Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Appearance
2.2. Sol-Gel Analysis
2.3. Determination of Drug Loading Efficiency (DLE, %)
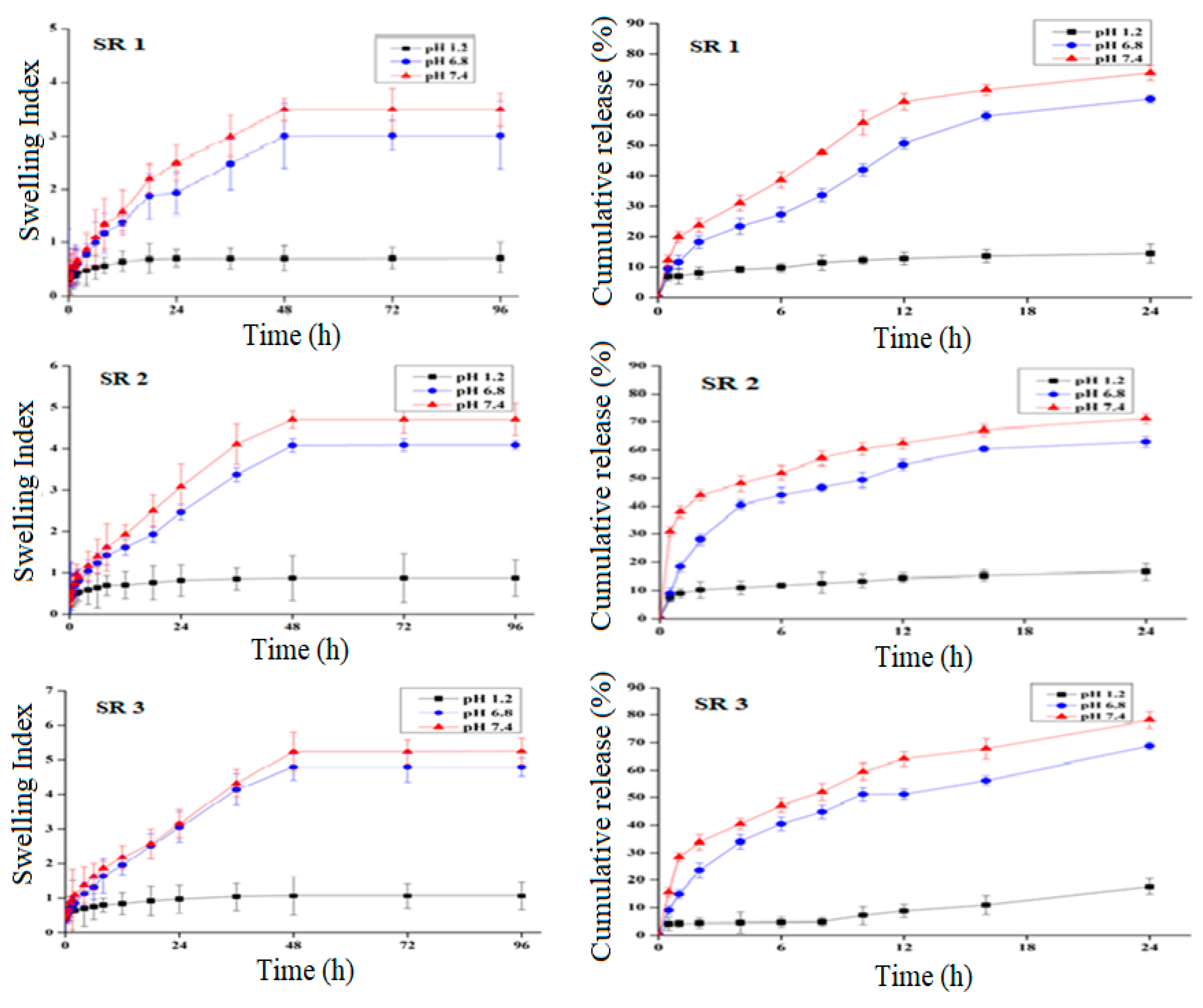
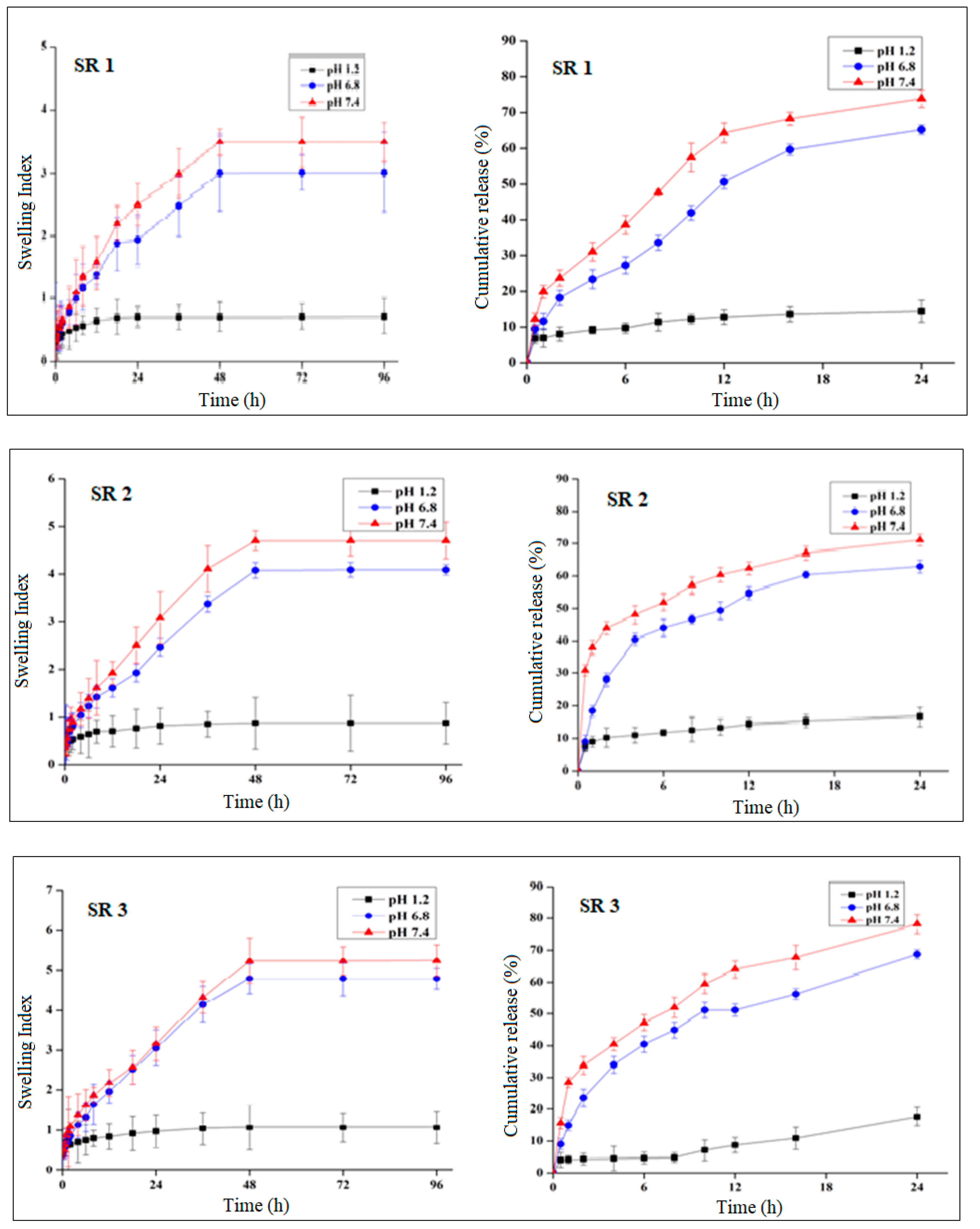
2.4. pH-Sensitivity, Swelling, and Drug Release
2.4.1. pH Effect on Swelling and Drug Release
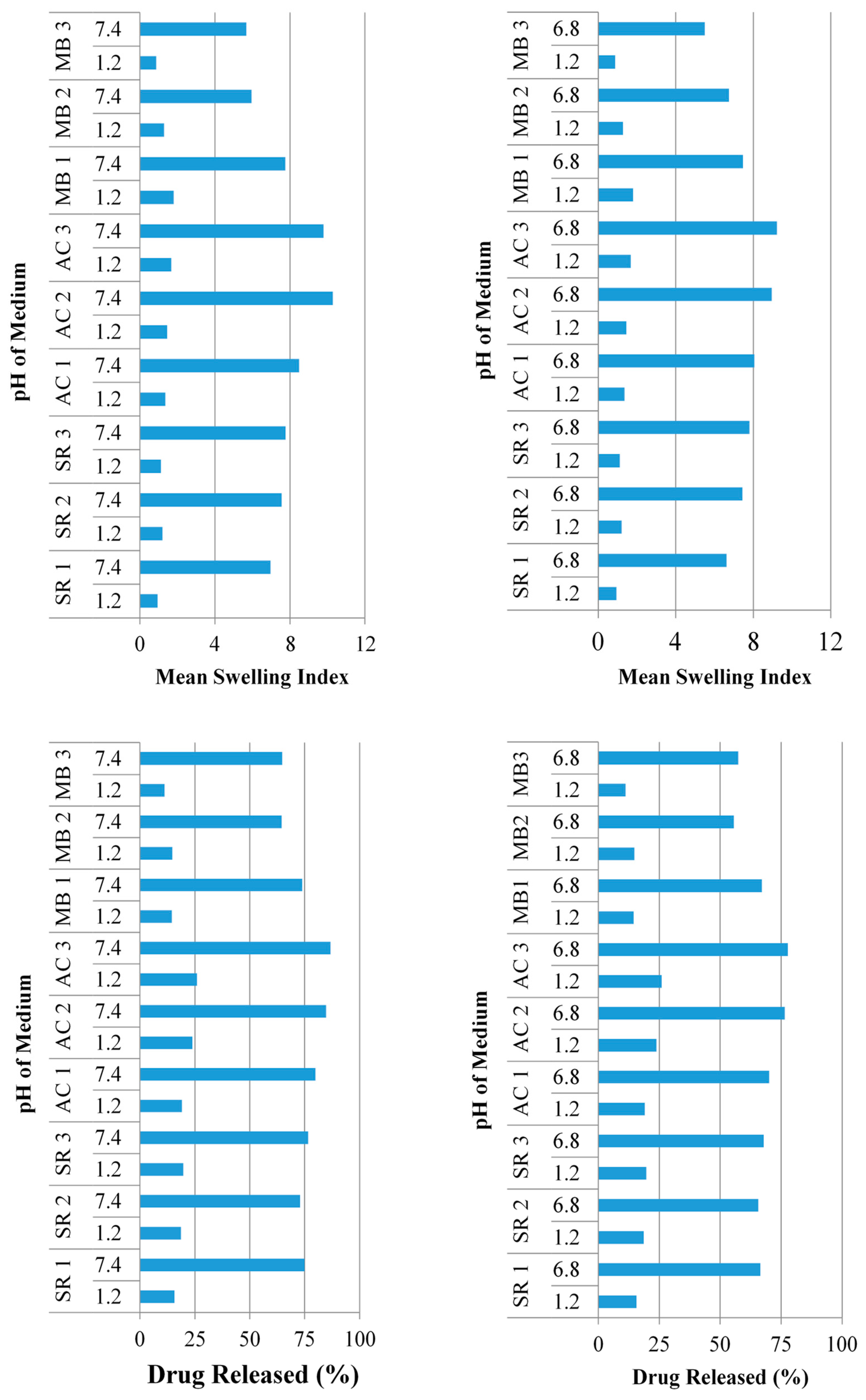
2.4.2. Effect of Polymer Concentration on Swelling and Drug Release
2.4.3. Effect of Monomer Concentration on Swelling and Drug Release
2.4.4. Effect of Cross-Linker Concentration on Swelling and Drug Release
2.5. Chemical Characterizations
2.5.1. Fourier Transform Infrared Spectroscopy (FTIR)
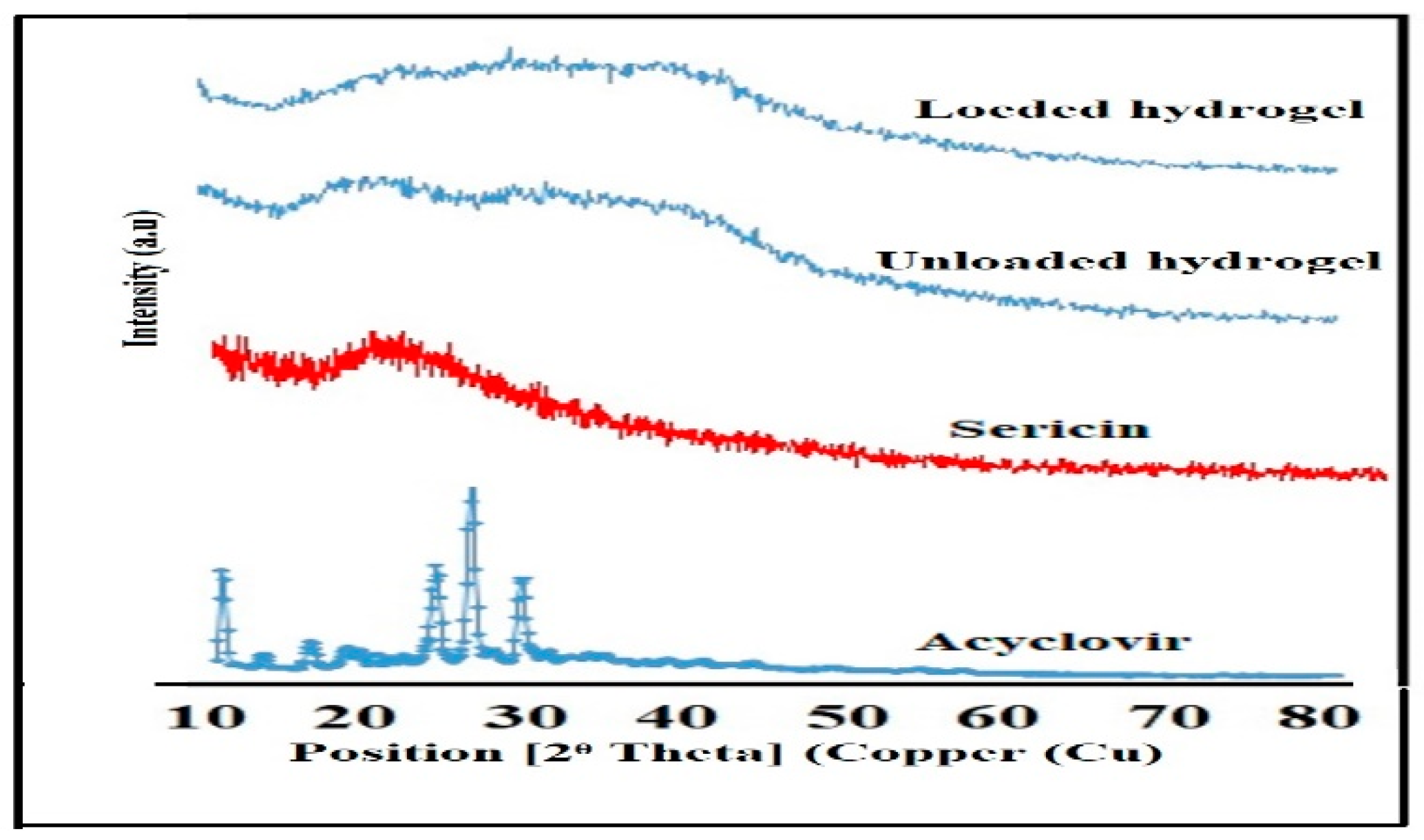
2.5.2. X-rays Diffraction Analysis (XRD)
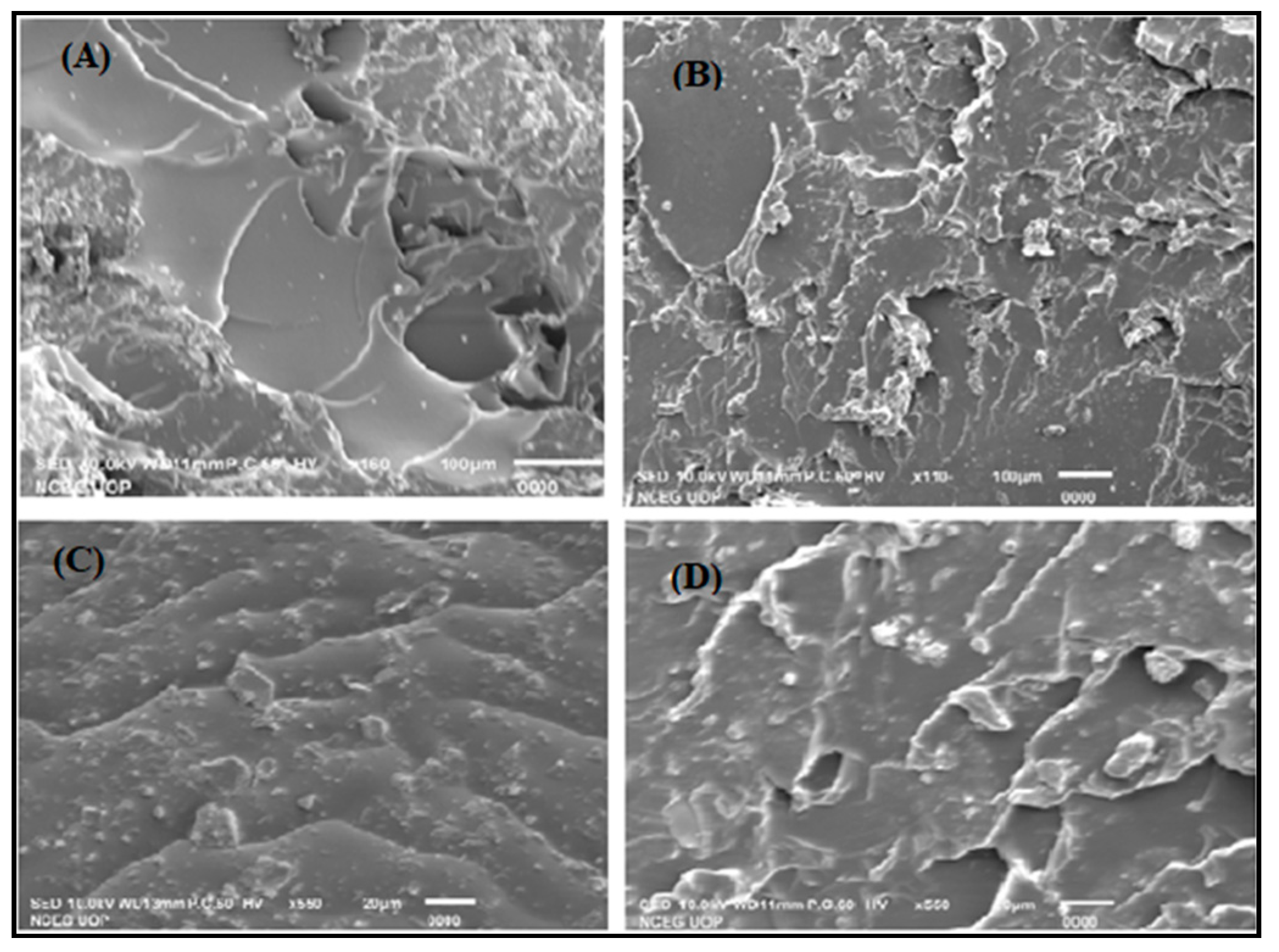
2.5.3. Scanning Electron Microscopy (SEM)
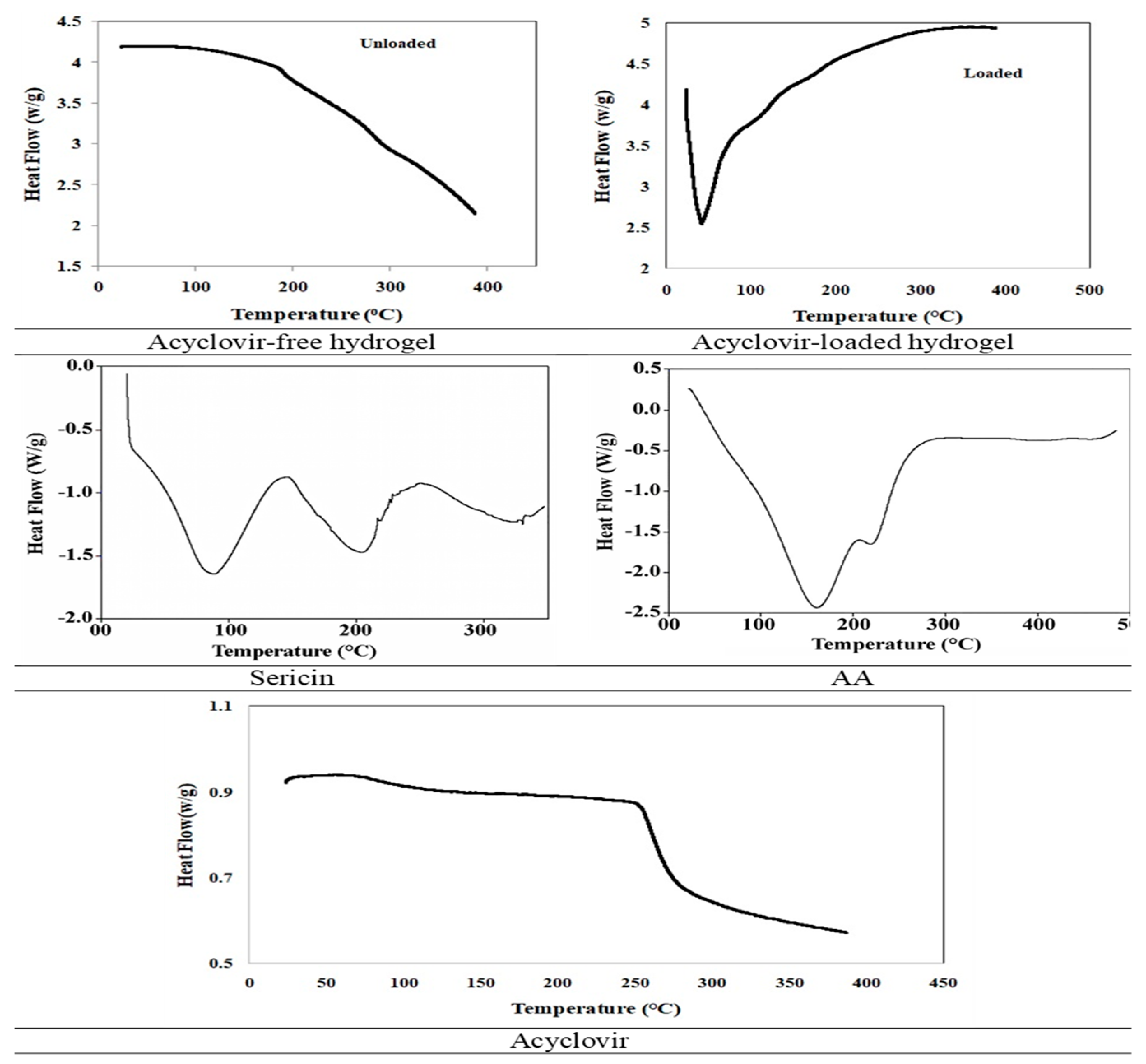
2.5.4. Differential Scanning Calorimetry (DSC)
2.6. Release Kinetics
2.7. Oral Tolerability and Safety Profiling
2.7.1. Determination of the Maximum Tolerance Dose (MTD)
2.7.2. Monitoring of the General Conditions
2.7.3. Serum Chemistry and Haematological Profiles
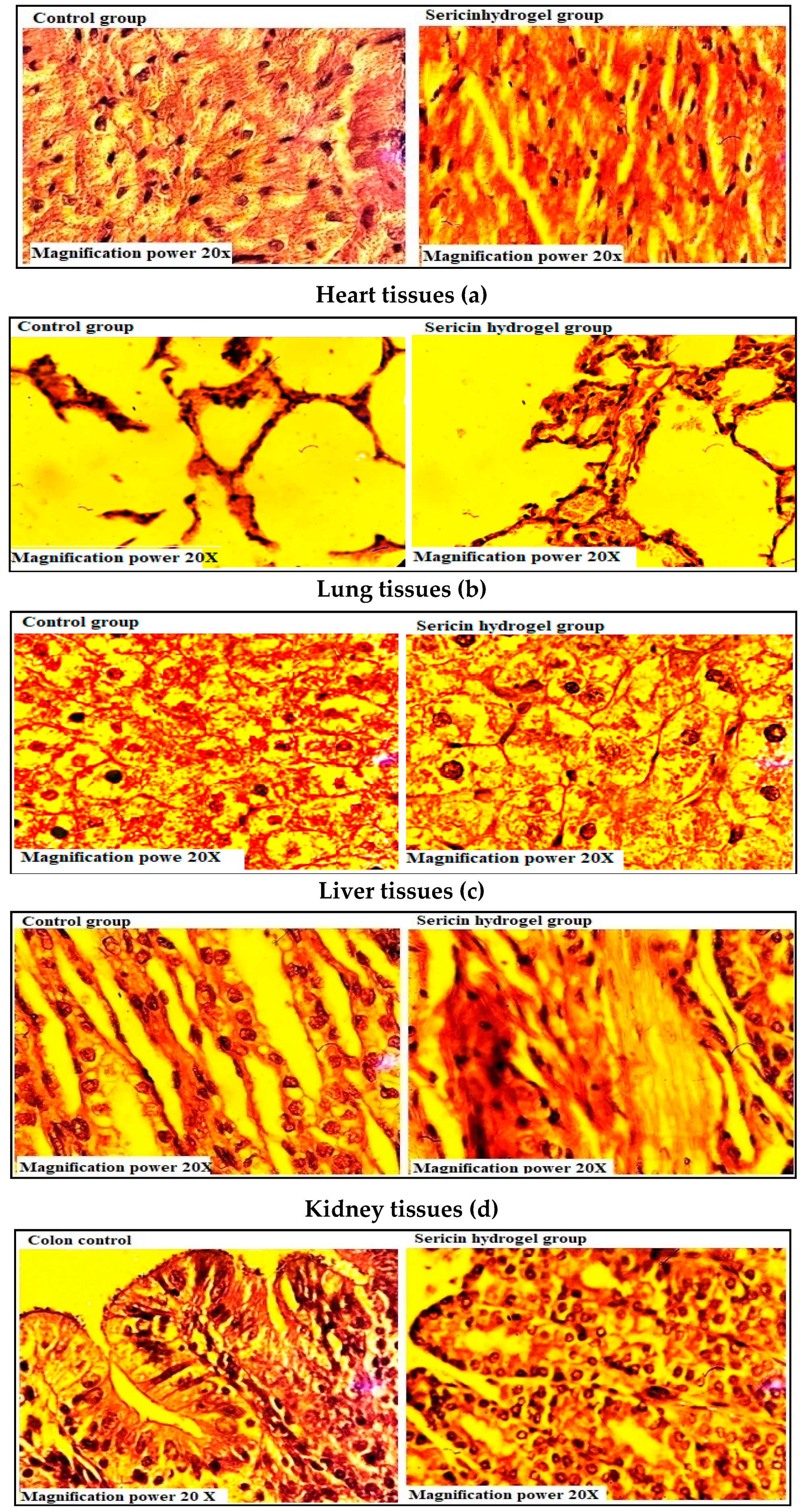
2.7.4. Histopathological Investigations
2.8. Comparison of Sericin Based Hydrogels with Other Delivery Techniques for Acyclovir
3. Materials and Methods
3.1. Chemicals
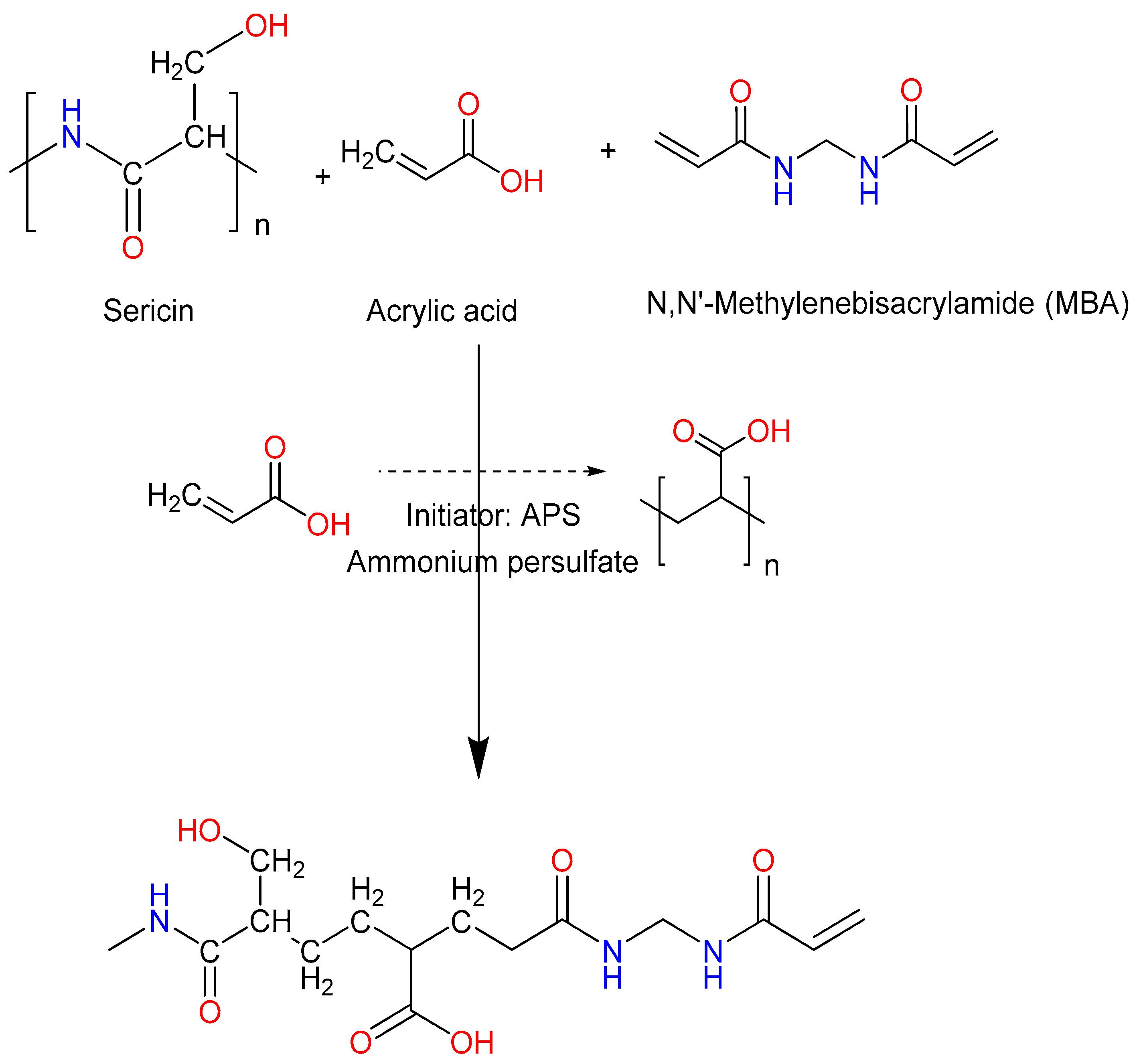
3.2. Synthesis of Hydrogel
3.3. Chemical characterizations
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.2. X-ray Diffraction (XRD)
3.3.3. Scanning Electron Microscopy (SEM)
3.3.4. Differential Scanning Calorimetry (DSC)
3.4. Sol-Gel Analysis
3.5. Drug Loading
3.6. Estimation of Acyclovir Content
3.7. Swelling Study
3.8. In-vitro Drug Release Study
3.9. Oral Tolerability and Safety Profiling
3.10. Hematology and Serum Chemistry
3.11. Histopathological Investigations
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandare, R.; Londhe, V.; Ashames, A.; Shaikh, N.; Zain Alabdin, S. Enhanced Solubility of Microwave-assisted Synthesized Acyclovir Co-crystals. Res. J. Pharm. Tech. 2020, 13, 5979–5986. