Abstract
Hepatocellular carcinoma (HCC) is the primary tumour of the liver with the greatest incidence, particularly in the elderly. Additionally, improvements in the treatments for chronic liver diseases have increased the number of elderly patients who might be affected by HCC. Little evidence exists regarding HCC in old patients, and the elderly are still underrepresented and undertreated in clinical trials. In fact, this population represents a complex subgroup of patients who are hard to manage, especially due to the presence of multiple comorbidities. Therefore, the choice of treatment is mainly decided by the physician in the clinical practice, who often tend not to treat elderly patients in order to avoid the possibility of adverse events, which may alter their unstable equilibrium. In this context, the clarification of the optimal treatment strategy for elderly patients affected by HCC has become an urgent necessity. The aim of this review is to provide an overview of the available data regarding the treatment of HCC in elderly patients, starting from the definition of “elderly” and the geriatric assessment and scales. We explain the possible treatment choices according to the Barcelona Clinic Liver Cancer (BCLC) scale and their feasibility in the elderly population.
1. Introduction
Hepatocellular carcinoma (HCC) is the primary tumour of the liver with the greatest incidence worldwide, and almost 13,000 new diagnoses of HCC are expected in Italy in 2020 [1,2]. It arises especially in patients with previous liver diseases (cirrhosis from viral infections, alcohol abuse, etc.), and the risk of developing HCC increases with age, reaching the highest incidence in the seventh decade of life. Despite therapeutic advances, the prognosis of HCC is severe, especially in patients with advanced disease [1,3]. For this reason, each HCC patient requires a careful and global evaluation in dedicated and high-volume centers by a multidisciplinary team. This team should include oncologists, radiologists, radiotherapists, surgeons, nutritionists and gastroenterologists due to the complexity of the management of HCC patients, who often show liver impairment, gastrointestinal bleeding, ascites or hepato-renal syndrome due to cirrhosis, and/or cachexia and malnutrition also due to the alcohol abuse. Each patient should be carefully staged, and even if multiple staging systems do exist, the Barcelona Clinic Liver Cancer (BCLC) classification is the most used in Europe [4,5].
The majority of patients affected by HCC are elderly, and age is one of the risk factors in HCC development. Improvements in the treatments of chronic liver diseases in recent decades have increased life expectations and the number of elderly patients who develop HCC. Therefore, a comprehensive geriatric assessment could be useful for oncologists to distinguish between fit and frail patients and to choose patients eligible to receive an active treatment. However, elderly patients are underrepresented in clinical trials [6], and this has led to undertreatment of these patients in clinical practice. In fact, they also represent a hard-to-manage, complex subgroup of patients due to the presence of multiple comorbidities. Therefore, since there is a lack of recommendations regarding HCC in the elderly, the choice of the treatment is mainly decided by each physician in the clinical practice, who often does not compare them to identify an active treatment that best avoids the possibility of drug-related adverse events (AEs), which may alter their unstable balance.
Based on this background, the clarification of the optimal treatment strategy for elderly patients with HCC has become a pressing need. The aim of this narrative review is to provide an overview of the available data regarding the treatment of HCC in elderly patients, starting from the definition of “elderly” and the geriatric assessment and scales. Then, we explain the possible treatment choices according to the BCLC algorithm and their feasibility in the elderly population.
2. Geriatric Assessment and Scales: A Tool to Choose a Tailored Treatment for Elderly HCC Patients
The definition of “elderly patient” is a controversial issue [7], due to the fact that chronological age alone cannot describe the complexity of biological events that drive the ageing process. Chronological age is a simple way of defining a target population and, according to the International Society of Geriatric Oncology (SIOG), 70 years is the cut-off currently used to define a patient as elderly [8]. Accordingly, the scientific literature on liver disease, including HCC, refers to 70 years old as the cut-off to distinguish elderly and non-elderly patients [9].
More than 60% of patients who are newly diagnosed with cancer are ≥70 years of age; this finding, along with the trend of ageing of the global population, requires a better definition of the correct approach to these patients [10]. Therefore, the challenge for oncologists is to estimate the risk-benefit ratio, meaning to define when the expected benefit of treatment is superior to the risk of toxicity and/or dose reduction/discontinuation of treatment in the elderly population, which has a limited life expectancy and a reduced ability to adapt to physical and psychological stress. This evaluation could be difficult, and the routine clinical history and the standard performance status (PS) scales, which are used daily in clinical practice (such as Karnofsky Performance Status (KPS) or Eastern Cooperative Oncology Group Performance Status (ECOG PS)), are often not adequate to evaluate the “individual reserve” and to differentiate the frail patient from the fit patient.
