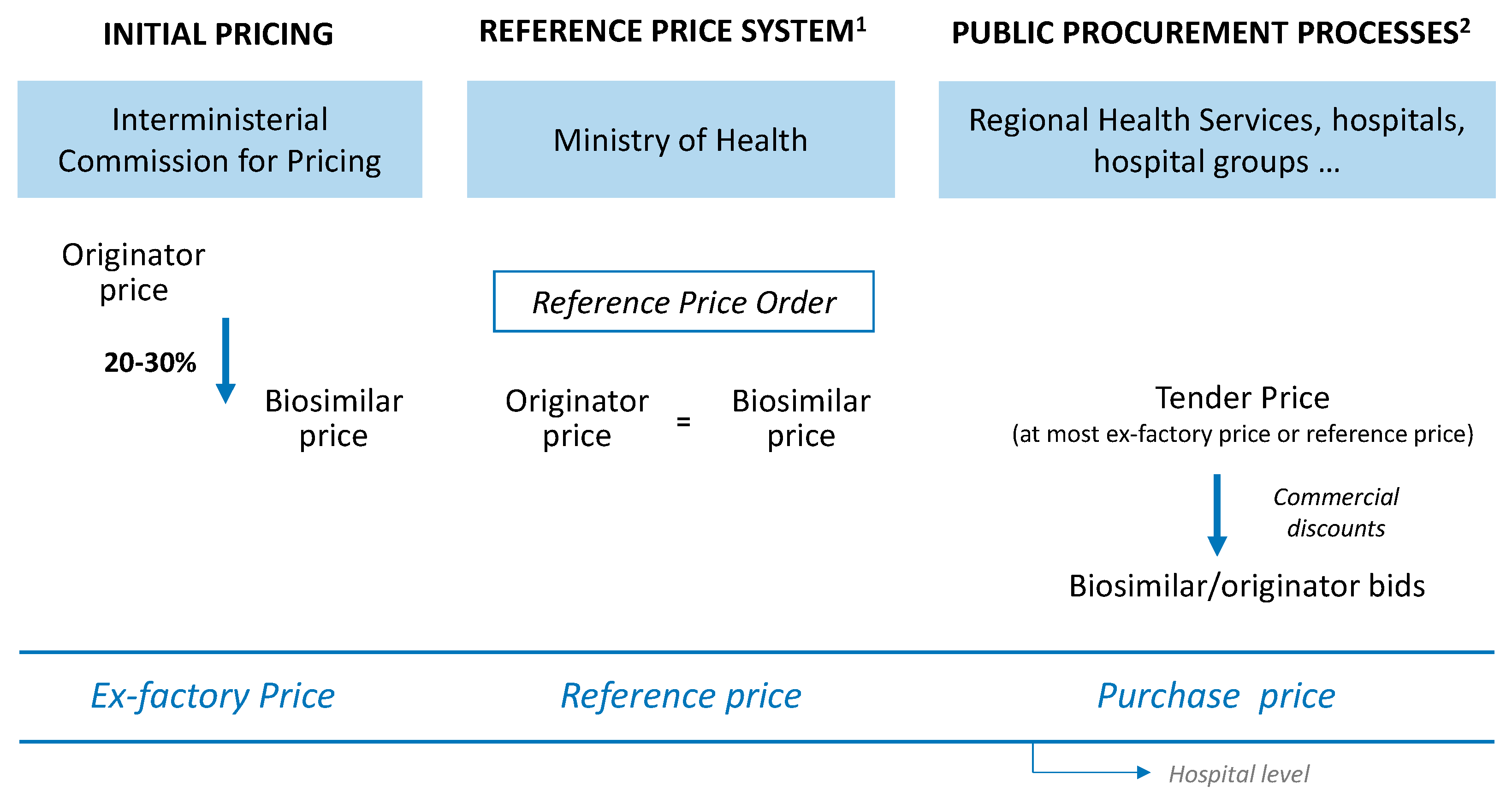
4. Materials and Methods
4.1. Model Design
4.2. Uptake Estimation
4.3. Price Estimation
4.4. Molecule-Specific Assumptions
4.5. Sensitivity Analsyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaw, J.; Horrace, W.; Vogel, R. The determinants of life expectancy: An analysis of the OECD health data. South. Econ. J. 2005, 71, 768–783. Available online: https://www.researchgate.net/publication/23545170_The_Determinants_of_Life_Expectancy_An_Analysis_of_the_OECD_Health_Data (accessed on 3 June 2020). [CrossRef]
- Powrie-Smith, A. From innovation to outcomes. In Medicines Costs in Context; European Federation of Pharmaceutical Industries and Associations: Brussels, Belgium, 2016; Available online: https://www.efpia.eu/about-medicines/use-of-medicines/value-of-medicines/ (accessed on 3 June 2020).
