Improving the Quality of Oocytes with the Help of Nucleolotransfer Therapy
Abstract
1. Introduction
2. Fibrillar Sphere Handling
3. NLBs/NPBs and Their Impact on Oocyte Maturation and Embryonic Development
4. Preservation of NLB/NPB
5. NLB/NPB Transfer and Its Perspective in Therapy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crozet, N. Nucleolar structure and RNA synthesis in mammalian oocytes. J. Reprod. Fertil. Suppl. 1989, 38, 9–16. [Google Scholar]
- Olson, M.O.; Dundr, M.; Szebeni, A. The nucleolus: An old factory with unexpected capabilities. Trends Cell Biol. 2000, 10, 189–196. [Google Scholar] [CrossRef]
- Carmo-Fonseca, M.; Mendes-Soares, L.; Campos, I. To be or not to be in the nucleolus. Nat. Cell Biol. 2000, 2, E107–E112. [Google Scholar] [CrossRef]
- Visintin, R.; Amon, A. The nucleolus: The magician’s hat for cell cycle tricks. Curr. Opin. Cell Biol. 2000, 12, 372–377. [Google Scholar] [CrossRef]
- Watanabe, Y. Monopolar attachment by polo. Nat. Cell Biol. 2003, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kyogoku, H.; Ogushi, S.; Miyano, T. Nucleoli from growing oocytes support the development of enucleolated full-grown oocytes in the pig. Mol. Reprod. Dev. 2010, 77, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, L.A. A light- and electron-microscope study of the nucleolus during growth of the oocyte in the prepubertal mouse. J. Cell Sci. 1971, 9, 637–663. [Google Scholar] [PubMed]
- Chouinard, L.A. A light- and electron-microscope study of the oocyte nucleus during development of the antral follicle in the prepubertal mouse. J. Cell Sci. 1975, 17, 589–615. [Google Scholar] [PubMed]
- Bonnet-Garnier, A.; Feuerstein, P.; Chebrout, M.; Fleurot, R.; Jan, H.-U.; Debey, P.; Beaujean, N. Genome organization and epigenetic marks in mouse germinal vesicle oocytes. Int. J. Dev. Biol. 2012, 56, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Shishova, K.V.; Khodarovich, Y.M.; Lavrentyeva, E.A.; Zatsepina, O.V. High-Resolution microscopy of active ribosomal genes and key members of the RRNA processing machinery inside nucleolus-like bodies of fully-grown mouse oocytes. Exp. Cell Res. 2015, 337, 208–218. [Google Scholar] [CrossRef]
- Szöllösi, M.S.; Debey, P.; Szöllösi, D.; Rime, H.; Vautier, D. Chromatin behaviour under influence of puromycin and 6-DMAP at different stages of mouse oocyte maturation. Chromosoma 1991, 100, 339–354. [Google Scholar] [CrossRef]
