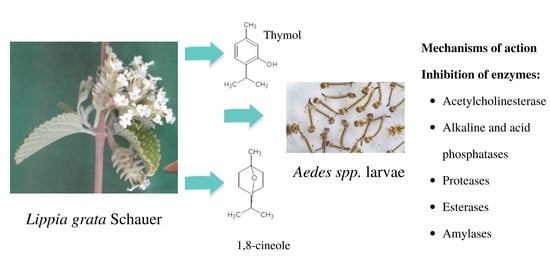
Chemical Composition, Larvicidal Activity, and Enzyme Inhibition of the Essential Oil of Lippia grata Schauer from the Caatinga Biome against Dengue Vectors
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis of Lippia grata Essential Oils
2.2. Larvicidal Activity of L. grata Essential Oils against Larvae of Ae. aegypti and Ae. albopictus
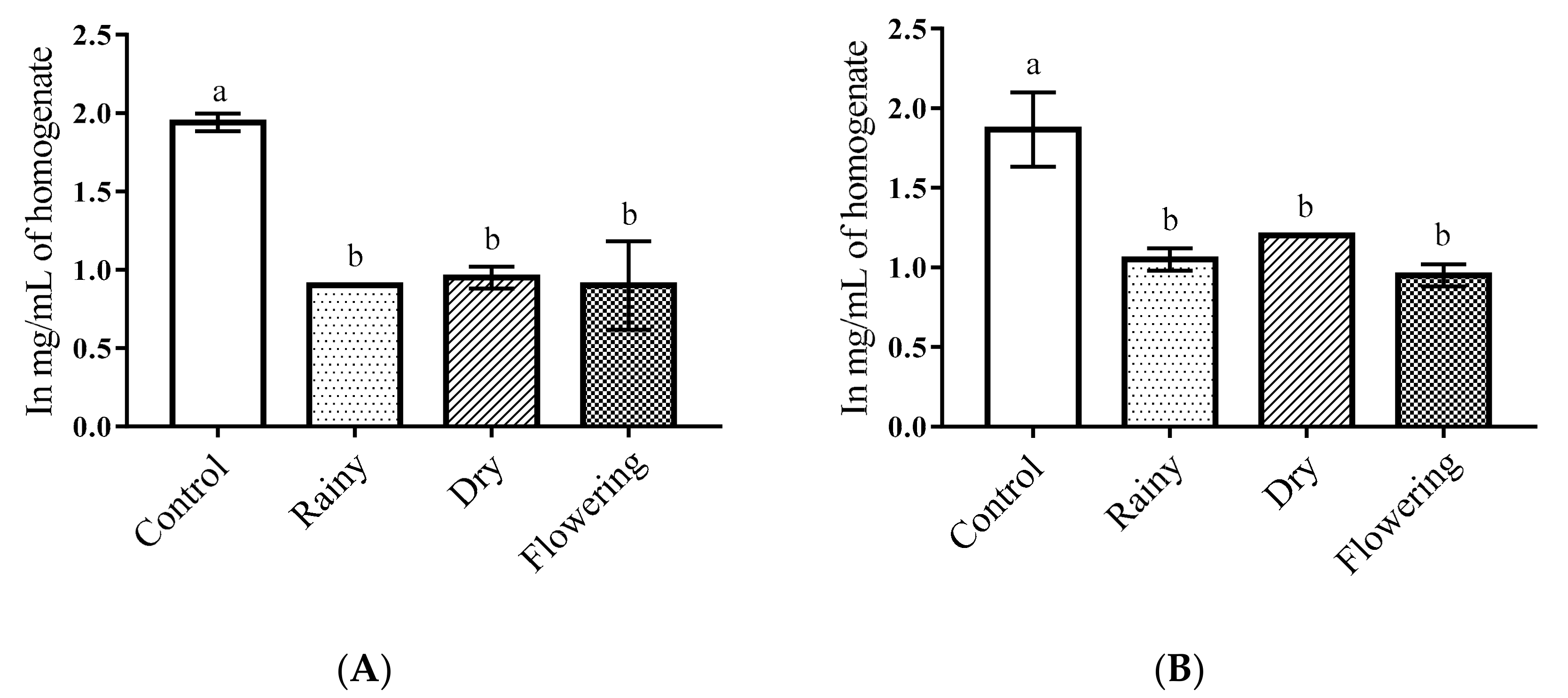
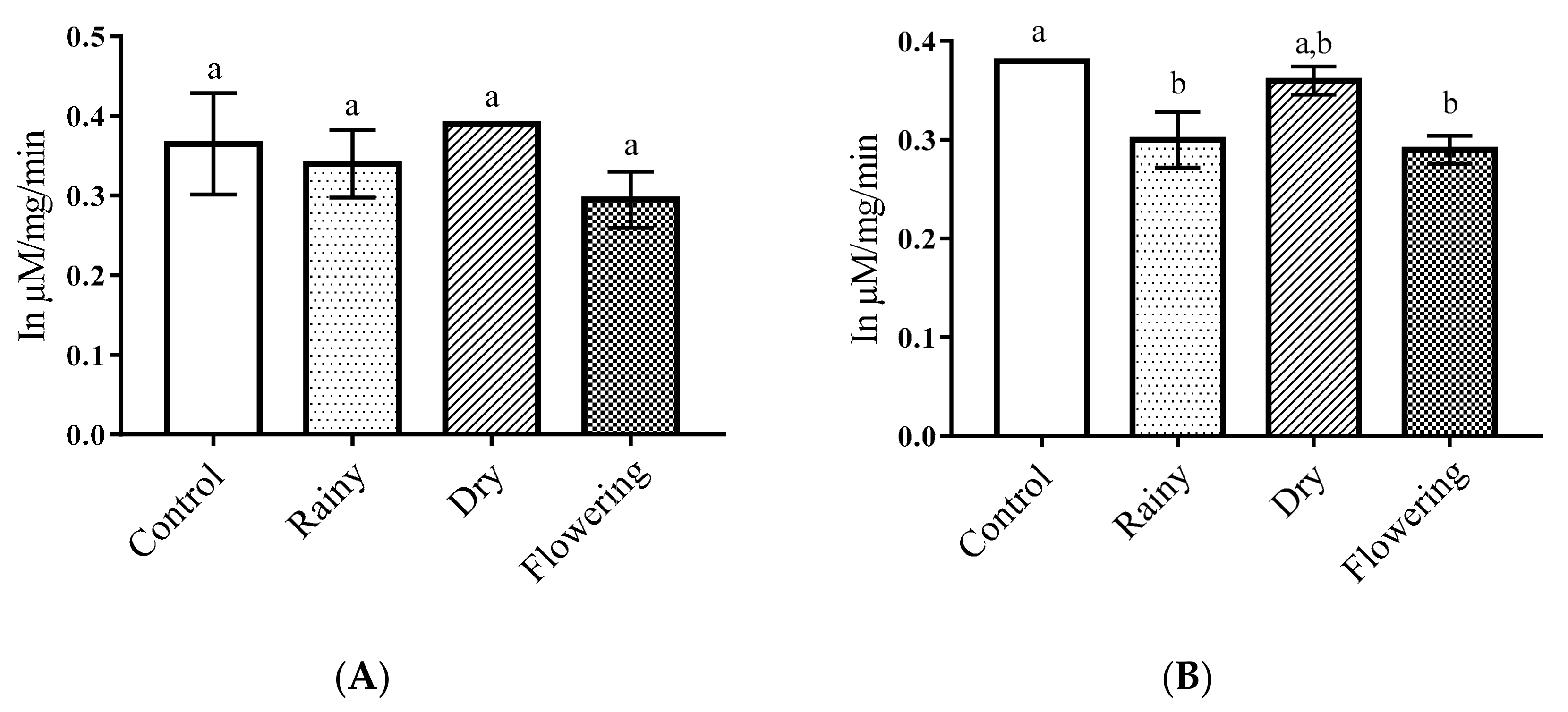
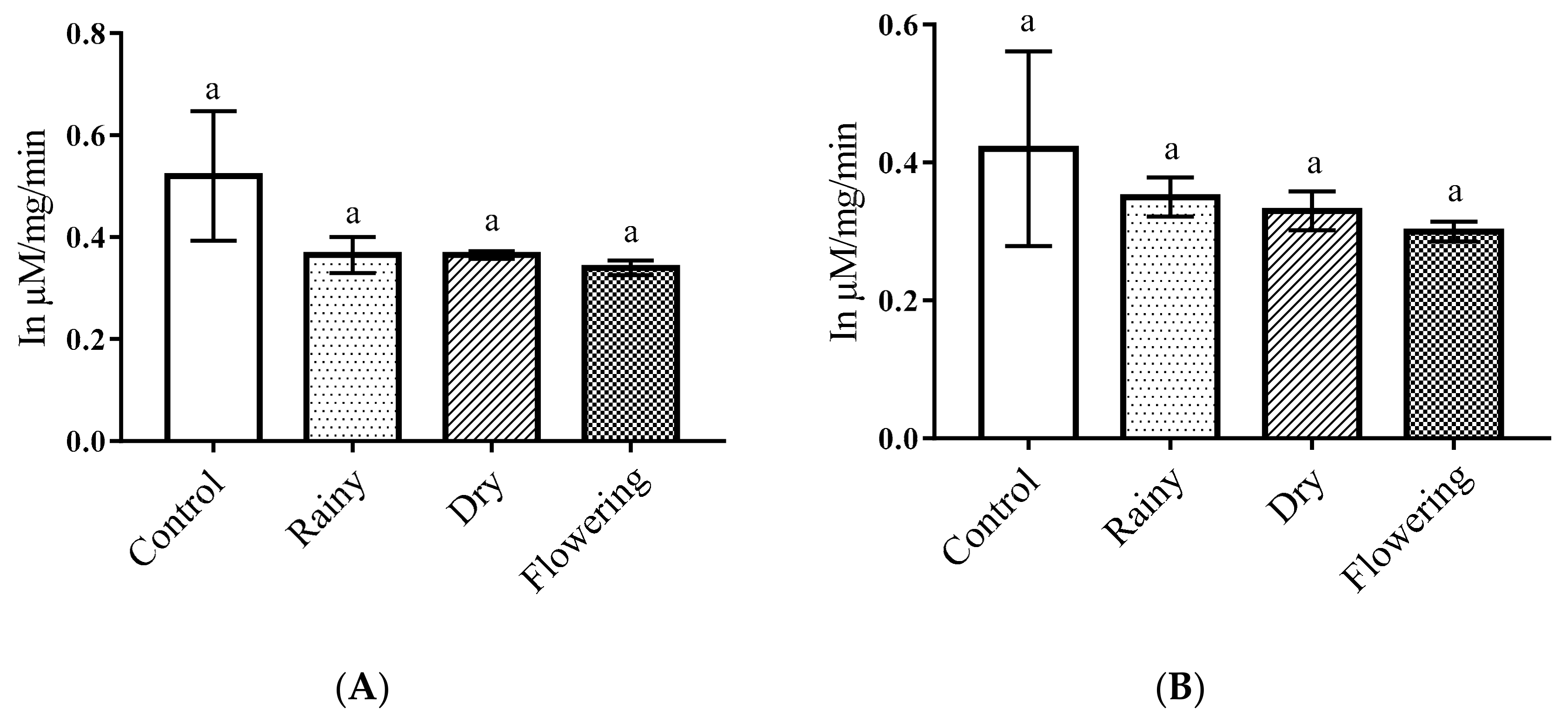
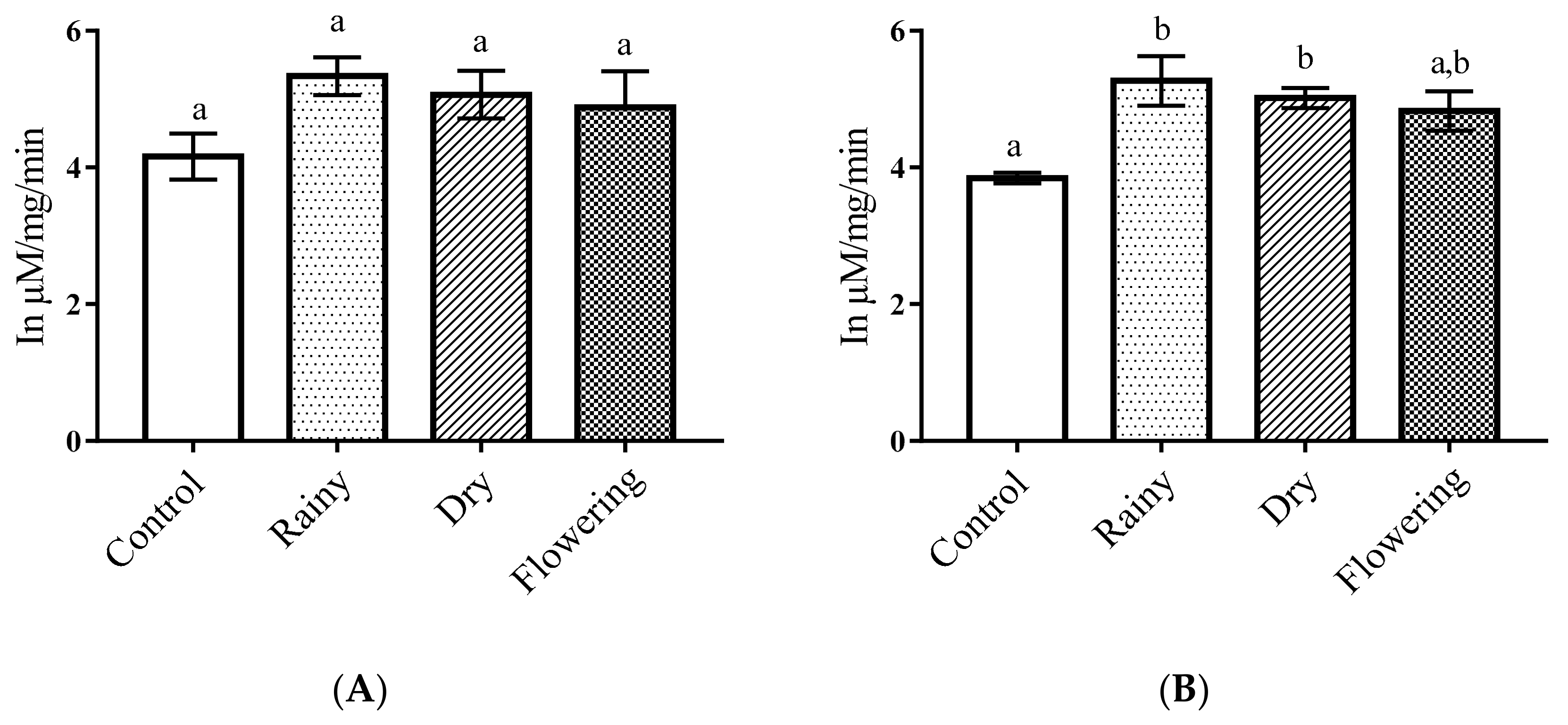
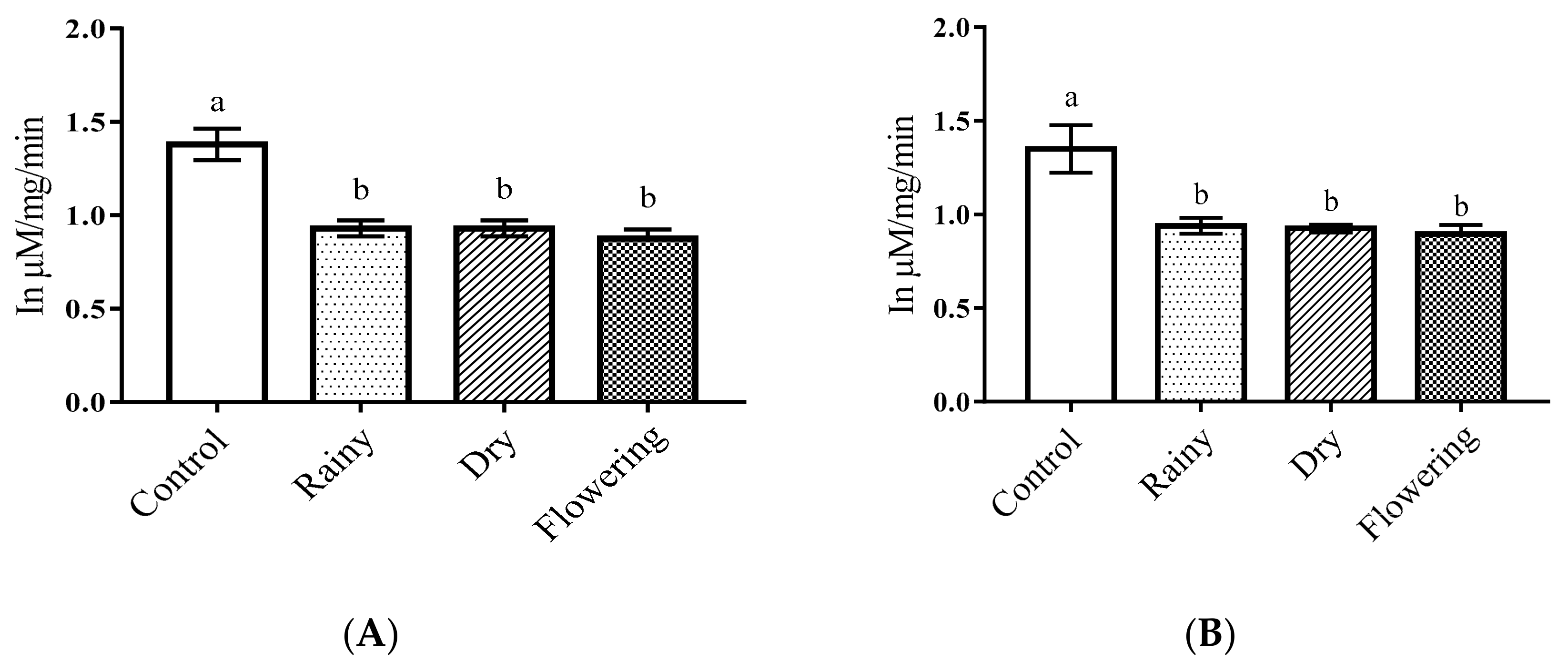
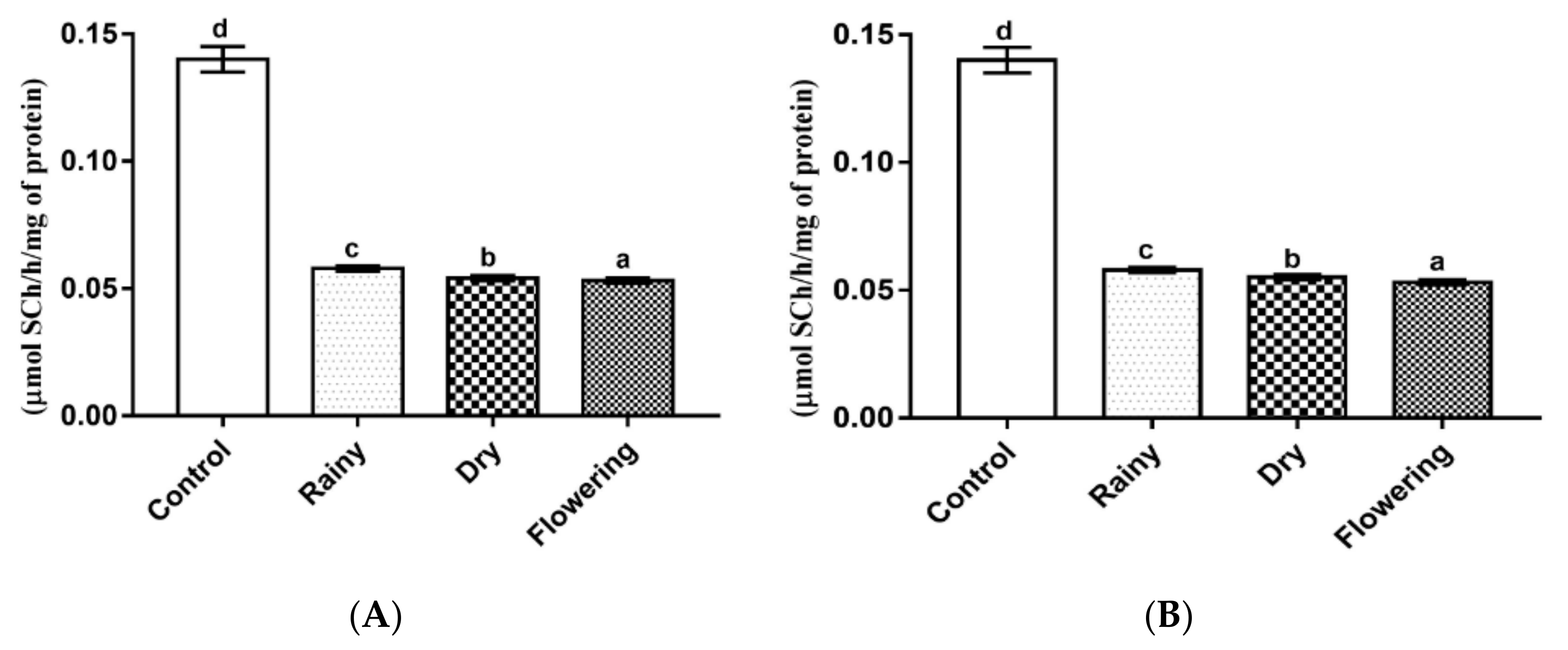
2.3. Activity of the Essential Oils of L. grata on Enzymes of the Larvae of Ae. aegypti and Ae. albopictus
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of the Essential Oils
3.3. Chemical Analysis of the Essential Oils
3.4. Larvicidal Activity
3.5. Activity of Essential Oils on Enzymes of Ae. aegypti and Ae. albopictus Larvae
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourenço-de-Oliveira, R.; Vazeille, M.; de Filippis, A.M.B.; Failloux, A.B. Aedes aegypti in Brazil: Genetically differentiated populations with high susceptibility to dengue and yellow fever viruses. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 43–54. [Google Scholar] [CrossRef]
- Souza, M.L.A.; Andrade, L.M.B.; Spyrides, M.H.C.; Justino, J.R. Bayesian estimates for the mapping of dengue hotspots and estimation of the risk of disease epidemic in Northeast Brazil. Urban Clim. 2018, 26, 198–211. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Secretaria de Vigilância em Saúde; Ministério da Saúde. Boletim epidemiológico: Monitoramento de casos de arbovírus transmitidos por Aedes aegypti (dengue, chikungunya e zika). Sem. Epidemiológicas 2020, 1, 46. Available online: https://www.gov.br/saude/pt-br/media/pdf/2020/dezembro/11/boletim_epidemiologico_svs_48.pdf (accessed on 28 December 2020).
- Morais, S.M.; Cavalcanti, E.S.B.; Bertini, L.M.; Oliveira, C.L.L.; Rodrigues, J.R.B.; Cardoso, J.H.L. Larvicidal activity of essential oils from Brazilian Croton species against Aedes aegypti L. J. Am. Mosq. Control Assoc. 2006, 22, 161–164. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.A. Review of biopesticides and their mode of action against insect pests. In Environmental Sustainability; Thangavel, P., Sridevi, G., Eds.; Springer: New Delhi, India, 2015; pp. 49–63. [Google Scholar] [CrossRef]
- Gross, A.D.; Norris, E.J.; Kimber, M.J.; Coats, J.R.; Bartholomay, L.C. Essential oils enhance the toxicity of permethrin against Aedes aegypti and Anopheles gambiae. Med. Vet. Entomol. 2017, 31, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Astari, S.; Tan, M. Resistance of Aedes aegypti (Diptera: Culicidae) in 2006 to pyrethroid insecticides in indonesia and its association with oxidase and esterase leveis. Pak. J. Biol. Sci. 2007, 10, 3688–3692. [Google Scholar] [CrossRef]