In this context, several tools, such as the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) and the Cancer and Aging Research Group (CARG) chemotherapy toxicity calculator, have been developed to assist oncologists in the prediction of chemotherapy tolerance in this population [11]. According to American Society of Clinical Oncology (ASCO) guidelines, a comprehensive geriatric assessment (CGA) should be performed in all the older patients diagnosed with cancer before starting treatment [4,8,12].
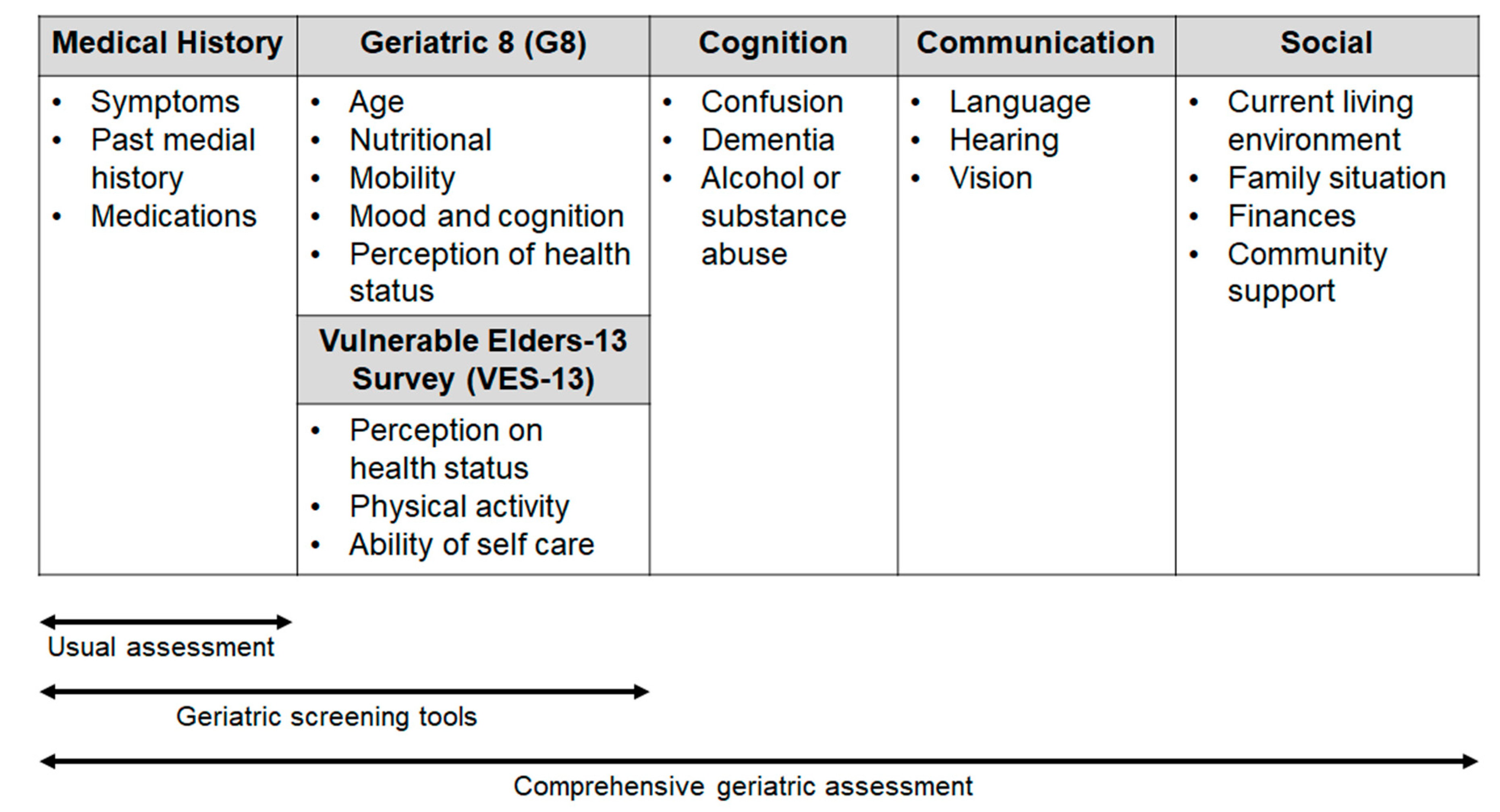
Comprehensive geriatric assessment (CGA) includes an evaluation of several issues (comorbidity, polypharmacy, functional status and nutritional status, psychological health, family and social support, cognition; see Figure 1) with the use of different tests (e.g., Activities of Daily Living, Mini Mental State Examination, Geriatric Depression Scale, Mini Nutritional Assessment), that can help to predict the adverse events (AEs) of treatment and the patient outcome.