- Fléchon, J.E.; Kopecný, V. The nature of the “nucleolus precursor body” in early preimplantation embryos: A review of fine-structure cytochemical, immunocytochemical and autoradiographic data related to nucleolar function. Zygote 1998, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, S.; Palmieri, C.; Fulka, H.; Saitou, M.; Miyano, T.; Fulka, J. The maternal nucleolus is essential for early embryonic development in mammals. Science 2008, 319, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Fulka, H.; Langerova, A. Nucleoli in embryos: A central structural platform for embryonic chromatin remodeling? Chromosome Res. 2019, 27, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Schultz, R.M.; Montgomery, R.R.; Belanoff, J.R. Regulation of Mouse oocyte meiotic maturation: Implication of a decrease in oocyte CAMP and protein dephosphorylation in commitment to resume meiosis. Dev. Biol. 1983, 97, 264–273. [Google Scholar] [CrossRef]
- Bornslaeger, E.A.; Mattei, P.; Schultz, R.M. Involvement of CAMP-dependent protein kinase and protein phosphorylation in regulation of mouse oocyte maturation. Dev. Biol. 1986, 114, 453–462. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Lu, Q.; Breitbart, H.; Chen, D.Y. CAMP inhibits mitogen-activated protein (MAP) kinase activation and resumption of meiosis, but exerts no effects after spontaneous germinal vesicle breakdown (GVBD) in mouse oocytes. Reprod. Fertil. Dev. 1999, 11, 81–86. [Google Scholar] [CrossRef]
- Fulka, H.; Rychtarova, J.; Loi, P. The Nucleolus-like and Precursor Bodies of Mammalian Oocytes and Embryos and Their Possible Role in Post-Fertilization Centromere Remodelling. Biochem. Soc. Trans. 2020, 48, 581–593. [Google Scholar] [CrossRef]
- Fulka, J.; Moor, R.M.; Loi, P.; Fulka, J. Enucleolation of porcine oocytes. Theriogenology 2003, 59, 1879–1885. [Google Scholar] [CrossRef]
- Benc, M.; Fulka, J.J.; Strejček, F.; Morovič, M.; Murín, M.; Martínková, S.; Jettmarová, D.; Laurinčík, J. Enucleolation and Nucleolus Transfer in Mammalian Oocytes and Zygotes. Int. J. Dev. Biol. 2019, 63, 253–258. [Google Scholar] [CrossRef]
- Kyogoku, H.; Kitajima, T.S.; Miyano, T. Nucleolus precursor body (NPB): A distinct structure in mammalian oocytes and zygotes. Nucleus 2014, 5, 493–498. [Google Scholar] [CrossRef]
- Fulka, H.; Langerova, A. The maternal nucleolus plays a key role in centromere satellite maintenance during the oocyte to embryo transition. Development 2014, 141, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.D.; Kay, B.K.; Gall, J.G. In vitro rna synthesis in oocyte nuclei of the newt notophthalmus. Chromosoma 1981, 82, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, S.; Saitou, M. The nucleolus in the mouse oocyte is required for the early step of both female and male pronucleus organization. J. Reprod. Dev. 2010, 56, 495–501. [Google Scholar] [CrossRef]
