Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN)
Abstract
1. Introduction
2. Results
2.1. Histology Review
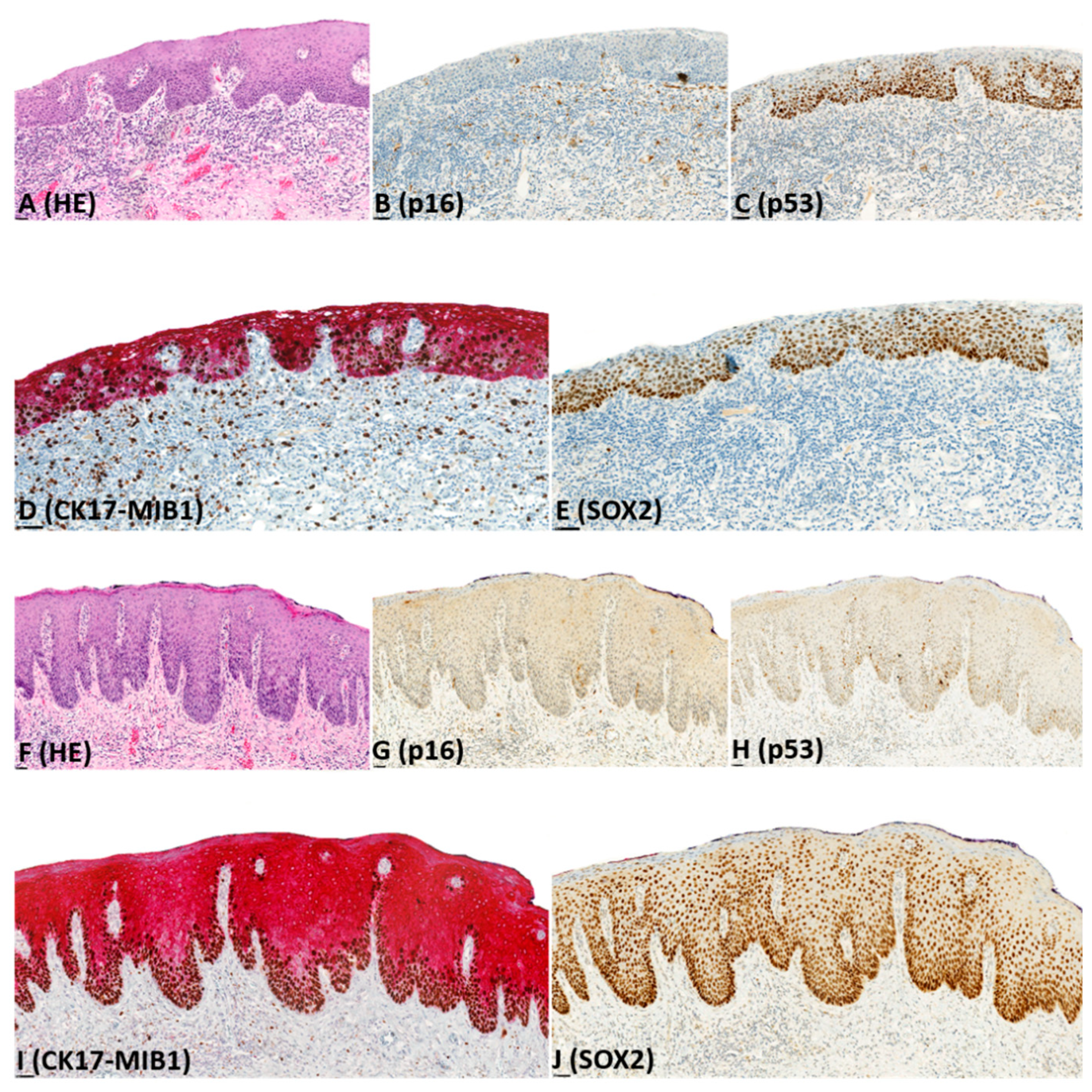
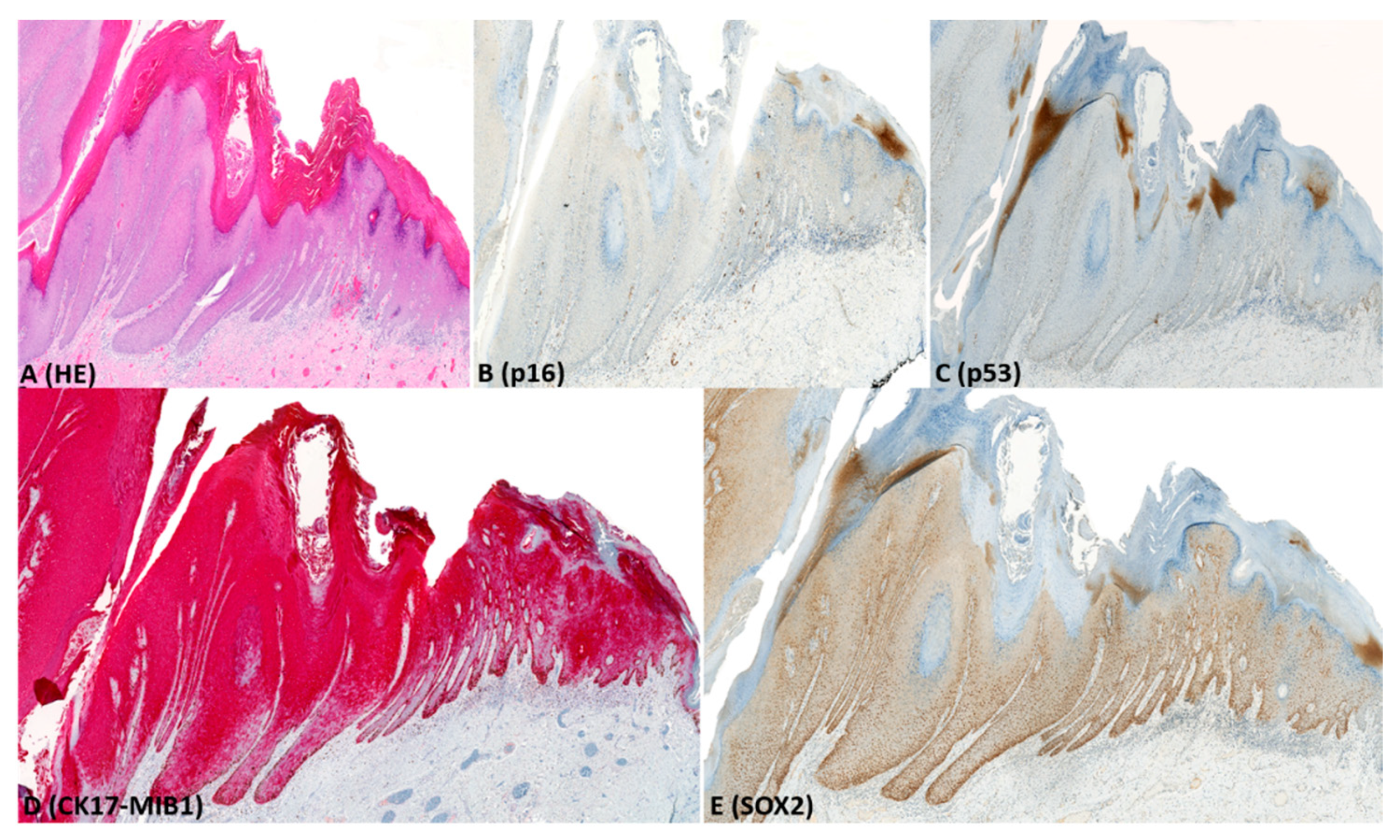
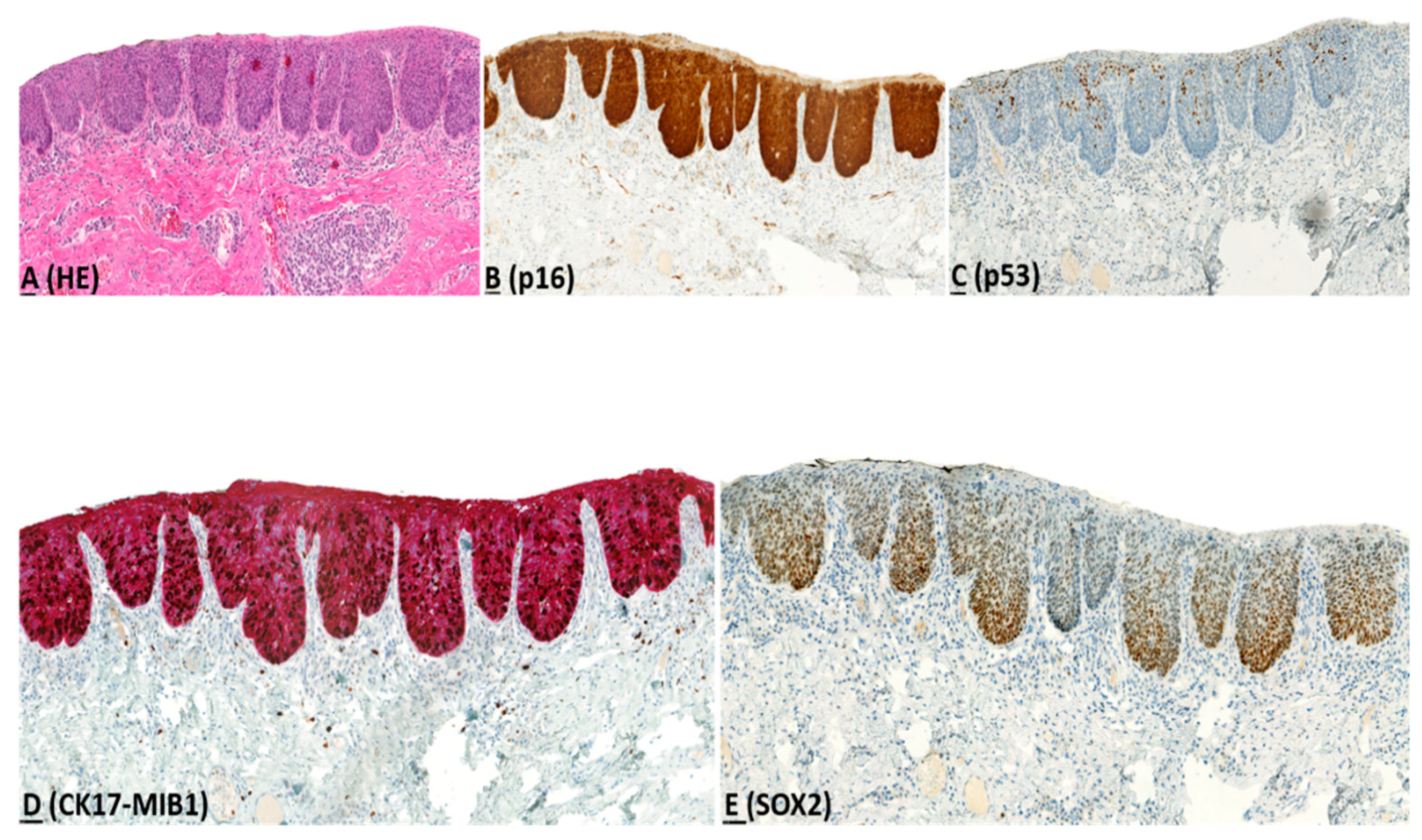
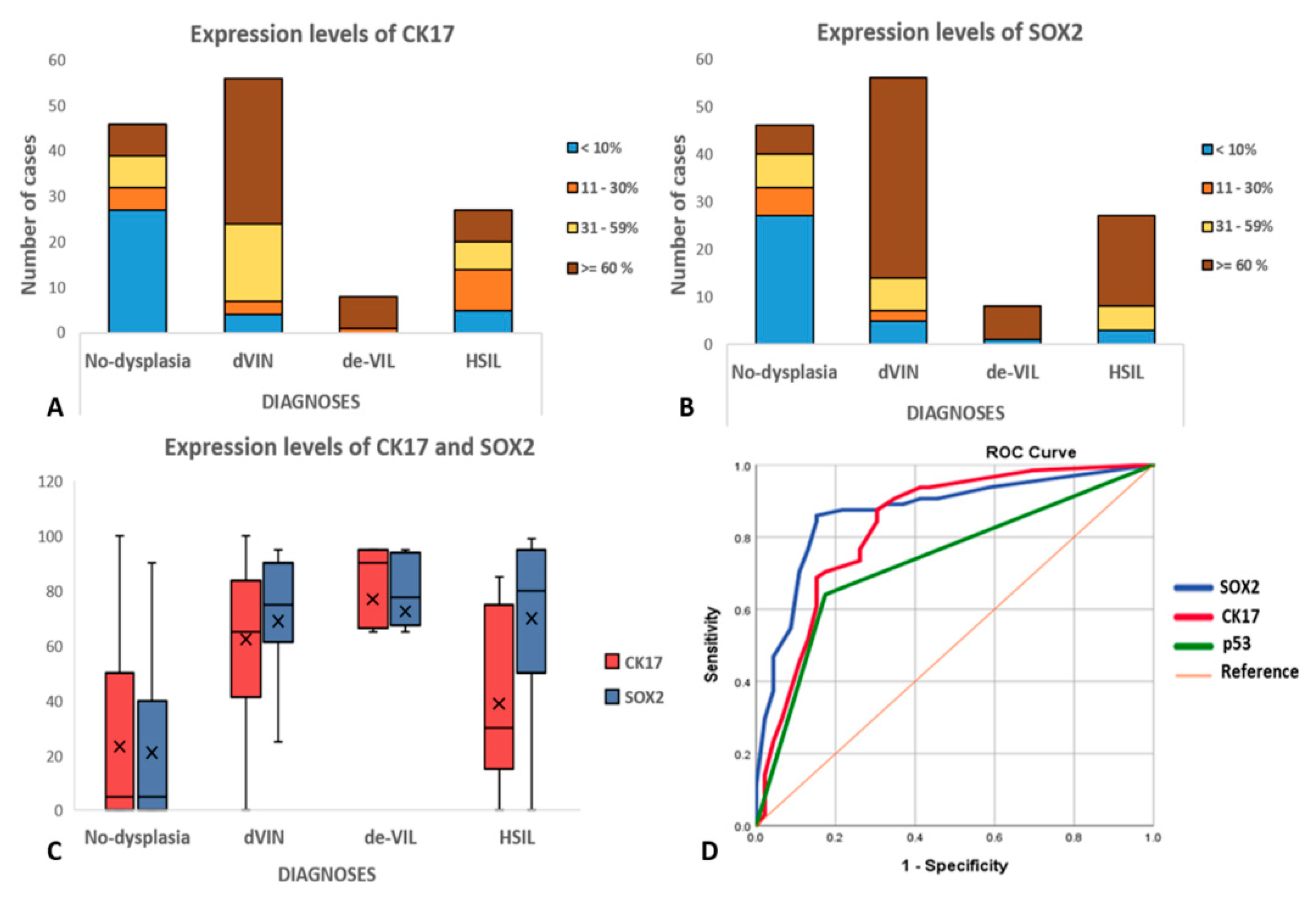
2.2. Immunohistochemistry (IHC)
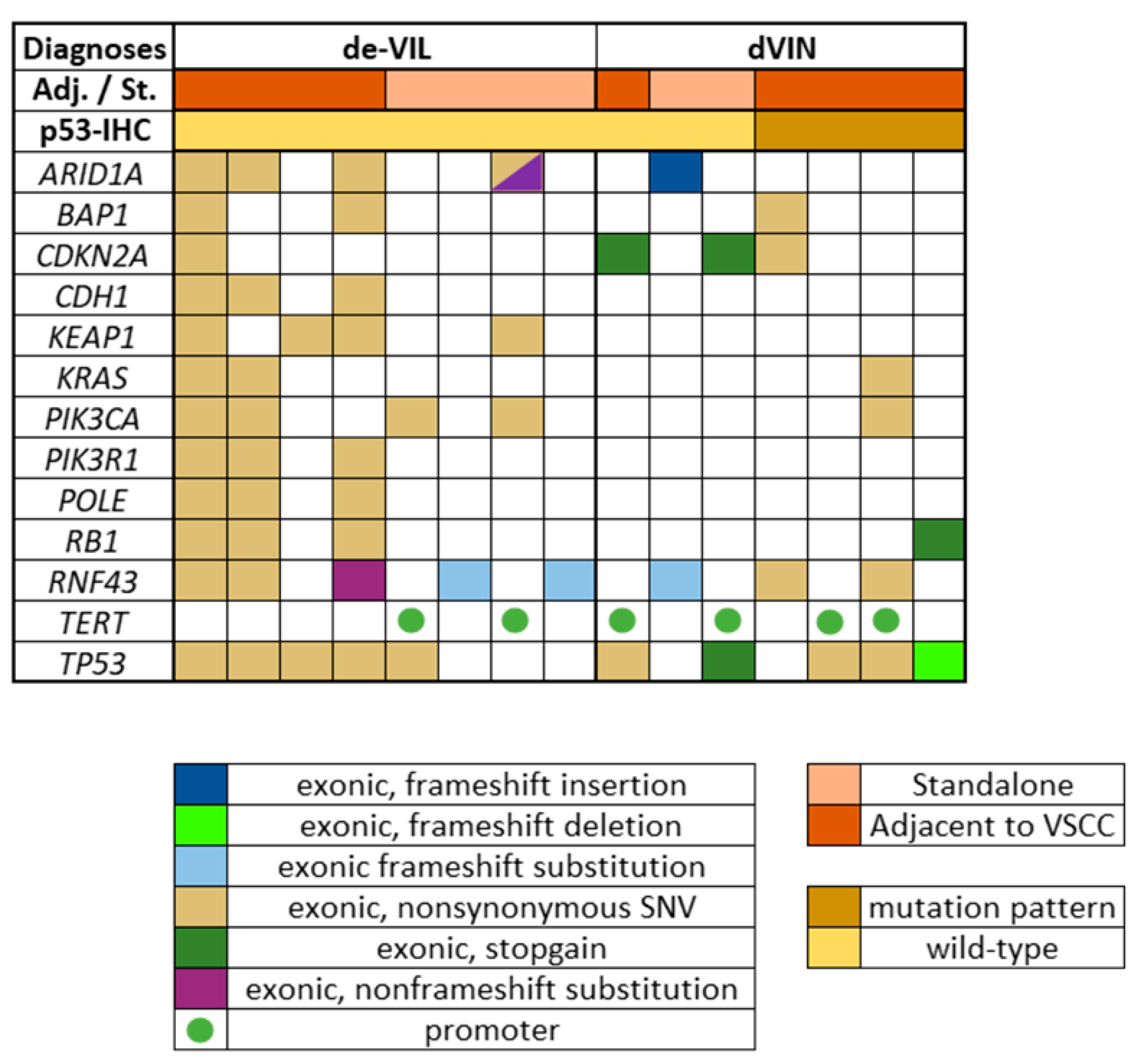
2.3. Next Generation Targeted Sequencing
3. Discussion
4. Materials and Methods
4.1. Histology Review
4.2. Immunohistochemistry (IHC)
4.3. Next Generation Targeted Sequencing (NGTS)
4.4. Ethics Statement
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, A.; Syed, S.; Velangi, S.; Ganesan, R. New Directions in Vulvar Cancer Pathology. Curr. Oncol. Rep. 2019, 21, 88. [Google Scholar] [CrossRef]
- Heller, D.S.; Day, T.; Allbritton, J.I.; Scurry, J.; Radici, G.; Welch, K.; Preti, M.; the ISSVD Difficult Pathologic Diagnoses Committee. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J. Low. Genit. Tract Dis. 2020, 25, 57. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014.
- Singh, N.; Gilks, C.B. Vulval squamous cell carcinoma and its precursors. Histopathology 2020, 76, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Bigby, S.M.; Eva, L.J.; Leng Fong, K.; Jones, R.W. The natural history of vulvar intraepithelial neoplasia, differentiated type: Ev-idence for progression and diagnostic challenges. Int. J. Gynecol. Pathol. 2016, 35, 574–584. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, J.N.; Kim, S.Y.; Akbari, A.; Eshragh, S.; Reuschenbach, M.; von Knebel Doeberitz, M.; Prigge, E.S.; Jordan, S.; Singh, N.; Miller, D.M.; et al. HPV-independent Differentiated Vulvar Intraepithelial Neoplasia (dVIN) is Associated with an Aggressive Clinical Course. Int. J. Gynecol. Pathol. 2017, 36, 507–516. [Google Scholar] [CrossRef]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N.; et al. Brit-ish gynaecological cancer society (BGCS) vulval cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef]
- Dockery, L.E.; Soper, J.T. Vulvar Intraepithelial Neoplasia: A Review of the Disease and Current Management. Obstet. Gynecol. Surv. 2021, 76, 55–62. [Google Scholar] [CrossRef]
- van den Einden, L.C.; de Hullu, J.A.; Massuger, L.F.; Grefte, J.M.; Bult, P.; Wiersma, A.; van Engen-van Grunsven, A.C.; Sturm, B.; Bosch, S.L.; Hollema, H.; et al. Interobserver variability and the effect of education in the histopathological diagnosis of differ-entiated vulvar intraepithelial neoplasia. Mod. Pathol. 2013, 26, 874–880. [Google Scholar] [CrossRef]