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Murtaza, G.; Khalid, Q. Polysaccharide hydrogels for controlled release of acyclovir: Development, characterization and in vitro evaluation studies. Polym. Bull. 2017, 74, 4311–4328. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmad, M.; Sarfraz, R.M.; Minhas, M.U. Development of acyclovir loaded β-cyclodextrin-g-poly methacrylic acid hydrogel microparticles: An in vitro characterization. Adv. Polym. Technol. 2016, 37, 697–705. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Verma, S. Preparation and evaluation of Novelin SituGels containing acyclovir for the treatment of oral herpes simplex virus infections. Sci. World J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Ubaid, M.; Murtaza, G. In vitro evaluation of genipin-crosslinked Na-alginate/chitosan hydrogel films for delivery of metformin: Effect of chitosan molecular weight. Curr. Drug Deliv. 2018, 15, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Mahmood, S.; Buabeid, M.A.; Ullah, K.; Murtaza, G.; Mannan, A.; Khan, S.A. Synthesis, characterization and safety profiling of eudragit-based pH-responsive hydrogels: A promising platform for colonic delivery of losartan potassium. Curr. Drug Deliv. 2019, 16, 548–564. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Khanum, H.; Ullah, K.; Murtaza, G.; Khan, S.A. Fabrication and in vitro characterization of HPMC-g-poly(AMPS) hydrogels loaded with loxoprofen sodium. Int. J. Biol. Macromol. 2018, 120, 1624–1631. [Google Scholar] [CrossRef]
- Ubaid, M.; Shah, S.N.H.; Khan, S.A.; Murtaza, G. Synthesis and characterization of pH-sensitive genipin cross-linked chitosan/eudragit® L100 hydrogel for metform. Curr. Drug Deliv. 2018, 15, 1343–1358. [Google Scholar] [CrossRef]
- Shah, A.; Ali, M.; Arafa, E.A.; Hussain, I.; Li, L. The wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films loaded with moxifloxacin the wound healing and antibacterial potential of triple-component nanocomposite (chitosan-silver-sericin) films. Int. J. Pharm. 2019, 564, 22–38. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm sericin: Properties and biomedical applications. BioMed Res. Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, X. Synthesis and properties of silk sericin-g-poly (acrylic acid-co-acrylamide) superabsorbent hydrogel. Polym. Bull. 2010, 66, 447–462. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Zhaorigetu, S.; Sasaki, M.; Kato, N. Consumption of sericin suppresses colon oxidative stress and aberrant crypt foci in 1,2-dimethylhydrazine-treated rats by colon undigested sericin. J. Nutr. Sci. Vitaminol. 2007, 53, 297–300. [Google Scholar] [CrossRef]
- Dash, B.C.; Mandal, B.B.; Kundu, S.C. Silk gland sericin protein membranes: Fabrication and characterization for potential biotechnological applications. J. Biotechnol. 2009, 144, 321. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Murao, T.; Ito, Y.; Okamoto, N.; Sasaki, M. Enhancing effects of sericin on corneal wound healing in rat debrided corneal epithelium. Biol. Pharm. Bull. 2009, 32, 933. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef]
- Hasatsri, S.; Yamdech, R.; Aramwit, P.; Chanvorachote, P. Physical and biological assessments of the innovative bilayered wound dressing made of silk and gelatin for clinical applications. J. Biomater. Appl. 2014, 29, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar] [CrossRef]
- Wu, W.; Li, W.; Wang, L.Q.; Tu, K.; Sun, W. Synthesis and characterization of pH- and temperature-sensitive silk sericin/poly (N-isopropylacrylamide) interpenetrating polymer networks. Polym. Int. 2006, 55, 513–519. [Google Scholar] [CrossRef]
- Siritienthong, T.; Ratanavaraporn, J.; Aramwit, P. Development of ethyl alcohol-precipitated silk sericin/polyvinyl alcohol scaffolds for accelerated healing of full-thickness wounds. Int. J. Pharm. 2012, 439, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Siritientong, T.; Ratanavaraporn, J.; Srichana, T.; Aramwit, P. Preliminary characterization of genipin-cross-linked silk sericin/poly (vinyl alcohol) films as two-dimensional wound dressings for the healing of superficial wounds. BioMed Res. Int. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Aramwit, P.; Siritienthong, T.; Srichana, T.; Ratanavaraporn, J. Accelerated healing of full-thickness wounds by genipin-crosslinked silk sericin/PVA scaffolds. Cells Tissues Organs 2013, 197, 224. [Google Scholar] [CrossRef]