- Panini, M.; Manicardi, G.C.; Moores, G.D.; Mazzoni, E. An overview of the main pathways of metabolic resistance in insects. Inverteb. Surviv. J. 2016, 13, 326–335. [Google Scholar] [CrossRef]
- Sharhriari, M.; Sahebzadeh, N.; Zibaee, A. Effect of Teucrium polium (Lamiaceae) essential oil on digestive enzyme activities and energy reserves of Ephestia kuehniella (Lepidoptera: Pyralidae). Invertebr. Surviv. J. 2017, 14, 182–189. [Google Scholar]
- Senthil-Nathan, S.A. Review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol. 2020, 10, 1591. [Google Scholar] [CrossRef]
- Parthiban, E.; Arokiyaraj, C.; Ramanibai, R. Annona muricata: An alternate mosquito control agent with special reference to inhibition of detoxifying enzymes in Aedes aegypti. Ecotoxicol. Environ. Saf. 2020, 189, 110050. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops. Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Sanchez Mata, D.S.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Cavalcanti, E.S.; Morais, S.M.; Lima, M.A.; Santana, E.W. Larvicidal activity of essential oils from Brazilian plants against Aedes aegypti L. Mem. Inst. Oswaldo Cruz. 2004, 99, 541–544. [Google Scholar] [CrossRef]
- Carvalho, R.R.C.; Laranjeira, D.; Souza, P.E.D.; Blank, A.F.; Alves, P.B.; Jesus, H.C.R.D.; Warwick, D.R.N. In Vitro activity of essential oils of Lippia sidoides and Lippia gracilis and their major chemical components against Thielaviopsis paradoxa, causal agent of stem bleeding in coconut palms. Quím. Nova 2013, 36, 241–244. [Google Scholar] [CrossRef]
- De Lima, G.P.G.; Souza, T.M.; Freire, G.P.; Farias, D.F.; Cunha, A.P.; Ricardo, N.M.P.S.; Morais, S.M.; Carvalho, A.F.U. Further insecticidal activities of essential oils from Lippia sidoides and Croton species against Aedes aegypti L. Parasitol. Res. 2013, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Santiago, G.M.P.; Arriaga, A.M.C.; Lemos, T.L.G.; Pessoa, O.D.L.; Lima, M.C.L.; Matos, F.J.A.; Lima, M.A.S.; Santos, H.S.; Silveira, E.R.; Barbosa, F.G.; et al. Larvicidal activity against Aedes aegypti L. (Diptera: Culicidae) of essential oils of Lippia species from Brazil. Nat. Prod. Commun. 2006, 1, 517–576. [Google Scholar] [CrossRef]
- Vera, S.S.; Zambrano, D.F.; Méndez-Sanchez, S.C.; Rodríguez-Sanabria, F.; Stashenko, F.E.; Luna, J.E.D. Essential oils with insecticidal activity against larvae of Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2647–2654. [Google Scholar] [CrossRef]
- Gomes, S.V.F.; Nogueira, P.C.L.; Moraes, V.R.S. Aspectos químicos e biológicos do gênero Lippia enfatizando Lippia gracilis Schauer. Eclet. Quím. 2011, 36, 64–77. [Google Scholar] [CrossRef]
- Silva, W.J.; Doria, G.A.A.; Maiaetal, R.T. Effects of essential oils on Aedes aegypti larvae: Alternatives to environmentally safe insecticides. Bioresour. Technol. 2008, 99, 3251–3255. [Google Scholar] [CrossRef]