- Dasgupta, S.; de Jonge, E.; Van Bockstal, M.R.; Wong-Alcala, L.S.M.; Wilhelmus, S.; Makkus, L.A.C.F.; Schelfout, K.; Van de Vijver, K.K.; Smits, S.; Marbaix, E.; et al. Histological interpretation of differentiated vulvar intraepithelial neoplasia (dVIN) remains challenging—Observations from a bi-national ring-study. Virchows Arch. 2021, 1–11. [Google Scholar] [CrossRef]
- van de Nieuwenhof, H.P.; Bulten, J.; Hollema, H.; Dommerholt, R.G.; Massuger, L.F.; van der Zee, A.G.; de Hullu, J.A.; van Kempen, L.C. Differentiated vulvar intraepithelial neoplasia is often found in lesions, previously diagnosed as lichen sclerosus, which have progressed to vulvar squamous cell carcinoma. Mod. Pathol. 2011, 24, 297–305. [Google Scholar] [CrossRef]
- Jin, C.; Liang, S. Differentiated Vulvar Intraepithelial Neoplasia: A Brief Review of Clinicopathologic Features. Arch. Pathol. Lab. Med. 2019, 143, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C. Human Papillomavirus–Independent Squamous Lesions of the Vulva. Surg. Pathol. Clin. 2019, 12, 249–261. [Google Scholar] [CrossRef]
- Shalin, S.C.; Racher, L.M.; Campbell, K.K. Lichenoid dermatoses involving the vulva: A clinical-pathologic correlation. Semin. Diagn. Pathol. 2020, 38, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Ewing-Graham, P.C.; Swagemakers, S.M.A.; van der Spek, P.J.; van Doorn, H.C.; Noordhoek Hegt, V.; Koljenović, S.; van Kemenade, F.J. Precursor lesions of vulvar squamous cell carcinoma—Histology and biomarkers: A systematic review. Crit. Rev. Oncol. Hematol. 2020, 147, 102866. [Google Scholar] [CrossRef] [PubMed]
- Tessier-Cloutier, B.; Kortekaas, K.E.; Thompson, E.; Pors, J.; Chen, J.; Ho, J.; Prentice, L.M.; McConechy, M.K.; Chow, C.; Proc-tor, L.; et al. Major p53 immunohistochemical patterns in in situ and invasive squamous cell carcinomas of the vulva and correla-tion with TP53 mutation status. Mod. Pathol. 2020, 33, 1595–1605. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Pors, J.; Thompson, E.; Ho, J.; Prentice, L.; McConechy, M.; Aguirre-Hernandez, R.; Miller, R.; Leung, S.; Proctor, L.; et al. Molecular characterization of invasive and in situ squamous neoplasia of the vulva and implications for mor-phologic diagnosis and outcome. Mod. Pathol. 2021, 34, 508–518. [Google Scholar] [CrossRef]
- Kortekaas, K.E.; Solleveld-Westerink, N.; Tessier-Cloutier, B.; Rutten, T.A.; van Poelgeest, M.I.E.; Gilks, C.B.; Hoang, L.N.; Bosse, T. Performance of the pattern based interpretation of p53 immunohistochemistry as a surrogate for TP53 mutations in vul-var squamous cell carcinoma. Histopathology 2020, 77, 92–99. [Google Scholar] [CrossRef]