- Siritientong, T.; Angspatt, A.; Ratanavaraporn, J.; Aramwit, P. Clinical potential of a silk sericin-releasing bioactive wound dressing for the treatment of split-thickness skin graft donor sites. Pharm. Res. 2013, 31, 104–116. [Google Scholar] [CrossRef]
- Aramwit, P.; Ratanavaraporn, J.; Ekgasit, S.; Tongsakul, D.; Bang, N. A green salt-leaching technique to produce sericin/PVA/glycerin scaffolds with distinguished characteristics for wound-dressing applications. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2015, 103, 915. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Mudassir, J.; Majeed, S. Synthesis and characterization of polycaprolactone/acrylic acid (PCL/AA) hydrogel for controlled drug delivery. Bull. Mater. Sci. 2011, 34, 1537–1547. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Mudassir, J. Swelling and aspirin release study: Cross-linked pH-sensitive vinyl acetate-co-acrylic acid (VAC-co-AA) hydrogels. Drug Dev. Ind. Pharm. 2008, 34, 512–521. [Google Scholar] [CrossRef]
- Halib, N.; Amin, M.C.I.M.; Ahmad, I. Unique stimuli responsive characteristics of electron beam synthesized bacterial cellulose/acrylic acid composite. J. App. Polym. Sci. 2010, 116, 2920–2929. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Mudassir, J.; Sheikh, Z.Z. Synthesis and characterization of pH-sensitive pectin/acrylic acid hydrogels for verapamil release study. Iran. Polym. J. 2011, 20, 147–159. [Google Scholar]
- Ullah, K.; Khan, S.A.; Murtaza, G.; Sohail, M.; Manan, A.; Afzal, A. Gelatin-based hydrogels as potential biomaterials for colonic delivery of oxaliplatin. Int. J. Pharm. 2019, 556, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Sohail, M.; Murtaza, G.; Khan, S.A. Natural and synthetic materials based CMCh/PVA hydrogels for oxaliplatin delivery: Fabrication, characterization, in-vitro and in-vivo safety profiling. Int. J. Biol. Macromol. 2019, 122, 538–548. [Google Scholar] [CrossRef]
- Khalid, S.; Qadir, M.; Massud, A.; Ali, M.; Rasool, M. Effect of degree of cross-linking on swelling and drug release behaviour of poly (methyl methacrylate-co-itaconic acid) [P(MMA/IA)] hydrogels for site specific drug delivery. J. Drug Deliv. Sci. Technol. 2009, 19, 413–418. [Google Scholar] [CrossRef]