- Lichtenberg, F. Pharmaceutical innovation and longevity growth in 30 developing OECD and high-income countries, 2000–2009. Health Policy Technol. 2014, 3, 36–58. Available online: https://www.nber.org/system/files/working_papers/w18235/w18235.pdf (accessed on 3 June 2020). [CrossRef]
- Lichtenberg, F. The Benefits of Pharmaceutical Innovation: Health, Longevity, and Savings; Montreal Economic Institute: Montreal, QC, Canada, 2016; ISBN 978-2-922687-66-8. Available online: http://www.vises.org.au/documents/2016_Lichtenberg_Benefits_of_Pharma_Innovation.pdf (accessed on 3 June 2020).
- Newhouse, J.P. Medical Care Costs: How much Welfare Loss? J. Econ. Perspect. 1992, 6, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Willeme, P.; Dumont, M. Machines that go ‘ping’: Medical technology and health expenditures in OECD countries. Health Econ. 2015, 24, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- OECD. Improving Forecasting of Pharmaceutical Spending-Insights from 23 OECD and EU Countries; OECD: Paris, France, 2019; Available online: https://ec.europa.eu/health/sites/health/files/policies/docs/pharmaceutical-expenditure-analytical-report-april-2019_en.pdf (accessed on 8 February 2021).
- IQVIA Institute. The Global Use of Medicine in 2019 and Outlook to 2023. Forecasts and Areas to Watch. 2019. Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (accessed on 3 June 2020).
- Espin, J.; Schlander, M.; Godman, B.; Anderson, P.; Mestre-Ferrandiz, J.; Borget, I.; Hutchings, A.; Flostrand, S.; Parnaby, A.; Jommi, C. Projecting Pharmaceutical Expenditure in EU5 to 2021: Adjusting for the Impact of Discounts and Rebates. Appl. Health Econ. Health Policy 2018, 16, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Farfan-Portet, M.I.; Gerkens, S.; Lepage-Nefkens, I.; Vinck, I.; Hulstaert, F. Are biosimilars the next tool to guarantee cost-containment for pharmaceutical expenditures? Eur. J. Health Econ. 2014, 15, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Pugatch Consilium. Towards a Sustainable European Market for Off-Patent Biologics. Available online: https://www.pugatch-consilium.com/?p=2760 (accessed on 3 June 2020).
- IQVIA Institute. The Impact of Biosimilar Competition in Europe. 2019. Available online: https://ec.europa.eu/docsroom/documents/38461 (accessed on 3 June 2020).
- EMA (European Medicines Agency); European Commission. Biosimilars in the EU. Information Guide for Healthcare Professionals; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf (accessed on 3 June 2020).
- Biosimilar Medicines. Available online: https://www.ema.europa.eu/en/medicines/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar (accessed on 31 December 2020).
- Centro de Información Online de Medicamentos de la Agencia Española de Medicamentos Sanitarios (CIMA). Available online: https://cima.aemps.es/cima/publico/lista.html (accessed on 31 December 2020).
- Simoens, S.; Jacobs, I.; Popovian, R.; Isakov, L.; Shane, L.G. Assessing the Value of Biosimilars: A Review of the Role of Budget Impact Analysis. Pharm. Econ. 2017, 35, 1047–1062. [Google Scholar] [CrossRef] [PubMed]
- Rognoni, C.; Bertolani, A.; Jommi, C. Budget Impact Analysis of Rituximab Biosimilar in Italy from the Hospital and Payer Perspectives. Glob. Reg. Health Technol. Assess. 2018, XX, I–II. Available online: https://journals.sagepub.com/doi/pdf/10.1177/2284240318784289 (accessed on 3 June 2020). [CrossRef]
- Piras, M.; Naddeo, C.; Bettio, M.; Dragui, R.; Venturini, F. 1ISG-039 The cost-savings potential of biosimilar drugs: A budget impact analysis. Eur. J. Hosp. Pharm. 2019, 26, A18. Available online: https://ejhp.bmj.com/content/ejhpharm/26/Suppl_1/A18.2.full.pdf (accessed on 3 June 2020).