- Day, T.; Marzol, A.; Pagano, R.; Jaaback, K.; Scurry, J. Clinicopathologic diagnosis of differentiated vulvar intraepithelial neo-plasia and vulvar aberrant maturation. J. Low. Genit. Tract. Dis. 2020, 24, 392–398. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ewing-Graham, P.C.; van Kemenade, F.J.; van Doorn, H.C.; Noordhoek Hegt, V.; Koljenović, S. Differentiated vulvar intraepithelial neoplasia (dVIN): The most helpful histological features and the utility of cytokeratins 13 and 17. Virchows Arch. 2018, 473, 739–747. [Google Scholar] [CrossRef]
- Rakislova, N.; Alemany, L.; Clavero, O.; Saco, A.; Torné, A.; Del Pino, M.; Munmany, M.; Rodrigo-Calvo, M.T.; Guerrero, J.; Marimon, L.; et al. p53 immunohistochemical patterns in HPV-independent squamous cell carcinomas of the vulva and the asso-ciated skin lesions: A study of 779 cases. Int. J. Mol. Sci. 2020, 21, 8091. [Google Scholar] [CrossRef]
- Kashofer, K.; Regauer, S. Analysis of full coding sequence of the TP53 gene in invasive vulvar cancers: Implications for thera-py. Gynecol. Oncol. 2017, 146, 314–318. [Google Scholar] [CrossRef]
- Watkins, J.C.; Howitt, B.E.; Horowitz, N.S.; Ritterhouse, L.L.; Dong, F.; Macconaill, L.E.; Garcia, E.; Lindeman, N.I.; Lee, L.J.; Berkowitz, R.S.; et al. Differentiated exophytic vulvar intraepithelial lesions are genetically distinct from keratinizing squamous cell carcinomas and contain mutations in PIK3CA. Mod. Pathol. 2017, 30, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Pinto, A.; Amemiya, Y.; Seth, A.; Mirkovic, J.; Parra-Herran, C. Differentiated exophytic vulvar intrapeithelial le-sion: Clincopathologic and molecular analysis documenting its relationship with verrucous carcinoma of the vulva. Mod. Pathol. 2020, 33, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.M.; Mills, A.M. Putative precancerous lesions of vulvar squamous cell carcinoma. Semin. Diagn. Pathol. 2020, 38, 27–36. [Google Scholar] [CrossRef]
- Almadani, N.; Thompson, E.F.; Tessier-Cloutier, B.; Pors, J.; Hoang, L. An update of molecular pathology and shifting systems of classification in tumours of the female genital tract. Diagn. Histopathol. 2020, 26, 278–288. [Google Scholar] [CrossRef]
- Podoll, M.B.; Singh, N.; Gilks, C.B.; Moghadamfalahi, M.; Sanders, M.A. Assessment of CK17 as a Marker for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia. Int. J. Gynecol. Pathol. 2017, 36, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.; Moch, H.; Choschzick, M. SOX2 Gene Amplification and Overexpression is Linked to HPV-positive Vulvar Carcinomas. Int. J. Gynecol. Pathol. 2018, 37, 68–73. [Google Scholar] [CrossRef]