- Sohail, K.; Khan, I.U.; Shahzad, Y.; Hussain, T.; Ranjha, N.M. PH-sensitive polyvinylpyrrolidone-acrylic acid hydrogels: Impact of material parameters on swelling and drug release. Brazil. J. Pharm. Sci. 2014, 50, 173–184. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Y. Synthesis and swelling properties of silk sericin-g-poly (acrylic acid/attapulgite) composite superabsorbent. Polym. Bull. 2014, 72, 487–501. [Google Scholar] [CrossRef]
- Sapru, S.; Ghosh, A.K.; Kundu, S.C. Non-immunogenic, porous and antibacterial chitosan and Antheraea mylitta silk sericin hydrogels as potential dermal substitute. Carbohydr. Polym. 2017, 167, 196–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Meng, L.; Zhang, Y.; Ai, R.; Qi, N.; He, H.; Xu, H.; Tang, X. Thiolated eudragit nanoparticles for oral insulin delivery: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2012, 436, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Rehman, N.U.; Haider, A.; Kortz, U.; Afridi, S.; Sohail, M.; Joshi, S.A.; Iqbal, J. Novel pH responsive supramolecular hydrogels of chitosan hydrochloride and polyoxometalate: In-vitro, in-vivo and preliminary safety evaluation. Int. J. Pharm. 2017, 533, 125–137. [Google Scholar]
- Barkat, K.; Ahmad, M.; Minhas, M.U.; Khalid, I.; Nasir, B. Development and characterization of pH-responsive polyethylene glycol-co-poly (methacrylic acid) polymeric network system for colon target delivery of oxaliplatin: Its acute oral toxicity study. Ad. Polym. Technol. 2018, 37, 1806–1822. [Google Scholar] [CrossRef]
- Hassan, H.; Bello, R.O.; Adam, S.K.; Alias, E.; Affandi, M.; Shamsuddin, A.F.; Basir, R. Acyclovir-loaded solid lipid nanoparticles: Optimization, characterization and evaluation of its pharmacokinetic profile. Nanomaterials 2020, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, T.H.; Jeong, S.W.; Chung, S.E.; Lee, D.Y.; Kim, D.-H.; Shin, B.S. Development of a gastroretentive delivery system for acyclovir by 3D printing technology and its in vivo pharmacokinetic evaluation in Beagle dogs. PLoS ONE 2019, 14, e0216875. [Google Scholar] [CrossRef]