- Dias, C.N.; Alves, L.P.L.; Rodrigues, K.A.F.; Brito, M.C.A.; Rosa, C.S.; Amaral, F.M.M.; Monteiro, O.S.; Andrade, E.H.A.; Maia, J.G.S.; Moraes, D.F.C. Chemical composition and larvicidal activity of essential oils extracted from Brazilian Legal Amazon plants against Aedes aegypti L. (Diptera: Culicidae). Evid. Based Complementary Altern. Med. 2015, 2015, 490765. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Sampaio, C.G.; Souza, J.S.N.; Campos, A.R.; Silva, A.B.R.; Morais, S.M.; Martins, V.E.P. Different susceptibilities of Aedes aegypti and Aedes albopictus larvae to plant-derived products. Rev. Soc. Bras. Med. Trop. 2019, 52, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bitu, V.C.N.; Fecundo, H.D.T.F.; Costa, J.G.M.; Rodrigues, F.F.G.; Colares, A.V.; Coutinho, H.D.M.; Botelho, M.A.; Portela, A.C.; Santana, N.M.; Menezes, I.R.A. Effect of collection time on composition of essential oil of Lippia gracilis Schauer (Verbenaceae) growing in Northeast Brazil. J. Essent. Oil Bear. Plants. 2015, 18, 647–653. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatistica. Território. Available online: https://brasilemsintese.ibge.gov.br/territorio.html. (accessed on 19 January 2021).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4.1 ed.; Allured Publ. Corp: Carol Stream, IL, USA, 2017; p. 804. [Google Scholar]
- Barriga, I.B.; Gonzales, A.P.P.F.; Brasiliense, A.R.P.; Castro, K.N.C.; Tavares-Dias, M. Essential oil of Lippia grata (Verbenaceae) is effective in the control of monogenean infections in Colossoma macropomum gills, a large Serrasalmidae fish from Amazon. Aquac. Res. 2020, 51, 3804–3812. [Google Scholar] [CrossRef]
- Albuquerque, C.C.; Camara, T.R.; Sant’ana, A.E.G.; Ulisses, C.; Willadino, L.; Marcelino Júnior, C. Effects of the essential oil of Lippia gracilis Schauer on caulinary shoots of heliconia cultivated In Vitro. Rev. Bras. Pl. Med. 2012, 14, 26–33. [Google Scholar] [CrossRef][Green Version]
- Cruz, E.M.O.; Mendonça, M.C.; Blank, A.F.; Sampaio, T.S.; Pinto, J.A.O.; Gagliardi, P.R.; Junior, L.F.G.O.; Lima, R.S.N.; Nunes, R.S.; Warwick, D.R.N. Lippia gracilis Schauer essential oil nanoformulation prototype for the control of Thielaviopis paradoxa. Ind. Crops Prod. 2018, 117, 245–251. [Google Scholar] [CrossRef]
- Neto, R.M.; Matos, F.J.A.; Andrade, V.S.; Melo, M.C.N.; Carvalho, C.B.M.; Guimarães, S.B.; Pessoa, O.D.L.; Silva, S.L.; Silva, S.F.R.; Vasconcelos, P.R.L. The essential oil from Lippia gracilis Schauer, Verbenaceae, in diabetic rats. Braz. J. Pharmacogn. 2010, 20, 261–266. [Google Scholar] [CrossRef]
- Craveiro, A.A.; Alencar, J.W.; Matos, F.J.A.; Andrade, C.H.S.; Machado, M.I.L. Essential oils from brazilian Verbenaceae. genus Lippia. J. Nat. Prod. 1981, 44, 598–601. [Google Scholar] [CrossRef]
- Souza, A.V.V.; Santos, U.S.; Corrêa, R.M.; Souza, D.D.; Oliveira, F.J.V. Essential oil content and chemical composition of Lippia gracilis Schauer cultived in the Sub-meddle São Francisco Valley. J. Essent. Oil Bear. Plants. 2017, 20, 983–994. [Google Scholar] [CrossRef]