- Brustmann, H.; Brunner, A. Immunohistochemical Expression of SOX2 in Vulvar Intraepithelial Neoplasia and Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2013, 32, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Shima, K.; Kondo, T.; Semba, I. Atypical immunohistochemical patterns can complement the histopathological di-agnosis of oral premalignant lesions. J. Oral. Biosci. 2020, 62, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.J.; McCoy, M.J.; Hemmings, C.; Iacopetta, B.; Platell, C.F. Expression of PD-L1 and SOX2 during rectal tumourigene-sis: Potential mechanisms for immune escape and tumour cell invasion. Oncol. Lett. 2018, 16, 5761–5768. [Google Scholar]
- Ren, Z.H.; Zhang, C.P.; Ji, T. Expression of SOX2 in oral squamous cell carcinoma and the association with lymph node me-tastasis. Oncol. Lett. 2016, 11, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, R.M.; Primiani, A.; Doyle, L.A.; Linskey, K.R.; Duncan, L.M.; Odze, R.D.; Zukerberg, L.R. Cytokeratin 17: An ad-junctive marker of invasion in squamous neoplastic lesions of the anus. Am. J. Surg. Pathol. 2014, 38, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Kerem, R.; Rahat, M.A.; Madah, W.; Greenberg, E.; Sabo, E.; Elmalah, I. Cytokeratin-17 as a Potential Marker for Squamous Cell Carcinoma of the Larynx. Ann. Otol. Rhinol. Laryngol. 2004, 113, 821–827. [Google Scholar] [CrossRef]
- Hussein, A.A.; Forouzanfar, T.; Bloemena, E.; De Visscher, J.; Brakenhoff, R.H.; Leemans, C.R.; Helder, M.N. A review of the most promising biomarkers for early diagnosis and prognosis prediction of tongue squamous cell carcinoma. Br. J. Cancer 2018, 119, 724–736. [Google Scholar] [CrossRef]
- UniProt, C. Uniprot: A worldwide hub of protein knowledge. Nucleic. Acid. Res. 2019, 47, D506–D515. [Google Scholar]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H.; et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: Background and consensus recommendations from the college of american pathologists and the american society for colposcopy and cervical pa-thology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef]
- Goyal, A.; Zhang, G.; Yang, B. Differential expression patterns of GATA3 in usual and differentiated types of vulvar intraepi-thelial neoplasia: Potential diagnostic implications. Mod. Pathol. 2018, 31, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Rakislova, N.; Alemany, L.; Clavero, O.; Del Pino, M.; Saco, A.; Marimon, L.; Quirós, B.; Lloveras, B.; Ribera-Cortada, I.; Alejo, M.; et al. HPV-independent Precursors Mimicking High-grade Squamous Intraepithelial Lesions (HSIL) of the Vulva. Am. J. Surg. Pathol. 2020, 44, 1506–1514. [Google Scholar] [CrossRef]