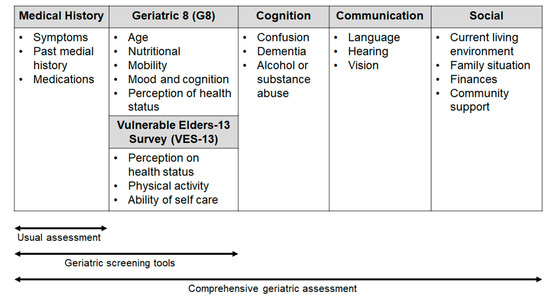
Figure 1.
Areas of investigation used in the geriatric scales and assessment.
To meet the needs of selected patients in the daily clinical practice, in 2005, SIOG recommended for the first time the use of a brief and simple screening test in the elderly, with the aim of identifying those patients with “a geriatric risk profile” for which CGA is necessary [8]. Therefore, in recent years, a few screening tools have been developed and validated [8]. Some consist of selected questions from validated geriatric scales (e.g., the Geriatric Depression Screen and Mini-Mental Status Exam, abbreviated CGA), whereas others, such as the Geriatric 8 (G8) and Senior Adult Oncology Program are made up of items that have been borrowed from different geriatric domains [13,14].
The updated SIOG guidelines on the screening geriatric tests suggest that the G8 has the highest sensitivity (ranging from 65% to 92% in the different studies) [15]. It consists of eight items: one item refers to the age of the patient (<80; 80–85; >85 years) and seven items, sampled from the Mini Nutritional Assessment (MNA) questionnaire, concern nutritional status, weight loss, body mass index, motor skills, psychological status, number of medications and self-perception of health. G8 has been shown to be able to predict the chemotherapy-related toxicity and to have a prognostic role in various cancer types [16,17].
Another questionnaire, the Vulnerable Elders-13 Survey (VES-13), validated for cancer patients includes 13 items regarding the perception of health status. The test reflects the everyday activity and difficulty in performing activities related to patient health or physical condition [18]. A score ≥3 identifies patients whose health is susceptible to deterioration, with an increased risk of functional decline or death over 2 years [9].
Two studies suggest that using a combination of the two aforementioned screening tools is significantly better than the use of G8 or VES-13 alone. Therefore, the combination might be a sensitive screening tool in older cancer patients [19,20]; if these tests suggest potential “frailty”, the optimal approach to choose the tailored treatment for the patients is to perform the CGA.
In conclusion, expanding the usual clinical evaluation with quick and simple screening tests and, eventually, CGA in patients recognized as at risk could be useful to identify areas of vulnerability, predict survival and toxicities and guide clinicians in treatment decisions.
3. HCC Treatment in Elderly Patients According to BCLC Stage
The BCLC stage system can be used as both a prognostic and a therapeutic tool. According to the characteristics of the tumour and the degree of liver failure, patients are grouped into the following categories: very early (BCLC 0), early (BCLC A), intermediate (BCLC B), advanced (BCLC C) and terminal (BCLC D). While the BCLC stage system has mainly been developed to be incorporated into the therapeutic algorithm of HCC, BCLC categories may also provide prognostic information. However, the majority of recent prognostic studies comparing BCLC to other systems, such as MESH, ITA.LI.CA, CLIP and HKLC have clearly shown the poor prognostic performance of BCLC [21,22], underlying the disadvantages of using that stage system as prognostic. For an example, both ITA.LI.CA and CLIP score performed better than BCLC in predicting overall survival (OS) in HCC patients who were candidates for surgery [21]. Additionally, best clinical practice has clearly unveiled the limitations of BCLC treatment categories, which do not include some clinically relevant parameters, such as AFP levels, a well-known predictor of OS in various clinical situations. In this regard, a prospective study has suggested that AFP may improve the prognostic efficacy of the BCLC system, suggesting the additive effect of this biomarker [23].