- Melo, J.O.; Blanck, A.F.; Oliveira, A.M.S.; Andrade, T.M.; Arrigoni-Blanck, M.F.; Alves, P.B. Content and chemical composition of the essential oil of Lippia gracilis Schauer accessions in different drying times. Biosci. J. 2019, 35, 1821–1828. [Google Scholar] [CrossRef]
- Franco, C.S.; Ribeiro, A.F.; Carvalho, N.C.C.; Monteiro, O.S.; Silva, J.K.R.; Andrade, E.H.A.; Maia, J.G.S. Composition antioxidante and antifungal and activities of the essential oil of Lippia gracilis Schauer. Afr. J. Biotechnol. 2014, 13, 3107–3113. [Google Scholar] [CrossRef]
- Botrel, P.P.; Pinto, J.E.B.P.; Ferraz, V.; Bertolucci, S.K.V.; Figueiredo, F.C. Teor e composição química do óleo essencial de Hyptis marrubioides Epl., Lamiaceae em função da sazonalidade. Acta Sci. Agron. 2010, 32, 533–538. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Lopes, N.P. Plantas medicinais: Fatores que influenciam o conteúdo dos metabólitos secundários. Quím. Nova. 2007, 30, 374–381. [Google Scholar] [CrossRef]
- Mar, J.M.; Silva, L.S.; Azevedo, S.G.; França, L.P.; Goes, A.F.F.; Santos, A.L.; Bezerra, J.A.; Nunomura, R.C.S.; Machado, M.B.; Sanches, E.A. Lippia origanoides essential oil: An efficient alternative to control Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. Ind. Crops Prod. 2018, 111, 292–297. [Google Scholar] [CrossRef]
- Mahanta, S.; Sarma, R.; Khanikor, B. The essential oil of Lippia alba Mill (Lamiales:Verbenaceae) as mosquitocidal and repellent agent against Culex quinquefasciatus Say (Diptera: Culicidae) and Aedes aegypti Linn (Diptera: Culicidae). JOBAZ 2019, 80, 64. [Google Scholar] [CrossRef]
- Liu, X.C.; Liu, Q.; Zhou, L.; Liu, Z.L. Evaluation of larvicidal activity of the essential oil of Allium macrostemon Bunge and its selected major constituent compounds against Aedes albopictus (Diptera: Culicidae). Parasit. Vectors. 2014, 7, 1–5. [Google Scholar] [CrossRef]
- França, L.P.; Amaral, A.C.F.; Ramos, A.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.J.; Branches, A.D.S.; Silva, J.N.; Silva, N.G.; et al. Piper capitarianum essential oil: A promising insecticidal agente for the management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. 2020, 1–17. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repelente capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- Lee, D.C.; Ahn, Y.J. Laboratory and Simulated Field Bioassays to Evaluate Larvicidal Activity of Pinus densiflora Hydrodistillate, Its Constituents and Structurally Related Compounds against Aedes albopictus, Aedes aegypti and Culex pipiens pallens in Relation to Their Inhibitory Effects on Acetylcholinesterase Activity. Insects 2013, 4, 217–229. [Google Scholar] [CrossRef]