- Rakislova, N.; Alemany, L.; Clavero, O.; Del Pino, M.; Saco, A.; Quirós, B.; Lloveras, B.; Alejo, M.; Halec, G.; Quint, W.; et al. Differentiated Vulvar Intraepithelial Neoplasia-like and Lichen Sclerosus-like Lesions in HPV-associated Squamous Cell Carcinomas of the Vulva. Am. J. Surg. Pathol. 2018, 42, 828–835. [Google Scholar] [CrossRef]
- Griesinger, L.M.; Walline, H.; Wang, G.Y.; Lorenzatti Hiles, G.; Welch, K.C.; Haefner, H.K.; Lieberman, R.W.; Skala, S.L. Expanding the Morphologic, Immunohistochemical, and HPV Genotypic Features of High-grade Squamous Intraepithelial Lesions of the Vulva with Morphology Mimicking Differentiated Vulvar Intraepithelial Neoplasia and/or Lichen Sclerosus. Int. J. Gynecol. Pathol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.A.; Ji, J.X.; Almadani, N.; Crawford, R.I.; Gilks, C.B.; Kinloch, M.; Hoang, L. Comparison of p53 immunohistochemical staining in differentiated vulvar intraepithelial neoplasia (dVIN) with that in inflammatory dermatoses and benign squamous lesions in the vulva. Histopathology 2021, 78, 424–433. [Google Scholar] [CrossRef]
- Wing-Cheuk Wong, R.; Palicelli, A.; Hoang, L.; Singh, N. Interpretation of p16, p53 and mismatch repair protein immuno-histochemistry in gynaecological neoplasia. Diagn. Histopathol. 2020, 26, 257–277. [Google Scholar] [CrossRef]
- Jeffreys, M.; Jeffus, S.K.; Herfs, M.; Quick, C.M. Accentuated p53 staining in usual type vulvar dysplasia—A potential diagnostic pitfall. Pathol. Res. Pract. 2018, 214, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Liegl, B.; Regauer, S. p53 immunostaining in lichen sclerosus is related to ischaemic stress and is not a marker of differentiated vulvar intraepithelial neoplasia (d-VIN). Histopathology 2006, 48, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnos-tic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef]
- Cohen, P.A.; Anderson, L.; Eva, L.; Scurry, J. Clinical and molecular classification of vulvar squamous pre-cancers. Int. J. Gynecol. Cancer 2019, 29, 821–828. [Google Scholar] [CrossRef]
- Leblebici, C.; Paşaoğlu, E.; Kelten, C.; Darakci, S.; Dursun, N. Cytokeratin 17 and Ki-67: Immunohistochemical markers for the differential diagnosis of keratoacanthoma and squamous cell carcinoma. Oncol. Lett. 2017, 13, 2539–2548. [Google Scholar] [CrossRef]