An alternative approach that is useful to overcome BCLC’s drawbacks is the so-called “stage migration strategy”, according to which the treatments recommended for a different stage are selected as the best first-line treatment option [24]. Within such a treatment strategy, therapy for the next more or less advanced tumour stage may be selected, which is referred to as left-to-right or right-to-left stage migration, respectively. Stage migration is rather frequent in clinical practice: in a recent retrospective case series of 1369 HCC patients, stage migration to therapies indicated for more advanced stages was reported in approximately 60.2% of therapy choices, which is higher than previous reports [25].
“Treatment stage alternative”, which has recently been suggested by the latest American Association for the stufy of liver diseases (AASLD) guidelines [26,27], also represents a different treatment approach that is based on several therapeutic options for each BCLC stage, classified according to their level of evidence. Conversely, a “treatment hierarchy” approach is followed by both the Asia-Pacific [28] and the multi-society Italian [29] treatment algorithms. However, this strategy is completely independent of HCC staging and rather relies on other factors such as, for instance, the technical feasibility of liver resection, resulting in hierarchically organized treatments based on their effectiveness [27].
3.1. Early Stage HCC (BCLC Stage 0 or A)
According to BCLC classification, in patients with good health status (ECOG PS 0) and well-preserved liver function (Child-Pugh A class), solitary HCC <2 cm without vascular invasion or satellites is classified as very early stage (BCLC stage 0). In the same patients, a single tumour >2 cm or three nodules <3 cm are classified as early HCC (BCLC stage A) [30]. There is a wide range of treatment options for BCLC stages 0 or A HCC, including liver resection, liver transplantation and local ablation [30]. The BCLC classification does not specify whether age should be considered in treatment allocation, and the scientific evidence available is controversial.
3.1.1. Liver Transplantation
Even though liver transplantation (LT) is superior in terms of long-term survival compared with hepatic resection or local ablation for its potential to cure both the tumour and the underlying liver disease [31], elderly patients are rarely transplanted because of low priority in a context of limited organ availability. Recent studies reported comparable survival outcomes between older and younger recipients, while others reported significantly worse survival outcomes in the elderly group [32,33,34]. The studies had a retrospective design with questionable validity, and the cut-off used for old age also varied, making direct comparison difficult. Nevertheless, the eligibility for LT should not be based only on age, but other factors must be considered in patients selection, such as the evaluation of liver function (through the model for end-stage liver disease—MELD), functional reserve (with the indocyanine green kinetics (ICG) test) and the assessment of liver stiffness (with transient elastography—LSM) [35,36,37,38]. In addition, recent studies showed that elderly HCC patients have different clinical and pathological characteristics compared to younger patients. They are more likely to have NASH-cirrhosis and HCV infections than younger patients [6,39]. They also tend to have a lower grade of background liver fibrosis and lower Child–Pugh scores compared to younger HCC patients [40]. Furthermore, they have fewer but larger HCC nodules, associated with less aggressive disease than younger patients [41,42]. These findings have to be considered and discussed within a multidisciplinary team in order to guide the patient allocation to the best-tailored treatment. However, LT as a treatment option for the aged population is still subject to debate, due to the lack of liver donors, and the upper age limit for undergoing LT is yet to be defined [43].
3.1.2. Liver Resection
The older population is also under-represented in the cohort of patients undergoing liver resection for HCC [44,45], and they are usually more likely to receive conservative treatment [46]. In the majority of the studies conducted over the last twenty years, 0–14% of patients undergoing liver resection belong to the elderly population, whereas 12–28% were younger patients [44,45,46]. This fact could be likely related to the concerns regarding the increased operative risk for the older patients, with shorter expected survival [47,48].