- Ogushi, S.; Yamagata, K.; Obuse, C.; Furuta, K.; Wakayama, T.; Matzuk, M.M.; Saitou, M. Reconstitution of the oocyte nucleolus in mice through a single nucleolar protein, NPM2. J. Cell Sci. 2017, 130, 2416–2429. [Google Scholar] [CrossRef]
- Kyogoku, H.; Fulka, J.; Wakayama, T.; Miyano, T. De novo formation of nucleoli in developing mouse embryos originating from enucleolated zygotes. Development 2014, 141, 2255–2259. [Google Scholar] [CrossRef]
- Fulka, H.; Fulka, J. Nucleolar transplantation in oocytes and zygotes: Challenges for further research. Mol. Hum. Reprod. 2010, 16, 63–67. [Google Scholar] [CrossRef]
- Kimura, Y.; Yanagimachi, R. Intracytoplasmic sperm injection in the mouse. Biol. Reprod. 1995, 52, 709–720. [Google Scholar] [CrossRef]
- Fulka, J.J.; Benc, M.; Loi, P.; Langerova, A.; Fulka, H. Function of atypical mammalian oocyte/zygote nucleoli and its implications for reproductive biology and medicine. Int. J. Dev. Biol. 2019, 63, 105–112. [Google Scholar] [CrossRef]
- Fulka, H.; Martinkova, S.; Kyogoku, H.; Langerova, A.; Fulka, J. Production of giant mouse oocyte nucleoli and assessment of their protein content. J. Reprod. Dev. 2012, 58, 371–376. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Roberts, K.; Raff, M.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2008; ISBN 978-0-8153-4111-6. [Google Scholar]
- Kovalská, M.; Petrovičová, I.; Strejček, F.; Adamkov, M.; Halašová, E.; Lehotský, J.; Laurinčík, J.; Østrup, O. The role of RNA-polymerase II transcription in embryonic nucleologenesis by bovine embryos. Biologia 2010, 65, 552–557. [Google Scholar] [CrossRef]
- Morovic, M.; Strejcek, F.; Nakagawa, S.; Deshmukh, R.S.; Murin, M.; Benc, M.; Fulka, H.; Kyogoku, H.; Pendovski, L.; Fulka, J.; et al. Mouse oocytes nucleoli rescue embryonic development of porcine enucleolated oocytes. Zygote 2017, 25, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Benc, M.; Martinkova, S.; Rychtarova, J.; Fulka, J.; Bartkova, A.; Fulka, H.; Laurincik, J. Assessing the effect of interspecies oocyte nucleolar material dosage on embryonic development. Theriogenology 2020, 155, 17–24. [Google Scholar] [CrossRef]