- Ravasio, R.; Mazzi, S.; Esposito, M.; Fiorino, G.; Migliore, A. A Budget impact analysis of adalimumab biosimilar: The Italian context. AboutOpen 2019, 5, 16–23. Available online: https://journals.aboutscience.eu/index.php/aboutopen/article/view/280 (accessed on 3 June 2020). [CrossRef][Green Version]
- Aladul, M.I.; Fitzpatrick, R.W.; Chapman, S.R. The effect of new biosimilars in rheumatology and gastroenterology specialties on UK healthcare budgets: Results of a budget impact analysis. Res. Soc. Adm. Pharm. 2019, 15, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Agirrezabal, I.; Sánchez-Iriso, E.; Mandar, K.; Cabasés, J.M. Real-World Budget Impact of the Adoption of Insulin Glargine Biosimilars in Primary Care in England (2015–2018). Diabetes Care 2020, 43, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Mansell, K.; Bhimji, H.; Eurich, D.; Mansell, H. Potential cost-savings from the use of the biosimilars filgrastim, infliximab and insulin glargine in Canada: A retrospective analysis BMC. Health Serv. Res. 2019, 19, 827. [Google Scholar] [CrossRef] [PubMed]
- Patented Medicine Prices Review Board. Biologics in Canada Part 2: Biosimilar Savings, 2018. Chartbook. National Prescription Drug Utilization Information System. 2020. Available online: https://www.canada.ca/content/dam/pmprb-cepmb/documents/reports-and-studies/chartbooks/biologics-part2-biosimilar-savings2018.pdf (accessed on 3 June 2020).
- González Domínguez, A.; Ivanova Markova, Y.; Zozaya Gonzále, N.; Jiménez Torres, M.; Hidalgo Vega, A.; La Introducción de los Biosimilares en España. Estimación del Ahorro Para el Sistema Nacional de Salud; Fundación Weber: Madrid, España, 2017; Available online: http://weber.org.es/wp-content/uploads/2018/04/DT-002-Introducci%C3%B3n-de-los-Biosimilares-en-Espa%C3%B1a_vf.pdf (accessed on 3 June 2020).
- IQVIA Institute for Human Data Science (2018). Advancing Biosimilar Sustainability in Europe. September 2018. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institutereports/advancing-biosimilar-sustainability-in-europe.pdf (accessed on 3 June 2020).
- Real Decreto 177/2014, de 21 de Marzo, por el que se Regula el Sistema de Precios de Referencia y de Agrupaciones Homogéneas de Medicamentos en el Sistema Nacional de Salud, y Determinados Sistemas de Información en Materia de Financiación y Precios de los Medicamentos y Productos Sanitarios. Available online: https://www.boe.es/boe/dias/2014/03/25/pdfs/BOE-A-2014-3189.pdf (accessed on 3 June 2020).
- World Health Organization. Medicines Reimbursement Policies in Europe. 2018. Available online: https://www.euro.who.int/__data/assets/pdf_file/0011/376625/pharmaceutical-reimbursement-eng.pdf (accessed on 3 June 2020).
- IQVIA Institute. Evolución y Tendencias del Mercado Farmacéutico Español. 2019. Available online: https://statics-correofarmaceutico.uecdn.es/cms/sites/11/2019/02/evolucionytendencias-iqvia.pdf (accessed on 3 June 2020).
- Ley 9/2017, de 8 de Noviembre, de Contratos del Sector Público, por la que se Transponen al Ordenamiento Jurídico Español las Directivas del Parlamento Europeo y del Consejo 2014/23/UE y 2014/24/UE, de 26 de Febrero de 2014. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2017-12902 (accessed on 3 June 2020).
- Real Decreto Legislativo 3/2011, de 14 de Noviembre, por el que se Aprueba el Texto Refundido de la Ley de Contratos del Sector Público. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2011-17887 (accessed on 3 June 2020).
- Ley 30/2007, de 30 de Octubre, de Contratos del Sector Público. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2007-18874 (accessed on 3 June 2020).