- Sari Aslani, F.; Safaei, A.; Pourjabali, M.; Momtahan, M. Evaluation of Ki67, p16 and CK17 Markers in Differentiating Cervical Intraepithelial Neoplasia and Benign Lesions. Iran. J. Med. Sci. 2013, 38, 15–21. [Google Scholar]
- Chang, X.; Zhang, J.; Huang, C.; Pang, X.; Luo, Q.; Zhang, H.; Zhang, S. Sex-determining region Y-related high mobility group box (SOX)-2 is overexpressed in cervical squamous cell carcinoma and contributes cervical cancer cell migration and invasion in vitro. Tumour Biol. 2015, 36, 7725–7733. [Google Scholar] [CrossRef]
- Liu, K.; Xie, F.; Zhao, T.; Zhang, R.; Gao, A.; Chen, Y.; Li, H.; Zhang, S.; Xiao, Z.; Li, J.; et al. Targeting SOX2 protein with pep-tide aptamers for therapeutic gains against esophageal squamous cell carcinoma. Mol. Ther. 2020, 28, 901–913. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Xiao, X.; Wang, C.; Zhang, X.; Wang, L.; Zhang, X.; Li, W.; Zheng, G.; Wang, S.; et al. SOX2 nuclear expres-sion is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral. Oncol. 2011, 47, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Flores, A. Cytokeratin 17 immunoexpression in actinic keratosis (bowenoid and nonbowenoid) and in bowen dis-ease. Ann. Diagn. Pathol. 2016, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zięba, S.; Chechlińska, M.; Kowalik, A.; Kowalewska, M. Genes, pathways and vulvar carcinoma—New insights from next-generation sequencing studies. Gynecol. Oncol. 2020, 158, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Trietsch, M.D.; Nooij, L.S.; Gaarenstroom, K.N.; Van Poelgeest, M.I. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: A review of the current literature. Gynecol. Oncol. 2015, 136, 143–157. [Google Scholar] [CrossRef]
- Williams, E.A.; Werth, A.J.; Sharaf, R.; Montension, M.; Sokol, E.S.; Pavlick, D.C.; McLaughlin-Drubin, M.; Erlich, R.; Toma, H.; Jon Williams, K.; et al. Vulvar squamous cell carcinoma: Comprehensive genomic profling of HPV+ versus HPV- forms reveals distinct sets of potentially actionable targets. JCO Precis. Oncol. 2020, 4, 647–661. [Google Scholar] [CrossRef]
- Nooij, L.S.; Ter Haar, N.T.; Ruano, D.; Rakislova, N.; van Wezel, T.; Smit, V.T.; Trimbos, B.J.; Ordi, J.; van Poelgeest, M.I.E.; Bosse, T. Genomic Characterization of Vulvar (Pre)cancers Identifies Distinct Molecular Subtypes with Prognostic Significance. Clin. Cancer Res. 2017, 23, 6781–6789. [Google Scholar] [CrossRef]