- Bhosale, U.; Devi, V.K.; Jain, N. Formulation and optimization of mucoadhesive nanodrug delivery system of acyclovir. J. Young- Pharm. 2011, 3, 275–283. [Google Scholar] [CrossRef]
- Md, S.; Ahuja, A.; Khar, R.K.; Baboota, S.; Chuttani, K.; Mishra, A.K.; Ali, J. Gastroretentive drug delivery system of acyclovir-loaded alginate mucoadhesive microspheres: Formulation and evaluation. Drug Deliv. 2011, 18, 255–264. [Google Scholar] [CrossRef] [PubMed]



| Formulation Code | Entrapped Acyclovir (mg)/Hydrogel Disc ± SEM |
|---|---|
| SR-1 | 62.4 ± 0.606 |
| SR-2 | 64.5 ± 0.548 |
| SR-3 | 65.8 ± 0.404 |
| AC-1 | 69.3 ± 0.669 |
| AC-2 | 72.4 ± 0.721 |
| AC-3 | 73.8 ± 0.721 |
| MB-1 | 59.4 ± 1.270 |
| MB-2 | 56.2 ± 1.334 |
| MB-3 | 52.1 ± 1.228 |
| Formulations | pH | R2 | Value of “n” | |||
|---|---|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi | Koresemayer–Peppas | |||
| SR-1 | 1.2 | 0.673 | 0.699 | 0.88 | 0.454 | 0.388 |
| 6.8 | 0.923 | 0.966 | 0.976 | 0.650 | 0.75 | |
| 7.4 | 0.855 | 0.939 | 0.976 | 0.574 | 0.731 | |
| SR-2 | 1.2 | 0.646 | 0.674 | 0.857 | 0.413 | 0.393 |
| 6.8 | 0.747 | 0.848 | 0.940 | 0.573 | 0.728 | |
| 7.4 | 0.632 | 0.807 | 0.856 | 0.312 | 0.530 | |
| SR-3 | 1.2 | 0.901 | 0.911 | 0.914 | 0.661 | 0.510 |
| 6.8 | 0.934 | 0.982 | 0.974 | 0.800 | 0.901 | |
| 7.4 | 0.825 | 0.935 | 0.978 | 0.543 | 0.713 | |
| AC-1 | 1.2 | 0.911 | 0.910 | 0.796 | 0.519 | 0.373 |
| 6.8 | 0.843 | 0.940 | 0.986 | 0.568 | 0.656 | |
| 7.4 | 0.813 | 0.949 | 0.971 | 0.427 | 0.572 | |
| AC-2 | 1.2 | 0.594 | 0.637 | 0.792 | 0.274 | 0.323 |
| 6.8 | 0.683 | 0.861 | 0.893 | 0.298 | 0.474 | |
| 7.4 | 0.404 | 0.613 | 0.661 | 0.204 | 0.415 | |
| AC-3 | 1.2 | 0.983 | 0.951 | 0.951 | 0.714 | 0.576 |
| 6.8 | 0.829 | 0.937 | 0.978 | 0.618 | 0.732 | |
| 7.4 | 0.753 | 0.907 | 0.949 | 0.481 | 0.643 | |
| MB-1 | 1.2 | 0.638 | 0.658 | 0.827 | 0.432 | 0.359 |
| 6.8 | 0.888 | 0.954 | 0.984 | 0.651 | 0.765 | |
| 7.4 | 0.767 | 0.955 | 0.950 | 0.547 | 0.721 | |
| MB-2 | 1.2 | 0.833 | 0.849 | 0.944 | 0.569 | 0.408 |
| 6.8 | 0.906 | 0.943 | 0.974 | 0.805 | 0.871 | |
| 7.4 | 0.833 | 0.911 | 0.978 | 0.577 | 0.709 | |
| MB-3 | 1.2 | 0.559 | 0.575 | 0.793 | 0.42 | 0.323 |
| 6.8 | 0.847 | 0.906 | 0.977 | 0.646 | 0.746 | |