Apart from the influence of life expectancy, elderly patients are generally characterized by higher incidences of comorbidities, such as diabetes, hypertension, cerebrovascular and cardiovascular disease, according to the studies comparing surgical resection in the elderly versus young HCC patients [49]. However, data regarding the efficacy and safety of surgical and locoregional treatments in elderly patients with HCC are limited, and current guidelines do not suggest separating treatment for older age groups [30]. The morbidity and mortality rates after hepatectomy in elderly HCC patients range from 9% to 51% and from 0% to 42.9%, respectively [46]. Due to recent advances in surgical procedures and perioperative management, most recent studies and meta-analyses have shown comparable short-term outcomes, such as hospital stay, post-operative morbidity and mortality, between younger and elderly patients undergoing hepatectomy for HCC [6,35,46,50,51,52,53]. This could probably be explained by the more careful selection criteria of elderly patients. Publication bias and heterogeneity of study design could also have led to these results.
In clinical practice, careful patient selection, irrespective of age, is crucial in achieving acceptable mortality and morbidity. Tailoring of care is appropriate, and the multidisciplinary assessment has been shown to increase the proportion of patients offered therapy with curative intent [50,51,52,53,54]. Tools that allow better selection of candidates for hepatectomy play a central role. There are different scoring systems for assessing elderly patients, including the American Society of Anesthesiologists (ASA), POSSUM/P-POSSUM, E-PASS and APACHE II score [35]. In addition, in a recent study, the use of cardiopulmonary exercise testing (CPET), enhanced recovery (ERAS) and parenchymal sparing liver surgery in the selection, management and treatment of octogenarian patients with liver tumours allowed to achieve similar post-operative outcomes to younger patients [44]. In fact, studies and meta-analyses of randomized controlled trials on the efficacy of the ERAS in liver surgery showed an improved rate of post-operative morbidity (p < 0.01) and length of stay (p < 0.01) and accelerated functional recovery (time to first flatus p < 0.01) [55].
The positive impact of parenchymal sparing surgery is probably related to the high incidence of hospital death due to hepatic failure after major hepatectomy in elderly patients with HCC [46]. Although the degree of liver regeneration at one month after right lobectomy is similar in younger and elderly patients, it is possible that remnant liver regeneration immediately after major hepatectomy in elderly patients is impaired [46].
In order to reduce surgical stress and improve outcomes, in the last ten years, laparoscopic and robotic techniques have been applied in liver surgery, which have been shown to be safe and effective approaches, with morbidity and mortality in major laparoscopic-robotic series in very early and early HCC of 10–15% and 1%, respectively [35,56,57,58]. Laparoscopic-robotic resection of HCC, in particular when the tumours are mainly located in superficial peripheral positions of the liver, provides optimal survival outcomes and minimizes complications and hospital stay [57,59]. Additionally, several studies have demonstrated that laparoscopic and robotic resections of HCC also in cirrhotic patients are associated with a reduced risk of post-operative liver decompensation, post-operative ascites and morbidities [57,60]. In a recent retrospective study, Wang et al. compared minor laparoscopic hepatectomy in elderly (48 patients, ≥70 years) and younger patients (97 patients, <70 years). The authors showed no significant difference in operation time, intraoperative blood loss, length of hospital stay, incidence of complications, recurrence rates, overall survival (OS) and disease-free survival (DFS) rates between the groups. Multivariate analysis showed that age was not an independent predictor of OS and DFS [61]. In another study from Korea, a propensity score matching analysis between patients ≥70 years old and <70 years old (41 patients in each arm) showed shorter hospital stay (7 versus 11 days, p = 0.002) in the elderly, as well as similar resections with microscopic margins involvement (so-called R1 resections) and complications rate, and no difference in 5 year OS (86.7% versus 62.2%, p = 0.221) and DFS (43.4% versus 30.8%, p = 0.500) [62]. Nomi et al. also showed a lower rate of major complications and shorter hospital stay in octogenarians with HCC treated with laparoscopic liver resection compared to younger patients [63]. For this potentially feasible and safe alternative to open hepatectomy, selecting criteria for elderly patients needs to be determined in order to benefit as many patients as possible.
Regarding long-term outcomes of liver resection, most studies showed that 5-year OS rates of elderly HCC patients after hepatectomy ranged between 26% and 75.9%, whereas those in younger HCC patients ranged from 31.4 to 68% [35,46,53]. Recent reports demonstrated comparable results between young and elderly patients for 1, 3 and 5 year OS and DFS rates [6,35,46,53,64,65,66,67,68,69,70,71,72,73,74]. In the study of Hirokawa et al., the DFS was worse in elderly patients than in the younger ones [66]. Additionally, some trials found a high prevalence of HCV infection, and a subgroup analysis showed that responders to interferon had significantly increased survival. It is well known that elderly patients respond poorly to interferon therapy due to severe side effects; nevertheless, the newer oral antiviral agents could possibly lead to improvement in DFS in elderly HCC patients with HCV infection [67].