- Murin, M.; Strejcek, F.; Bartkova, A.; Morovic, M.; Benc, M.; Prochazka, R.; Lucas-Hahn, A.; Pendovski, L.; Laurincik, J. Intranuclear characteristics of pig oocytes stained with brilliant cresyl blue and nucleologenesis of resulting embryos. Zygote 2019, 27, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H.; Viveiros, M.M.; Ren, Y.; Wang, P.; DeMayo, F.J.; Frail, D.E.; Eppig, J.J.; Matzuk, M.M. Roles of NPM2 in chromatin and nucleolar organization in oocytes and embryos. Science 2003, 300, 633–636. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E. The probability of abnormal preimplantation development can be predicted by a single static observation on pronuclear stage morphology. Hum. Reprod. 1999, 14, 1318–1323. [Google Scholar] [CrossRef]
- Gianaroli, L.; Magli, M.C.; Ferraretti, A.P.; Fortini, D.; Grieco, N. Pronuclear morphology and chromosomal abnormalities as scoring criteria for embryo selection. Fertil. Steril. 2003, 80, 341–349. [Google Scholar] [CrossRef]
- Hyttel, P. Electron microscopy of mammalian oocyte development, maturation and fertilization. In Oocyte Maturation and Fertilization: A Long History for a Short Event; Bentham Books: Sharjah, United Arab Emirates, 2011; pp. 1–37. ISBN 978-1-60805-062-8. [Google Scholar]
- Miyano, T.; Manabe, N. Oocyte growth and acquisition of meiotic competence. Soc. Reprod. Fertil. Suppl. 2007, 63, 531–538. [Google Scholar]
- Kyogoku, H.; Ogushi, S.; Miyano, T.; Fulka, J. Nucleoli from growing oocytes inhibit the maturation of enucleolated, full-grown oocytes in the pig. Mol. Reprod. Dev. 2011, 78, 426–435. [Google Scholar] [CrossRef]
- Mangia, F.; Epstein, C.J. Biochemical studies of growing mouse oocytes: Preparation of oocytes and analysis of glucose-6-phosphate dehydrogenase and lactate dehydrogenase activities. Dev. Biol. 1975, 45, 211–220. [Google Scholar] [CrossRef]
- Ericsson, S.A.; Boice, M.L.; Funahashi, H.; Day, B.N. Assessment of Porcine oocytes using brilliant cresyl blue. Theriogenology 1993, 39, 214. [Google Scholar] [CrossRef]
- Andersen, J.S.; Lyon, C.E.; Fox, A.H.; Leung, A.K.L.; Lam, Y.W.; Steen, H.; Mann, M.; Lamond, A.I. Directed proteomic analysis of the human nucleolus. Curr. Biol. 2002, 12, 1–11. [Google Scholar] [CrossRef]
- Christians, E.; Boiani, M.; Garagna, S.; Dessy, C.; Redi, C.A.; Renard, J.P.; Zuccotti, M. Gene expression and chromatin organization during mouse oocyte growth. Dev. Biol. 1999, 207, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bachant, J.B.; Elledge, S.J. Mitotic treasures in the nucleolus. Nature 1999, 398, 757–758. [Google Scholar] [CrossRef]
- Combelles, C.M.H.; Cekleniak, N.A.; Racowsky, C.; Albertini, D.F. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum. Reprod. 2002, 17, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Yuswiati, E.; Holtz, W. Work in progress: Successful transfer of vitrified goat embryos. Theriogenology 1990, 34, 629–632. [Google Scholar] [CrossRef]