| 7.4 | 0.672 | 0.770 | 0.900 | 0.483 | 0.658 | |
| Parameter | Group-I (Control) | Group-II (Treatment) | ||
|---|---|---|---|---|
| Male Rabbits (n = 3) | Female Rabbits (n = 3) | Male Rabbits (n = 3) | Female Rabbits (n = 3) | |
| Total Protein (g/L) | 72.37 ± 0.71 | 71.15 ± 0.53 | 74.12 ± 0.22 | 67.57 ± 0.40 |
| Globulin (g/L) | 21.42 ± 0.44 | 20.85 ± 1.14 | 22.26 ± 0.43 | 20.81 ± 0.12 |
| Albumin (g/L) | 54.43 ± 0.62 | 52.56 ± 1.61 | 56.66 ± 0.83 | 53.92 ± 0.43 |
| ALT (U/L) | 83.10 ± 1.81 | 81.21 ± 1.68 | 84.54 ± 0.58 | 82.34 ± 0.45 |
| ALP (U/L) | 135.07 ± 0.18 | 131.97 ± 1.63 | 136.91 ± 0.70 | 132.66 ± 0.75 |
| AST (U/L) | 58.99 ± 0.46 | 60.06 ± 0.70 | 61.44 ± 1.80 | 63.32 ± 0.34 |
| Cholesterol (mmol/L) | 109.87 ± 1.44 | 108.56 ± 0.80 | 106.74 ± 1.39 | 107.45 ± 1.40 |
| Glucose (mmol/L) | 8.63 ± 0.35 | 9.01 ± 0.44 | 7.99 ± 0.19 | 8.25 ± 0.97 |
| Creatinine (µmol/L) | 154.38 ± 2.13 | 157.26 ± 0.78 | 156.98 ± 0.38 | 158.32 ± 0.70 |
| Urea (mmol/L) | 16.08 ± 0.53 | 14.67 ± 0.86 | 16.12 ± 1.97 | 13.80 ± 0.86 |
| Uric acid (mg/dL) | 3.87 ± 0.13 | 3.91 ± 0.66 | 3.95 ± 0.75 | 3.93 ± 0.07 |
| Magnesium (mmol/L) | 0.86 ± 0.14 | 0.96 ± 0.44 | 1.12 ± 0.19 | 1.06 ± 0.12 |
| Phosphorus (mmol/L) | 2.82 ± 0.11 | 2.80 ± 0.86 | 2.45 ± 0.25 | 2.67 ± 0.17 |
| Potassium (mmol/L) | 5.99 ± 0.12 | 6.29 ± 0.26 | 6.34 ± 0.25 | 6.35 ± 0.05 |
| Sodium (mmol/L) | 159.62 ± 1.36 | 158.45 ± 0.36 | 155.60 ± 0.81 | 157.83 ± 0.45 |
| Biochemical Analysis | Group-I (Control) | Group-II (Treatment) | ||
|---|---|---|---|---|
| Male Rabbits (n = 3) | Female Rabbits (n = 3) | Male Rabbits (n = 3) | Female Rabbits (n = 3) | |
| pH | 7.11 ± 0.07 | 7.01 ± 0.17 | 7.71 ± 0.13 | 7.50 ± 0.09 |
| Hemoglobin (g/L) | 114.33 ± 1.53 | 109.87 ± 0.78 | 112.87 ± 4.81 | 106.23 ± 1.18 |
| RBCs × 1012/L | 6.10 ± 0.12 | 6.00 ± 0.21 | 5.87 ± 0.09 | 6.04 ± 0.11 |
| Eosinophils × 109/L | 8.30 ± 0.07 | 7.98 ± 0.16 | 8.24 ± 0.18 | 7.05 ± 0.73 |
| WBCs × 109/L | 0.04 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.00 |
| Lymphocytes × 109/L | 2.43 ± 0.06 | 2.38 ± 0.13 | 2.72 ± 0.18 | 2.52 ± 0.28 |
| Neutrophils × 109/L | 4.11 ± 0.04 | 4.31± 0.71 | 4.41 ± 0.18 | 4.51 ± 0.27 |
| Basophils × 109/L | 0.33 ± 0.05 | 0.29 ± 0.12 | 0.43 ± 0.02 | 0.39 ± 0.13 |