- Pouwer, A.-F.W.; Einden, L.C.V.D.; Van Der Linden, M.; Hehir-Kwa, J.Y.; Yu, J.; Hendriks, K.M.; Kamping, E.J.; Eijkelenboom, A.; Massuger, L.F.; Bulten, J.; et al. Clonal Relationship Between Lichen Sclerosus, Differentiated Vulvar Intra-epithelial Neoplasia and Non HPV-related Vulvar Squamous Cell Carcinoma. Cancer Genom. Proteom. 2020, 17, 151–160. [Google Scholar] [CrossRef]
- Zięba, S.; Pouwer, A.-F.W.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Bakuła-Zalewska, E.; Kopczyński, J.; Pijnenborg, J.M.A.; De Hullu, J.A.; Kowalewska, M. Somatic Mutation Profiling in Premalignant Lesions of Vulvar Squamous Cell Carcinoma. Int. J. Mol. Sci. 2020, 21, 4880. [Google Scholar] [CrossRef]
- Giannakis, M.; Hodis, E.; Mu, X.J.; Yamauchi, M.; Rosenbluh, J.; Cibulskis, K.; Saksena, G.; Lawrence, M.S.; Qian, Z.R.; Nishihara, R.; et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014, 46, 1264–1266. [Google Scholar] [CrossRef]
- Sarris, E.G.; Saif, M.W.; Syrigos, K.N. The Biological Role of PI3K Pathway in Lung Cancer. Pharmaceuticals 2012, 5, 1236–1264. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; De Vet, H.C.W.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hart, W.R. Vulvar intraepithelial neoplasia of the simplex (differentiated) type: A clinicopathologic study including analysis of HPV and p53 expression. Am. J. Surg. Pathol. 2000, 24, 429–441. [Google Scholar] [CrossRef] [PubMed]

| Mean Age (95% CI) | Adjacent * | Standalone | Vulvar Skin with Adnexa | Vulvar Skin without Adnexa | |
|---|---|---|---|---|---|
| Number of Cases (Percentage) | |||||
| dVIN (n = 56) | 69.3 (66.0–72.8) | 35 (63) | 21 (38) | 37 (66) | 19 (34) |
| de-VIL (n = 8) | 68.3 (58.9–77.6) | 4 (50) | 4 (50) | 6 (75) | 2 (25) |
| HSIL (n = 27) | 64.2 (57.8–70.5) | 8 (30) | 19 (70) | 24 (89) | 3 (11) |
| Non-dysplastic vulvar tissue (n = 46) | 63.2 (58.2–68.2) | 23 (50) | 23 (50) | 38 (83) | 8 (17) |
| Immunohistochemical Marker | Expression Patterns | Diagnoses | ||||
|---|---|---|---|---|---|---|
| dVIN (n = 56) | de-VIL (n = 8) | HSIL (n = 27) | Non-Dysplastic Vulvar Tissue (n = 46) | |||
| Number of Cases (Percentage) | ||||||
| p53 | mutant patterns | Parabasal/diffuse overexpression | 30 (54) | 0 (0) | 0 (0) | 1 (2) |
| Basal overexpression | 1 (2) | 0 (0) | 0 (0) | 7 (15) | ||
| Null-pattern | 10 (18) | 0 (0) | 0 (0) | 0 (0) | ||
| wild-type patterns | Wild-type (scattered) | 15 (26) | 8 (100) | 1 (4) | 38 (83) | |
| Wild-type (mid-epithelial) | 0 (0) | 0 (0) | 26 (96) | 0 (0) | ||
| p16 | Block-type expression | 0 (0) | 0 (0) | 26 (96) | 0 (0) | |
| Non-block-type expression | 2 (4) | 0 (0) | 1 (4) | 7 (15) | ||