- Tachikawa, S.; Otoi, T.; Kondo, S.; Machida, T.; Kasai, M. Successful vitrification of bovine blastocysts, derived by in vitro maturation and fertilization. Mol. Reprod. Dev. 1993, 34, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Takei, M.; Kano, M.; Tomita, M.; Leibo, S.P. Piglets produced by transfer of vitrified porcine embryos after stepwise dilution of cryoprotectants. Cryobiology 1998, 36, 20–31. [Google Scholar] [CrossRef]
- Kyogoku, H.; Wakayama, T.; Kitajima, T.S.; Miyano, T. Single nucleolus precursor body formation in the pronucleus of mouse zygotes and SCNT embryos. PLoS ONE 2018, 13, e0202663. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Kashiwazaki, N.; Ashman, R.J.; Grupen, C.G.; Seamark, R.F.; Nottle, M.B. Removal of cytoplasmic lipid enhances the tolerance of porcine embryos to chilling. Biol. Reprod. 1994, 51, 618–622. [Google Scholar] [CrossRef]
- Park, K.-E.; Kwon, I.-K.; Han, M.-S.; Niwa, K. Effects of partial removal of cytoplasmic lipid on survival of vitrified germinal vesicle stage pig oocytes. J. Reprod. Dev. 2005, 51, 151–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moffa, F.; Comoglio, F.; Krey, L.C.; Grifo, J.A.; Revelli, A.; Massobrio, M.; Zhang, J. Germinal Vesicle transfer between fresh and cryopreserved immature mouse oocytes. Hum. Reprod. 2002, 17, 178–183. [Google Scholar] [CrossRef][Green Version]
- He, Z.; Liu, H.C.; Rosenwaks, Z. Cryopreservation of nuclear material as a potential method of fertility preservation. Fertil. Steril. 2003, 79, 347–354. [Google Scholar] [CrossRef]
- Kren, R.; Fulka, J.; Fulka, H. Cryopreservation of isolated mouse germinal vesicles. J. Reprod. Dev. 2005, 51, 289–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fulka, H.; Langerova, A.; Barnetova, I.; Novakova, Z.; Mosko, T.; Fulka, J. How to repair the oocyte and zygote? J. Reprod. Dev. 2009, 55, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Verdun, D. The nucleolus: A Model for the organization of nuclear functions. Histochem. Cell Biol. 2006, 126, 135–148. [Google Scholar] [CrossRef]
- Oestrup, O.; Hall, V.; Petkov, S.G.; Wolf, X.A.; Hyldig, S.; Hyttel, P. From zygote to implantation: Morphological and molecular dynamics during embryo development in the pig. Reprod. Domest. Anim. Zuchthyg. 2009, 44 (Suppl. 3), 39–49. [Google Scholar] [CrossRef]