- Furtado, R.S.; de Lima, M.G.A.; Andrade Neto, M.; Bezerra, J.N.S.; Silva, M.G.V. Atividade larvicida de óleos essenciais contra Aedes aegypti L. (Diptera: Culicidae). Neotrop. Entomol. 2005, 34, 843–847. [Google Scholar] [CrossRef]
- Norris, E.J.; Johnson, J.B.; Gross, A.D.; Bartholomay, L.C. Plant essential oils enhance diverse pyrethroids against multiple strains of mosquitoes and inhibit detoxification enzyme processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Intirach, J.; Junkum, A.; Lumjuan, N.; Chaithong, U.; Somboon, P.; Jitpakdi, A.; Riyong, D.; Champakaew, D.; Muangmoon, R.; Chansang, A.; et al. Biochemical effects of Petroselinum crispum (Umbellifereae) essential oil on the pyrethroid resistant strains of Aedes aegypti (Diptera: Culicidae). Insects 2019, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Deshpande, S.; Tare, V.; Sabharwal, S. Larvicidal activity of the α-amylase inhibitor from the seeds of Macrotyloma uniflorum (Leguminosae) against Aedes aegypti (Diptera: Culicidae). Int. J. Trop. Insect Sci. 2011, 31, 69–74. [Google Scholar] [CrossRef]
- Braga, L.A.; Valle, D.A. Aedes aegypti: Inseticidas, mecanismos de ação e resistência. Epidemiol. Serv. Saúde. 2007, 16, 279–293. [Google Scholar] [CrossRef]
- Agra-Neto, A.C.; Napoleão, T.H.; Pontual, E.V.; de Lima Santos, N.D.; de Andrade Luz, L.; de Oliveira, C.M.F.; Melo-Santos, M.A.V.; Coelho, L.C.B.B.; Navarro, D.M.A.F.; Paiva, P.M.G. Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol. Res. 2014, 113, 175–184. [Google Scholar] [CrossRef]
- Bakr, R.F.; Helmy, N.; Nawwar, G.A.; Ibrahim, S.E.; Helmy, O.M. Changes in protein content of Culex pipiens mosquito treated with two agriculture waste extracts. Egypt. Acad. J. Biol. Sci. A Entomol. 2010, 3, 95–103. [Google Scholar] [CrossRef]
- Maheswaran, R.; Ignacimuthu, S. A novel herbal formulation against dengue vector mosquitoes Aedes aegypti and Aedes albopictus. Parasitol. Res. 2012, 110, 1801–1813. [Google Scholar] [CrossRef]
- Devi, U.; Bora, D. Growth inhibitory effect of phenolic extracts of Ziziphus jujuba Mill. in dengue vector Aedes aegypti (L) in parent and F1 generation. Asian Pac. J. Trop. Med. 2017, 10, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Koodalingam, A.; Mullainadhan, P.; Arumugam, M. Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae). Acta Trop. 2011, 118, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.P.; Pinto, J.E.A.O.; dos Santos, C.A.; Cruz, E.M.O.; de Fátima Arrigoni-Blank, M.; Andrade, T.M.; Santos, D.A.; Alves, P.B.; Blank, A.F. Harvest time and geographical origin affect the essential oil of Lippia gracillis Schauer. Ind. Crops Prod. 2016, 79, 205–210. [Google Scholar] [CrossRef]