| Platelets × 109/L | 354.50 ± 4.40 | 350.97 ± 1.74 | 356.80 ± 0.97 | 352.73 ± 1.71 |
| PCV (L/L) | 0.47 ± 0.01 | 0.44 ± 0.02 | 0.45 ± 0.02 | 0.46 ± 0.04 |
| MCV (L/L) | 66.84 ± 0.78 | 65.50 ± 0.93 | 67.77 ± 0.59 | 62.39 ± 0.79 |
| S. No. | Formulations | Intended Amount of Drug Loaded (mg) | Drug Loading (%) | Maximum Drug Release (%) | Time (h) for Maximum Drug Release | Reference |
|---|---|---|---|---|---|---|
| 1 | Solid Lipid nanoparticles | 10 | 85 | 100 | 24 | [42] |
| 2 | SR tablets | 100 | 100 | 100 | 12 | [43] |
| 3 | Thermo-responsive in-situ gels | 600 | 98.15–99.75 | 97 | 6 | [6] |
| 4 | Muco-adhesive nanoparticles | 25 | 71 | 94 | 12 | [44] |
| 5 | Gastro-retentive muco-adhesive microspheres | 200 | 51.42−80.46 | 75 | 8 | [45] |
| 6 | Sericin-based hydrogels | 100 | 73.8 | 86.85 | 24 | Present study |
| Formulations | SR (g)/100 g | AA (g)/100 g | MBA (g)/100 g | APS (g)/100 g |
|---|---|---|---|---|
| SR-1 | 3 | 15 | 0.3 | 1 |
| SR-2 | 6 | 15 | 0.3 | 1 |
| SR-3 | 9 | 15 | 0.3 | 1 |
| AC-1 | 9 | 18 | 0.3 | 1 |
| AC-2 | 9 | 21 | 0.3 | 1 |
| AC-3 | 9 | 24 | 0.3 | 1 |
| MB-1 | 9 | 15 | 0.5 | 1 |
| MB-2 | 9 | 15 | 0.8 | 1 |
| MB-3 | 9 | 15 | 1.0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Tabakha, M.M.; Khan, S.A.; Ashames, A.; Ullah, H.; Ullah, K.; Murtaza, G.; Hassan, N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals 2021, 14, 234. https://doi.org/10.3390/ph14030234
Al-Tabakha MM, Khan SA, Ashames A, Ullah H, Ullah K, Murtaza G, Hassan N. Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals. 2021; 14(3):234. https://doi.org/10.3390/ph14030234
Chicago/Turabian StyleAl-Tabakha, Moawia M., Shujaat Ali Khan, Akram Ashames, Hamid Ullah, Kaleem Ullah, Ghulam Murtaza, and Nageeb Hassan. 2021. "Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir" Pharmaceuticals 14, no. 3: 234. https://doi.org/10.3390/ph14030234
APA StyleAl-Tabakha, M. M., Khan, S. A., Ashames, A., Ullah, H., Ullah, K., Murtaza, G., & Hassan, N. (2021). Synthesis, Characterization and Safety Evaluation of Sericin-Based Hydrogels for Controlled Delivery of Acyclovir. Pharmaceuticals, 14(3), 234. https://doi.org/10.3390/ph14030234