Finally, post-operative recurrence of HCC is one of the most important factors affecting survival [68,69]. Repeated hepatectomy has also been suggested to be the most effective treatment for recurrence within the liver for elderly patients [70].
3.1.3. Local Ablative Approaches
In the past decade, local ablative therapy has evolved as an alternative to resection for early-stage HCC, in particular in the case of single nodules ≤2 cm deeply or centrally located. Radiofrequency ablation (RFA) can induce coagulative necrosis of the tumour with an adequate margin (heating of tissue to 60–100 °C) [6,54]. Percutaneous RFA has been widely used and almost completely replaced other local treatments, such as PEI (percutaneous ethanol injection) and PAI (percutaneous acetic acid injection). In a network meta-analysis of treatment of early HCC, including 2096 patients in 14 randomized clinical trials, RFA caused larger ablation volume in fewer sessions of treatment and better OS and DFS compared to PEI and PAI [71]. For very early HCC, the European Association for the Study of the Liver (EASL) guidelines suggest considering RFA as a first-line option, leaving surgery to those patients who fail treatment or with nodules that are not suitable for RFA [30]. In a recent meta-analysis, subgroup analysis according to patient’s age (65–75 vs. >75 years) and tumour size (≤3 vs. 3–5 cm) revealed that liver resection achieved better OS than RFA, except for patients older than 75 years with tumours ≤3 cm [72]. In a Japanese trial, Kaibori et al. used a propensity score analysis to match elderly patients treated with RFA or liver resection with similar liver function and tumour characteristics [73]. They showed that liver resection decreased recurrence risk and improved OS in patients >75 years with HCC <3 cm. Another meta-analysis comparing surgical outcomes, quality of life and costs between patients with HCC treated with RFA or liver resection concluded that, for very early HCC (single nodule <2 cm) in Child–Pugh class A patients, RFA provided similar life expectancy and quality-adjusted life expectancy at a lower cost [74]. Another retrospective study by Jiang et al. concluded that in elderly patients, RFA should be recommended for those with HCCs ≤ 2 cm, while surgical resection would be a better treatment for those with HCCs of 2–5 cm [75].
Significant advantages of surgical resection are mainly attributable to the removal of potential venous tumour thrombi, complete eradication of the primary tumour with clean resection margins and the possibility to have information about histopathology [30]. The presence of satellites, microvascular invasion or poor differentiation could guide the clinician in the post-operative management, including the possibility to consider an “ab initio” liver transplantation [76]. Given that elderly patients are generally not candidates for liver transplantation, the availability of the pathology characteristics does not usually affect the treatment strategy for this group of patients.
In addition, elderly patients have more comorbidities and may be poorer candidates for liver surgery. For these reasons, RFA has many advantages, such as minimal invasiveness, less blood loss, lower perioperative risk, and fewer negative effects on liver function. Therefore, RFA may be more feasible than surgical resection in elderly patients [72].
Recent studies and meta-analyses did not demonstrate any significant differences in terms of duration of hospitalization and serious adverse events among the elderly HCC patients and their younger counterparts treated with RFA [6,77]. The most common complications were hemoperitoneum, liver abscess, hemothorax, subcutaneous hematoma, and asymptomatic biloma. Less common complications included pneumothorax, hemobilia, massive hepatic infarction and gastrointestinal perforation [6].
Regarding the outcomes, several studies have reported five-year survival beyond 70% in well-selected patients with very early HCC treated with RFA [30]. In a recent meta-analysis, Hung et al. showed no significant differences in 1-year (odd ratio (OR) = 1.5, 95% confidence interval (CI): 0.788–2.885, p = 0.217) and 3-year OS (OR = 1.352, 95% CI: 0.940–1.944, p = 0.104) in elderly and younger patients who underwent RFA for HCC. At 5 years, the younger patients had significantly better clinical outcomes (OR = 1.379, 95% CI: 1.079–1.763, p = 0.01) [6]. Fujiwara et al. showed higher mortality at 5 years in elderly patients. However, an additional risk analysis demonstrated that there was a significant difference in liver-unrelated deaths between the elderly and younger patients [78]. This suggests that elderly patients tend to die from liver-unrelated causes at 5 years. Nishikawa et al. demonstrated decreased cumulative OS and DFS at 1-, 3- and 5-year intervals in the elderly patient group [79].