- García-Goñi, M.; Carcedo, D.; Villacampa, A.; Lores, M. Análisis de Impacto Presupuestario de los Medicamentos Biosimilares en el SNS de España 2009–2022. Informe Encargado por Biosim. Madrid, 2020. Available online: https://www.biosim.es/analisis-de-impacto-presupuestario-de-los-medicamentos-biosimilares-en-el-sistema-nacional-de-salud-de-espana-2009-2022/ (accessed on 8 February 2021).
- Ministerio de Sanidad. Prestación Farmacéutica Informe Anual del Sistema Nacional de Salud 2018. Available online: https://www.mscbs.gob.es/estadEstudios/estadisticas/sisInfSanSNS/tablasEstadisticas/InfAnualSNS2018/Cap.7_Farmacia.pdf (accessed on 3 June 2020).
- Ministerio de Hacienda. Serie Gasto Farmacéutico y Sanitario: Periodo Junio-2014 a Enero-2021. Indicadores Sobre Gasto Farmacéutico y Sanitario. 2021. Available online: https://www.hacienda.gob.es/es-ES/CDI/Paginas/EstabilidadPresupuestaria/InformacionAAPPs/Indicadores-sobre-Gasto-Farmacéutico-y-Sanitario.aspx (accessed on 5 April 2021).
- Aladul, M.I.; Fitzpatrick, R.W.; Chapman, S.R. Impact of Infliximab and Etanercept Biosimilars on Biological Disease-Modifying Antirheumatic Drugs Utilization and NHS Budget in the UK. BioDrugs 2017, 31, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Kanters, T.A.; Stevanovic, J.; Huys, I.; Vulto, A.G.; Simoens, S. Adoption of Biosimilar Infliximab for Rheumatoid Arthritis, Ankylosing Spondylitis, and Inflammatory Bowel Diseases in the EU5: A Budget Impact Analysis Using a Delphi Panel. Front Pharmacol. 2017, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Gulácsi, L.; Brodszky, V.; Baji, P.; Rencz, F.; Péntek, M. The Rituximab Biosimilar CT-P10 in Rheumatology and Cancer: A Budget Impact Analysis in 28 European Countries. Adv. Ther. 2017, 34, 1128–1144. [Google Scholar] [CrossRef] [PubMed]
- IQVIA Institute. The Impact of Biosimilar Competition in Europe. 2020. Available online: https://ec.europa.eu/health/sites/health/files/human-use/docs/biosimilar_competition_en.pdf (accessed on 3 February 2021).
- Autoridad Independiente de Responsabilidad Fiscal (AIReF). Evaluación del Gasto Público 2019. Estudio. Gasto Hospitalario del Sistema Nacional de Salud: Farmacia e Inversión en Bienes de Equipo. 2020. Available online: https://www.airef.es/wp-content/uploads/2020/10/SANIDAD/PDF-WEB-Gasto-hospitalario-del-SNS.pdf (accessed on 3 June 2020).
- Ministerio de Sanidad, Consumo y Bienestar Social, Plan de Acción Para Fomentar la Utilización de los Medicamentos Reguladores del Mercado en el Sistema Nacional de Salud: Medicamentos Biosimilares y Medicamentos Genéricos. 11 April 2019. Available online: https://www.mscbs.gob.es/profesionales/farmacia/pdf/PlanAccionSNSmedicamentosReguladoresMercado.pdf (accessed on 3 June 2020).
- Comité Asesor Para la Prestación Farmacéutica. Comentarios Sobre el Documento: Plan de Acción Para Fomentar la Utilización de los Medicamentos Reguladores del Mercado en el Sistema Nacional de Salud: Medicamentos Biosimilares y Medicamentos Genéricos. Documento de Consenso (Emitido el 22 de Mayo, Finalizado el 22 de Julio de 2019). Available online: https://www.mscbs.gob.es/en/profesionales/farmacia/pdf/20190722_Documento_CAPF_consenso_genericos_biosimilares.pdf (accessed on 8 February 2021).
- Almarza, C. Mercado de Medicamentos Biosimilares. Previsiones de Futuro e Impacto Sobre los Sistemas Nacionales de Salud. IMS Health. 2016. Available online: http://www.diariofarma.com/wp-content/uploads/2016/03/03-Concha-Almarza-IMS.pdf. (accessed on 9 April 2021).