- Saraiva, A.G.Q.; Saraiva, G.D.; Albuquerque, R.L.; Nogueira, C.E.S.; Teixeira, A.M.R.; Lima, L.B.; Cruz, B.G.; de Sousa, F.F. Chemical analysis and vibrational spectroscopy study of essential oils from Lippia sidoides and of its major constituent. Vib. Spectrosc. 2020, 110, 103111. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Borase, H.P.; Narkhede, C.P.; Salunke, B.K.; Patil, S.V. Mosquito larvicidal and pupaecidal potential of prodigiosin from Serratia marcescens and understanding its mechanism of action. Pestic. Biochem. Physiol. 2015, 123, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Sneh, B.; Strizhov, N.; Prudovsky, E.; Regev, A.; Koncz, C.; Schell, J.; Zilberstein, A. Digestion of δ-endotoxin by gut proteases may explain reduced sensitivity of advanced instar larvae of Spodoptera littoralis to Cry1C. Insect Biochem. Mol. Biol. 1996, 26, 365–373. [Google Scholar] [CrossRef]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.G.G.; Vasconcelos, I.M.; Acrisio Filho, J.U.B.; Carvalho, A.F.; Souza, T.M.; Gondim, D.M.F.; Varela, A.L.N.; Oliveira, J.T.A. Castor bean cake contains a trypsin inhibitor that displays antifungal activity against Colletotrichum gloeosporioides and inhibits the midgut proteases of the dengue mosquito larvae. Ind. Crops Prod. 2015, 70, 48–55. [Google Scholar] [CrossRef]
- Shamsi, T.N.; Parveen, R.; Ahmad, A.; Samal, R.R.; Kumar, S.; Fatima, S. Inhibition of gut proteases and development of dengue vector, Aedes aegypti by Allium sativum protease inhibitor. Acta Ecol. Sin. 2018, 38, 325–328. [Google Scholar] [CrossRef]
- Almeida Filho, L.C.P.; Tabosa, P.M.S.; Hissa, D.C.; Vasconcelos, I.M.; Carvalho, A.F.U. First insights on insecticidal activity upon Aedes aegypti and partial biochemical characterization of a novel low molecular mass chymotrypsin-trypsin inhibitor purified from Lonchocarpus sericeus seeds. Pest. Manag. Sci. 2018, 74, 1362–1373. [Google Scholar] [CrossRef]
- Mukanganyama, S.; Figueroa, C.C.; Hasler, J.A.; Niemeyer, H.M. Effects of DIMBOA on detoxification enzymes of the aphid Rhopalosiphum padi (Homoptera: Aphididae). J. Insect Physiol. 2003, 49, 223–229. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Khosravi, R.; Sendi, J.J.; Honarmand, P.; Amini, R.M. Toxicity and physiological effects of essential oil from Agastache foeniculum (Pursh) Kuntze Against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) Larvae. Ann. Res. Rev. Biol. 2013, 3, 649–658. [Google Scholar]
- Hemingway, J.; Ranson, H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 2000, 45, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, M.; Jia, M.; Hu, J.; Zhu, L. Chemical composition and larvicidal activity against Aedes mosquitoes of essential oils from Arisaema fargesii. Pest. Manag. Sci. 2020, 76, 534–542. [Google Scholar] [CrossRef]
- Kumrungsee, N.; Pluempanupat, W.; Koul, O.; Bullangpoti, V. Toxicity of essential oil compounds against diamondback moth, Plutella xylostella, and their impact on detoxification enzyme activities. J. Pest Sci. 2014, 87, 721–729. [Google Scholar] [CrossRef]