- Fulka, J.; Fulka, H.; John, J.C.S. Transmission of mitochondrial DNA disorders: Possibilities for the elimination of mutated mitochondria. Cloning Stem Cells 2007, 9, 47–50. [Google Scholar] [CrossRef]
- Bredenoord, A.L.; Pennings, G.; de Wert, G. Ooplasmic and nuclear transfer to prevent mitochondrial DNA disorders: Conceptual and normative issues. Hum. Reprod. Update 2008, 14, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.W.; Grifo, J.A.; Krey, L.C.; Zhang, J. Reconstruction of mouse oocytes by germinal vesicle transfer: Maturity of host oocyte cytoplasm determines meiosis. Hum. Reprod. 1999, 14, 2357–2361. [Google Scholar] [CrossRef]
- Fulka, J.; Mrazek, M.; Fulka, H.; Loi, P. Mammalian oocyte therapies. Cloning Stem Cells 2005, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Borsos, M.; Torres-Padilla, M.-E. Building up the nucleus: Nuclear Organization in the establishment of totipotency and pluripotency during mammalian development. Genes Dev. 2016, 30, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, M.; Garagna, S.; Merico, V.; Monti, M.; Alberto Redi, C. Chromatin organisation and nuclear architecture in growing mouse oocytes. Mol. Cell. Endocrinol. 2005, 234, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Jachowicz, J.W.; Santenard, A.; Bender, A.; Muller, J.; Torres-Padilla, M.-E. Heterochromatin establishment at pericentromeres depends on nuclear position. Genes Dev. 2013, 27, 2427. [Google Scholar] [CrossRef]
- Lin, C.; Koh, F.; Wong, P.; Conti, M.; Ramalho-Santos, M. Hira-mediated H3.3 incorporation is required for DNA replication and ribosomal rna transcription in the mouse zygote. Dev. Cell 2014, 30, 268–279. [Google Scholar] [CrossRef]
- Martin, C.; Beaujean, N.; Brochard, V.; Audouard, C.; Zink, D.; Debey, P. Genome restructuring in mouse embryos during reprogramming and early development. Dev. Biol. 2006, 292, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Probst, A.V.; Santos, F.; Reik, W.; Almouzni, G.; Dean, W. Structural differences in centromeric heterochromatin are spatially reconciled on fertilisation in the mouse zygote. Chromosoma 2007, 116, 403–415. [Google Scholar] [CrossRef] [PubMed]
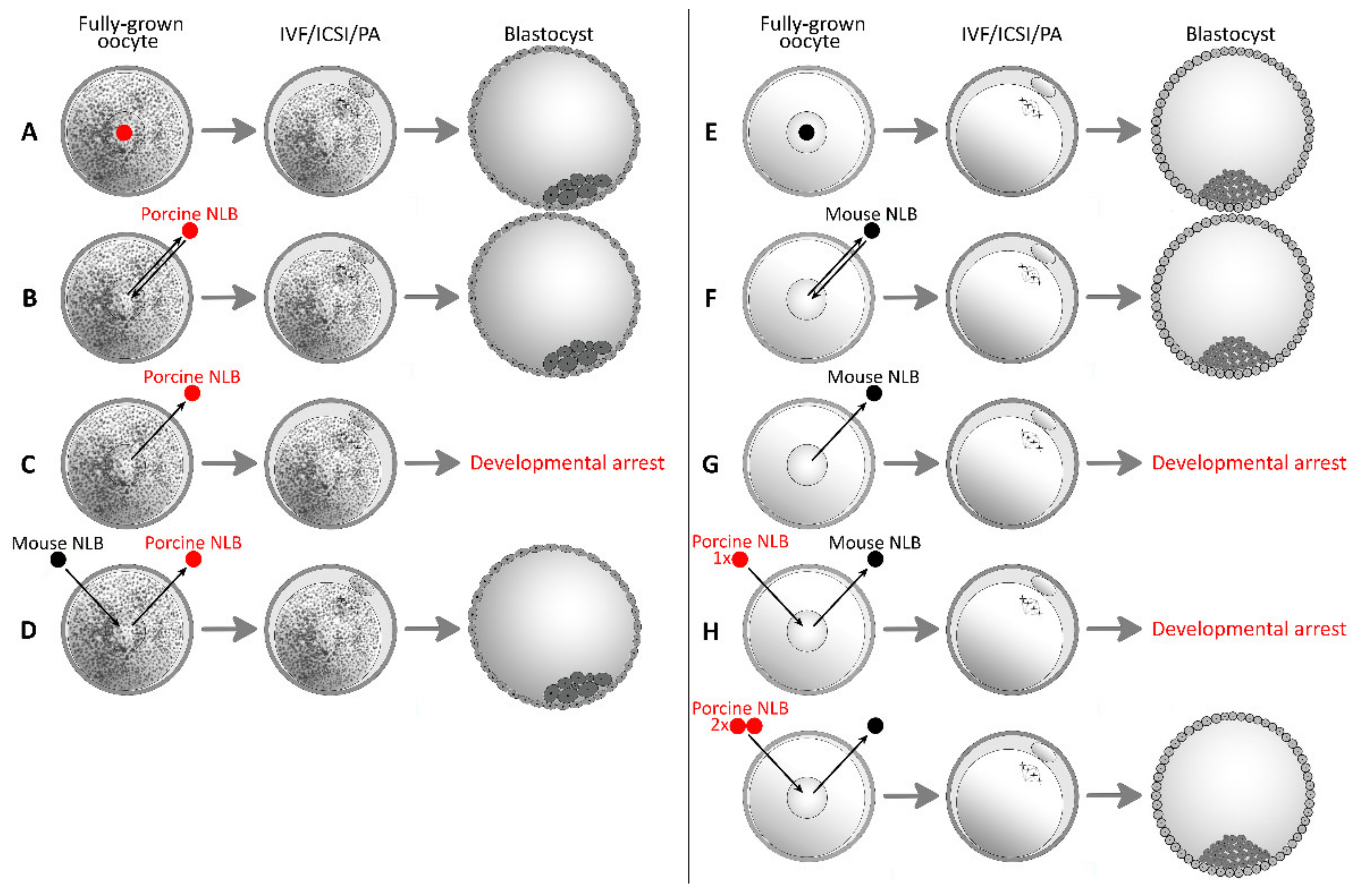
| Combination | Total Number of Oocytes | Total Number and % of Oocytes Forming Blastocyst, [Citation] | |
|---|---|---|---|
| Oocyte | NLB | ||
| Mouse | Mouse | 123 | 62 (50%) [34] |
| Mouse | Porcine (2 NLBs) | 281 | 126 (45%) [34] |
| Porcine | Porcine | 194 | 72 (37%) [13] |
| 313 | 37 (12%) [33] | ||
| Porcine | Mouse | 327 | 44 (14%) [33] |
| Year of Publication | Authors | Key Findings | Species |
|---|---|---|---|