- Rovira, J.; Espín, J.; García, L.; de Labry, A.O. The impact of biosimilars’ entry in the EU market. Andal. Sch. Public Health 2011, 30, 1–83. Available online: https://www.researchgate.net/publication/281504554_The_impact_of_biosimilars’_entry_in_the_EU_market (accessed on 3 June 2020).
- Conselleria de Sanitat Univesal I Salut Publica, Comunitat Valenciana. Memoria de Gestión de la Conselleria de Sanitat Universal i Salut Pública. Año 2015. Capítulo 10. Política Farmacéutica. Available online: http://www.san.gva.es/documents/157385/6697728/10.+Pol%C3%ADtica+Farmac%C3%A9utica.pdf (accessed on 3 June 2020).
- Ortega Eslava, A.; Marín Gil, R.; Fraga Fuentes, M.D.; López-Briz, E.; Puigventós Latorre, F.; GENESIS-SEFH. Guía de Evaluación Económica e Impacto Presupuestario en los Informes de Evaluación de Medicamentos; Guía Práctica Asociada al Programa MADRE v 4.0; SEFH: Madrid, Spain, 2016; ISBN 978-84-617-6757-1. Available online: https://gruposdetrabajo.sefh.es/genesis/genesis/Documents/GUIA_EE_IP_GENESIS-SEFH_19_01_2017.pdf (accessed on 3 June 2020).
- Puig-Junoy, J.; Oliva-Moreno, J.; Trapero-Bertrán, M.; Abellán-Perpiñán, J.M.; Brosa-Riestra, M.; Servei Català de la Salut (CatSalut). Guía y Recomendaciones Para la Realización y Presentación de Evaluaciones Económicas y Análisis de Impacto Presu-puestario de Medicamentos en el Ámbito del CatSalut; Generalitat de Catalunya. Departament de Salut. Servei Català de la Salut: Barcelona, España, 2014. Available online: https://scientiasalut.gencat.cat/bitstream/handle/11351/1057/guia_recomanacions_avaluacions_economiques_medicaments_catsalut_2014_cas.pdf?sequence=2&isAllowed=y (accessed on 3 June 2020).
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD International Language for Drug Utilization Research: The Anatomical Therapeutic Chemical (ATC) Classification System. Available online: https://www.whocc.no (accessed on 30 March 2020).
- Haustein, R.; Christoph de Millas, A.H.; Bertram, H. Saving money in the European healthcare systems with biosimilars. GaBI J. 2012, 1, 120–126. Available online: http://gabi-journal.net/saving-money-in-the-european-healthcare-systems-with-biosimilars.html (accessed on 3 June 2020). [CrossRef]
- Base de datos del Consejo General de Colegios Oficiales de Farmacéuticos. 2020. Available online: https://botplusweb.portalfarma.com/botplus.aspx (accessed on 30 March 2020).
- Briggs, A.H. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000, 17, 479–500. [Google Scholar] [CrossRef] [PubMed]


| Molecule 1 | Scenario without Biosimilars | Scenario with Biosimilars | Realized Savings |
| SOM | 992.5 | 617.32 | 375.18 |
| FIL | 469.62 | 180.22 | 289.4 |
| EPO | 993.01 | 403.86 | 589.15 |
| FOL | 119.2 | 64.27 | 54.92 |
| INF | 1054.47 | 604.18 | 450.3 |
| INS | 818.41 | 707.87 | 110.54 |
| ETA | 628.39 | 537.91 | 90.48 |
| CHO | 134.67 | 125.34 | 9.32 |
| RIT | 433.39 | 318.76 | 114.62 |
| TRA | 140.89 | 113.26 | 27.63 |
| ENO | 264.86 | 261.25 | 3.62 |
| ADA | 772.46 | 588.69 | 183.77 |
| PEG | 10.77 | 3.2 | 7.57 |
| TOTAL | 6832.63 | 4526.15 | 2306.48 |
| Molecule 1 | Base Case | Probabilistic Sensitivity Analysis | |||
| Mean | 95% CI | ||||
| SOM | 375.18 | 377.96 | 348.25 | - | 415.67 |
| FIL | 289.40 | 289.27 | 274.55 | - | 303.17 |