- Sharhriari, M.; Sahebzadeh, N.; Zibaee, A.; Khani, A.; Senthil-Nathan, S. Metabolic response of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) to essential oil of Ajwain and thymol. Toxin Rev. 2017, 36, 204–209. [Google Scholar] [CrossRef]
- Nasr, M.; Sendi, J.J.; Moharramipour, S.; Zibaee, A. Evaluation of Origanum vulgare L. essential oil as a source of toxicant and an inhibitor of physiological parameters in diamondback moth, Plutella xylustella L. (Lepidoptera: Pyralidae). J. Saudi Soc. Agric. Sci. 2017, 16, 184–190. [Google Scholar] [CrossRef]
- Farias, L.R.; Costa, F.T.; Souza, L.A.; Pelegrini, P.B.; Grossi-De-Sá, M.F.; Neto, S.M.; Bloch, C.J.; Laumann, R.A.; Nonha, E.F.; Franco, O.L. Isolation of novel Carica papaya a-amylase inhibitor with deleterious activity toward Callosobruchus maculatus. Pestic. Biochem. Physiol. 2007, 87, 255–260. [Google Scholar] [CrossRef]
- Bigham, M.; Hosseininaveh, V.; Nabavi, B.; Talebi, K.; Esmaeilzadeh, N.S. Effects of essential oil from Teucrium polium on some digestive enzyme activities of Musca domestica. Entomol. Res. 2010, 40, 37–45. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Shamakhi, L.; Sahebzadeh, N.; Naseri, D.; Hoda, H. Bio-efficacy and physiological effects of Eucalyptus globulus and Allium sativum essential oils against Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Tox. Rev. 2019, 34, 422–433. [Google Scholar] [CrossRef]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Sugauara, E.Y.; Rahal, I.L.; de Oliveira, H.L.M.; de Campos Bortolucci, W.; Fernandez, C.M.M.; Faria, M.G.I.; Ruiz, S.P.; Gonçalves, J.E.; Colauto, N.B.; Gazim, Z.C.; et al. Inga laurina crude extract to control Aedes aegypti. Res. Soc. Dev. 2020, 9, e1819119683–e1819119683. [Google Scholar] [CrossRef]
- Castillo-Morales, R.M.; Otero, A.L.C.; Mendez-Sanchez, S.C.; Da Silva, M.A.N.; Stashenko, E.E.; Duque, J.E. Mitochondrial affectation, DNA damage and AChE inhibition induced by Salvia officinalis essential oil on Aedes aegypti larvae. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 29–37. [Google Scholar] [CrossRef]
- Seo, S.M.; Jung, C.S.; Kang, J.; Lee, H.R.; Kim, S.W.; Hyun, J.; Park, I.K. Larvicidal and acetylcholinesterase inhibitory activity of apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-arami, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Teles, S.; Pereira, J.A.; Santos, C.H.; Menezes, R.V.; Malheiro, R.; Lucchese, A.M.; Silva, F. Geographical origin and drying methodology may affect the essential oil. Ind. Crops Prod. 2012, 37, 247–252. [Google Scholar] [CrossRef]
- National Institute of Standars and Technology. Chemistry Book on the Web, SRD 69. Available online: https://webbook.nist.gov/chemistry/ (accessed on 25 April 2019).
- World Health Organization (WHO) Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Available online: https://apps.who.int/iris/bitstream/handle/10665/69101/WHO_CDS_WHOPES_GCDPP_2005.13.pdf;jsessionid=ACA4BF09EDE97BB1D53DF249203894E0?sequence=1 (accessed on 13 July 2019).