| No expression | 54 (96) | 8 (100) | 0 (0) | 39 (85) | ||
| MIB1 | Increased expression | 29 (52) | 7 (88) | 27 (100) | 18 (39) | |
| Not increased expression | 27 (48) | 1 (12) | 0 (0) | 28 (61) | ||
| CK17 | Diffuse, moderate-strong, full epithelial expression | 27 (48) | 5 (63) | 6 (22) | 1 (2) | |
| Diffuse, moderate-strong, suprabasal expression | 18 (32) | 2 (25) | 11 (41) | 3 (7) | ||
| Patchy, moderate-strong, suprabasal expression | 8 (14) | 1 (12) | 10 (37) | 5 (11) | ||
| Patchy, weak, suprabasal expression | 2 (4) | 0 (0) | 0 (0) | 19 (41) | ||
| No expression | 1 (2) | 0 (0) | 0 (0) | 18 (39) | ||
| SOX2 | Diffuse, moderate-strong, full epithelial expression | 37 (66) | 5 (63) | 12 (44) | 2 (4) | |
| Diffuse, moderate-strong, basal and suprabasal expression | 11 (20) | 2 (25) | 12 (44) | 7 (15) | ||
| Scattered, weak, basal (predominant) and suprabasal expression | 7 (13) | 1 (12) | 2 (8) | 17 (37) | ||
| No expression | 1 (1) | 0 (0) | 1 (4) | 20 (44) | ||
| Median percentage of cells showing expression (95% CI) | ||||||
| CK17 | 65 (55.3–69.4) | 90 (53.7–95) | 45 (27.6–50.2) | 5 (14.4–32.1) | ||
| SOX2 | 75 (61.8–76.1) | 78 (48–96.9) | 80 (58.4–81.5) | 5 (12.8–29.2) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgupta, S.; Koljenović, S.; van den Bosch, T.P.P.; Swagemakers, S.M.A.; van der Hoeven, N.M.A.; van Marion, R.; van der Spek, P.J.; van Doorn, H.C.; van Kemenade, F.J.; Ewing-Graham, P.C. Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN). Pharmaceuticals 2021, 14, 324. https://doi.org/10.3390/ph14040324
Dasgupta S, Koljenović S, van den Bosch TPP, Swagemakers SMA, van der Hoeven NMA, van Marion R, van der Spek PJ, van Doorn HC, van Kemenade FJ, Ewing-Graham PC. Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN). Pharmaceuticals. 2021; 14(4):324. https://doi.org/10.3390/ph14040324
Chicago/Turabian StyleDasgupta, Shatavisha, Senada Koljenović, Thierry P. P. van den Bosch, Sigrid M. A. Swagemakers, Nick M. A. van der Hoeven, Ronald van Marion, Peter J. van der Spek, Helena C. van Doorn, Folkert J. van Kemenade, and Patricia C. Ewing-Graham. 2021. "Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN)" Pharmaceuticals 14, no. 4: 324. https://doi.org/10.3390/ph14040324
APA StyleDasgupta, S., Koljenović, S., van den Bosch, T. P. P., Swagemakers, S. M. A., van der Hoeven, N. M. A., van Marion, R., van der Spek, P. J., van Doorn, H. C., van Kemenade, F. J., & Ewing-Graham, P. C. (2021). Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN). Pharmaceuticals, 14(4), 324. https://doi.org/10.3390/ph14040324