| EPO | 589.15 | 590.26 | 546.85 | - | 634.32 |
| FOL | 54.92 | 54.92 | 53.71 | - | 56.07 |
| INF | 450.30 | 450.25 | 448.59 | - | 452.03 |
| INS | 110.54 | 110.40 | 106.08 | - | 115.03 |
| ETA | 90.48 | 90.60 | 83.26 | - | 99.25 |
| CHO | 9.32 | 9.32 | 8.99 | - | 9.68 |
| RIT | 114.62 | 114.63 | 103.36 | - | 126.12 |
| TRA | 27.63 | 27.61 | 26.24 | - | 29.04 |
| ENO | 3.62 | 3.61 | 2.27 | - | 4.83 |
| ADA | 183.77 | 184.08 | 161.25 | - | 207.42 |
| PEG | 7.57 | 7.57 | 6.79 | - | 8.32 |
| TOTAL | 2306.48 | 2310.47 | 2170.19 | - | 2460.96 |
| English Term | Spanish Term | Abbreviation in Spanish |
| National Health System (abbreviated in text as NHS) | Sistema Nacional de Salud | SNS |
| Interministerial Committee on Pricing of Medicines (abbreviated in text as ICPM) | Comisión Interministerial de Precios de Medicamentos | CIPM |
| Ex-factory price (abbreviated in text as EFP) | Precio de venta del laboratorio | PVL |
| Reference price (abbreviated in text as RP) | Precio de referencia | PR |
| Reference Price Order (abbreviated in text as RPO) | Orden de Precios de Referencia | OPR |
| Reference Price System (abbreviated in text as RPS) | Sistema de Precios de Referencia | SPR |
| Purchase price | Precio de adquisición | - |
| Official State Gazette (abbreviated in text as OSG) | Boletín Oficial del Estado | BOE |
| Molecule 1 | Year of First Biosimilar Launch | Public Tenders Analyzed 3 | Current Level of Discount | |
| Original | Biosimilar | |||
| SOM | 2009 2 | 9 | ++ | ++ |
| FIL | 2009 2 | 2 | +++ | +++ |
| EPO | 2009 2 | 2 | +++ | +++ |
| FOL | 2014 | - | - | - |
| INF | 2015 | 37 | + | +++ |
| INS | 2015 | - | - | - |
| ETA | 2016 | 55 | + | ++ |
| CHO | 2016 | - | - | - |
| RIT | 2017 | 36 | + | ++ |
| TRA | 2018 | 21 | + | +++ |
| ENO | 2018 | - | - | - |
| ADA | 2018 | 59 | + | +++ |
| PEG | 2019 | 2 | +++ | +++ |
| Scenario | Parameter | Variation with Respect to the Base Case |
| Scenario 1 | Originator and biosimilar prices | No commercial discounts applied |
| Scenario 2 | Commercial discounts (tenders) | No volume weighting applied |
| One-way | Parameter | Variation with Respect to the Base Case |
| One-way 1 | Biosimilar price prior RPO | ±50% |
| One-way 2 | Month of application of RPO | ±1 month |
| One-way 3 | Epoetin zeta market share distribution | ±20% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Goñi, M.; Río-Álvarez, I.; Carcedo, D.; Villacampa, A. Budget Impact Analysis of Biosimilar Products in Spain in the Period 2009–2019. Pharmaceuticals 2021, 14, 348. https://doi.org/10.3390/ph14040348
García-Goñi M, Río-Álvarez I, Carcedo D, Villacampa A. Budget Impact Analysis of Biosimilar Products in Spain in the Period 2009–2019. Pharmaceuticals. 2021; 14(4):348. https://doi.org/10.3390/ph14040348
Chicago/Turabian StyleGarcía-Goñi, Manuel, Isabel Río-Álvarez, David Carcedo, and Alba Villacampa. 2021. "Budget Impact Analysis of Biosimilar Products in Spain in the Period 2009–2019" Pharmaceuticals 14, no. 4: 348. https://doi.org/10.3390/ph14040348
APA StyleGarcía-Goñi, M., Río-Álvarez, I., Carcedo, D., & Villacampa, A. (2021). Budget Impact Analysis of Biosimilar Products in Spain in the Period 2009–2019. Pharmaceuticals, 14(4), 348. https://doi.org/10.3390/ph14040348