- Cheng, S.S.; Chang, H.T.; Chang, S.T.; Tsai, K.H.; Chen, W.J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresour. Technol. 2003, 89, 99–102. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Finney, D. Probit Analysis, 3rd ed.; Cambridge University Press: New York, NY, USA, 1971; p. 333. [Google Scholar]


| Constituents | KICal | Content (%) | ||
|---|---|---|---|---|
| REO | DEO | FEO | ||
| Α-Thujene | 926 | 0.19 | − | − |
| α-Pinene | 932 | 1.43 | − | 4.86 |
| Sabinene | 970 | 0.27 | − | − |
| β-Pinene | 972 | 0.26 | − | − |
| Myrcene | 988 | 1.74 | − | 1.32 |
| α-Terpinene | 1013 | 0.62 | − | − |
| p-Cymene | 1021 | 6.02 | 0.89 | 6.07 |
| Limonene | 1025 | 0.51 | − | − |
| 1,8-Cineole | 1028 | 9.43 | 13.58 | 7.00 |
| γ-Terpinene | 1056 | 2.82 | − | 1.33 |
| Terpinen-4-ol | 1180 | 1.74 | 1.21 | 1.09 |
| l-α-terpineol | 1194 | 0.98 | − | − |
| α-Terpineol | 1195 | 1.32 | 2.66 | 1.38 |
| Thymol methyl ether | 1240 | 7.02 | 5.05 | 6.51 |
| Thymol | 1303 | 58.46 | 73.49 | 65.82 |
| Carvacrol | 1309 | 0.45 | − | − |
| Thymol acetate | 1357 | 1.42 | 3.12 | 3.58 |
| α-Copaene | 1376 | 0.69 | − | − |
| Z-caryophyllene | 1417 | 2.57 | − | 1.04 |
| Delta-cadinene | 1511 | 0.71 | − | − |
| Caryophyllene oxide | 1562 | 0.58 | − | − |
| Total | 99.23 | 100 | 100 | |
| State | Chemotype/Percentage | Content (%) | Authors |
|---|---|---|---|
| Ceará | Thymol/carvacrol | 44.4–22.2 | Bitu et al., 2015 [25] |
| Carvacrol/p-cymene | 50.13–10.73 | Neto et al., 2010 [31] | |
| Thymol/carvacrol | 31–12 | Santiago et al., 2005 [19] | |
| Piauí | Carvacrol/p-cymene | 48.12–24.39 | Barriga et al., 2020 [28] |
| Paraiba | p-Cymene/carvacrol | 22.2–20 | Craveiro et al., 1981 [32] |
| Pernambuco | Carvacrol/thymol | 76.8–6.98 | Souza et al., 2017 [33] |
| Sergipe | Carvacrol/y-terpinene | 53.77–9.37 | Melo et al., 2019 [34] |
| Thymol/methyl thymol | 63.81–8.14 | ||
| Maranhão | Thymol/p-cymene | 73.5–9.2 | Franco et al., 2014 [35] |
| Oil | Larvae | LC50 ± SD (CI 95%) | LC90 ± SD (CI 95%) |
|---|---|---|---|
| REO | Ae. Aegypti | 21.77 a ± 0.68 (20.09–23.45) | 51.14 a ± 3.83 (41.62–60.66) |
| Ae. Albopictus | 35.99 c ± 0.54 (34.66–37.32) | 67.91 dc ± 2.25 (62.32–73.5) | |
| DEO | Ae. Aegypti | 36.28 c ± 3.14 (28.48–44.08) | 61.65 cb ± 1.30 (58.42–64.88) |
| Ae. Albopictus | 41.51 d ± 1.33 (38.21–44.81) | 71.11 d ± 4.16 (60.77–81.45) | |
| FEO | Ae. Aegypti | 30.00 b ± 1.89 (25.3–34.7) | 60.09 b ± 2.17 (54.69–65.49) |
| Ae. Albopictus | 46.06 d ± 0.80 (44.07–48.05) | 89.29 e ± 1.66 (85.16–93.43) | |
| Control | Vehicle (DMSO) | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felix, S.F.; Rodrigues, A.M.; Rodrigues, A.L.M.; de Freitas, J.C.C.; Alves, D.R.; da Silva, A.A.; dos Santos, D.L.; de Oliveira, K.R.L.; Montes, R.A.; da Silva, M.V.F.; et al. Chemical Composition, Larvicidal Activity, and Enzyme Inhibition of the Essential Oil of Lippia grata Schauer from the Caatinga Biome against Dengue Vectors. Pharmaceuticals 2021, 14, 250. https://doi.org/10.3390/ph14030250
Felix SF, Rodrigues AM, Rodrigues ALM, de Freitas JCC, Alves DR, da Silva AA, dos Santos DL, de Oliveira KRL, Montes RA, da Silva MVF, et al. Chemical Composition, Larvicidal Activity, and Enzyme Inhibition of the Essential Oil of Lippia grata Schauer from the Caatinga Biome against Dengue Vectors. Pharmaceuticals. 2021; 14(3):250. https://doi.org/10.3390/ph14030250
Chicago/Turabian StyleFelix, Stênio Freitas, Alzeir Machado Rodrigues, Ana Livya Moreira Rodrigues, José Claudio Carneiro de Freitas, Daniela Ribeiro Alves, Alice Araújo da Silva, Dayanne Lima dos Santos, Kethelly Rayne Lima de Oliveira, Renato Almeida Montes, Marcus Vinicius Ferreira da Silva, and et al. 2021. "Chemical Composition, Larvicidal Activity, and Enzyme Inhibition of the Essential Oil of Lippia grata Schauer from the Caatinga Biome against Dengue Vectors" Pharmaceuticals 14, no. 3: 250. https://doi.org/10.3390/ph14030250
APA StyleFelix, S. F., Rodrigues, A. M., Rodrigues, A. L. M., de Freitas, J. C. C., Alves, D. R., da Silva, A. A., dos Santos, D. L., de Oliveira, K. R. L., Montes, R. A., da Silva, M. V. F., da Silva Lopes, F. F., & de Morais, S. M. (2021). Chemical Composition, Larvicidal Activity, and Enzyme Inhibition of the Essential Oil of Lippia grata Schauer from the Caatinga Biome against Dengue Vectors. Pharmaceuticals, 14(3), 250. https://doi.org/10.3390/ph14030250