| 2003 | Fulka J. Jr. et al. | First successful microsurgical enucleolation of NLB from the oocyte [19]. | Pig |
| 2008 | Ogushi et al. | The maternal NLB is essential for early embryonic development in mammals [13]. | Mouse, pig |
| 2010 | Ogushi and Saitou | The nucleolus in the oocyte is required for the early step of both female and male pronucleus organization [24]. | Mouse |
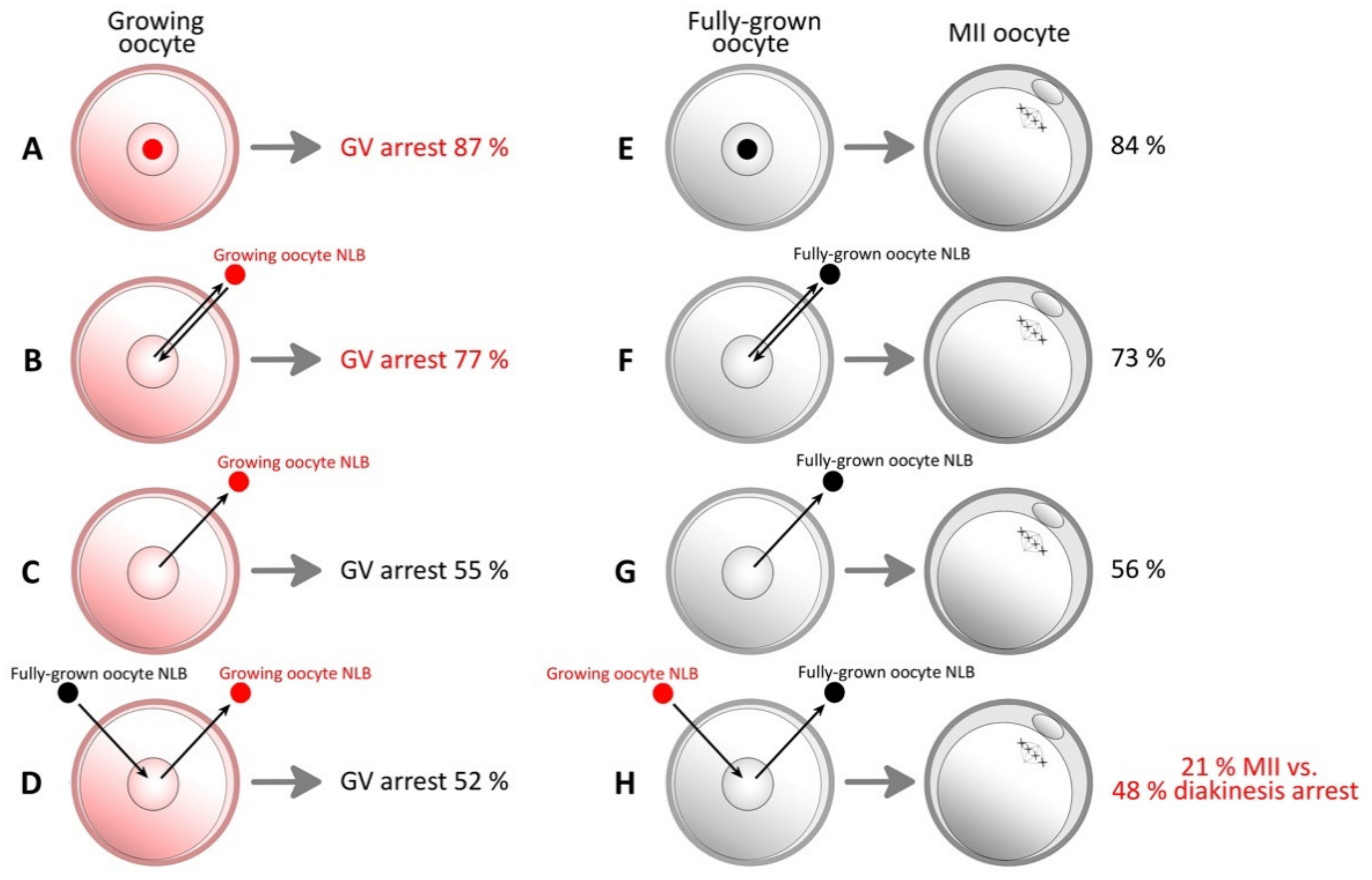
| 2011 | Kyogoku et al. | NLB from growing oocytes has an inhibitory effect on oocyte maturation [41]. | Pig |
| 2012 | Fulka H. et al. | First production of giant nucleolus and relative protein content analyse [30]. | Mouse |
| 2014 | Fulka H. and Langerova | The maternal nucleolus plays a key role in centromere satellite maintenance during the oocyte to embryo transition [22]. | Mouse |
| 2014 | Kyogoku et al. | NPBs start to function very soon after fertilization. Later NPBs’ enucleolation does not have a negative impact on embryonic development [26]. | Mouse |
| 2017 | Morovic et al. | First successful interspecies nucleolotransfer [33]. | Mouse, pig |
| 2017 | Ogushi et al. | NLB was reconstituted by single nucleolar protein Npm-2 [25]. | Mouse |
| 2020 | Benc et al. | Interspecies nucleolotransfer from porcine oocyte to mouse oocyte. Interspecies quantitative differences in RPC may be compensated by multiple NLB transfer [34]. | Mouse, pig |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benc, M.; Strejcek, F.; Morovic, M.; Bartkova, A.; Murin, M.; Gad, A.; Bonnet-Garnier, A.; Percinic, F.P.; Laurincik, J. Improving the Quality of Oocytes with the Help of Nucleolotransfer Therapy. Pharmaceuticals 2021, 14, 328. https://doi.org/10.3390/ph14040328
Benc M, Strejcek F, Morovic M, Bartkova A, Murin M, Gad A, Bonnet-Garnier A, Percinic FP, Laurincik J. Improving the Quality of Oocytes with the Help of Nucleolotransfer Therapy. Pharmaceuticals. 2021; 14(4):328. https://doi.org/10.3390/ph14040328
Chicago/Turabian StyleBenc, Michal, Frantisek Strejcek, Martin Morovic, Alexandra Bartkova, Matej Murin, Ahmed Gad, Amelie Bonnet-Garnier, Florina Popovska Percinic, and Jozef Laurincik. 2021. "Improving the Quality of Oocytes with the Help of Nucleolotransfer Therapy" Pharmaceuticals 14, no. 4: 328. https://doi.org/10.3390/ph14040328
APA StyleBenc, M., Strejcek, F., Morovic, M., Bartkova, A., Murin, M., Gad, A., Bonnet-Garnier, A., Percinic, F. P., & Laurincik, J. (2021). Improving the Quality of Oocytes with the Help of Nucleolotransfer Therapy. Pharmaceuticals, 14(4), 328. https://doi.org/10.3390/ph14040328