Nevertheless, RFA has a significant drawback of limited ablative margins, which is associated with a high risk of marginal recurrence [31]. This is particularly true in HCC > 3 cm as shown in previous studies, in which tumour size has been identified as an independent risk factor for local recurrence and OS [31,80,81,82]. In addition, RFA may not eradicate the tumour effectively in subcapsular HCC, or close to major bile ducts, large vessels or intestine. This may be associated with a higher risk of complications and recurrence, resulting in worse survival [83,84].
Although percutaneous RFA is the least invasive procedure, for tumours in difficult anatomical locations many centers have adopted laparoscopic radiofrequency ablation (LRFA). Two of the advantages of using this technique, compared with percutaneous RFA, are the real-time security monitoring of the ablation process and the accurate detection of tiny lesions [30]. However, a meta-analysis comparing LRFA to liver resection for early HCC (1691 patients in 11 studies) showed that patients undergoing hepatic resection had higher 3- and 5-year OS rates, higher 3-year DFS rate, and lower local recurrence rate than those undergoing LRFA. Nevertheless, patients undergoing LRFA had higher 3- and 5-year OS rates than those undergoing other minimally invasive ablation, although there was no statistical difference in local recurrence or DFS rate [85].
Interest in microwave ablation (MWA) has increased in recent years due to its potential physical advantages, which have been facilitated by modern high-powered devices [73]. In fact, MWA uses high-frequency microwave energy to heat the tumour to 60–100 °C and causes cell coagulation necrosis [86]. It is considered more effective than RFA in inducing higher intra-tumoural temperature, greater tumour ablation volume and faster ablation times, and it has a better convection profile than RFA [87]. However, complications such as liver failure, bleeding, infection, abscess, intercostal nerve injury, bile duct stenosis, organ injury and pneumothorax can develop at a rate of 2–3% [87,88]. It was reported that MWA could be as effective as RFA for single HCC < 3 cm and could have better tumour inactivation ability over RFA for 3–5 cm tumours and tumours adjacent to vessels and gallbladder [88,89].
A large study from China reported outcomes after MWA in 1007 patients with HCC. The 1- and 5-year survival rates were 91.2% and 59.8%, respectively. Subgroup analysis of the study indicated a 5-year survival rate of 29–68.6% for those with lesions >5 cm [90,91]. A recent meta-analysis comparing RFA and MWA therapy showed similar rates of complete response and local recurrence, with lower local recurrence rates in those with larger nodules treated with MWA and a lower 3-year survival rate, without statistical significance, compared to RFA. Major complications occurred more frequently with MWA than with RFA [92]. In a systematic review and meta-analysis including 26 studies (5 randomized clinical trials and 21 cohorts), the outcomes of 2393 patients treated with RFA were compared to 2003 patients treated with MWA [86]. Forty-seven percent of patients received treatment under general anesthesia in the MWA group and 84% in the RFA group (OR = 0.529, p < 0.001). The median ablation time was reduced in the MWA group (12 min) compared with the RFA group (29 min) (p < 0.001). In total, 17.6% patients exhibited progression during follow-up in the MWA group compared with 19.5% in the RFA group (OR = 0.877, p = 0.225). No statistically significant differences were observed between MWA and RFA groups in terms of OS and DFS (hazard ratio (HR) = 0.891 and 1.014, p = 0.222 and 0.852, respectively). One study that reported clinical outcomes after MWA suggested that this treatment option is safe and effective for older patients with HCC (>65 years) [93]. Finally, in a recent study, 510 elderly (≥65 years) and 1053 younger patients (<65 years) were diagnosed with early-stage HCC according to the Milan criteria. Similar survival outcomes were obtained in elderly and younger HCC patients treated by MWA, despite elderly patients having more comorbidities [94].
Data on other techniques, such as laser ablation, cryoablation and irreversible electroporation (IRE) are limited, and further studies are required in this field [95,96,97].
3.2. Intermediate-Stage HCC (BCLC Stage B)
According to current clinical guidelines, transcatheter arterial chemoembolization (TACE) is the recommended treatment for intermediate-stage HCC (multifocal, unresectable HCC, without macrovascular invasion or extrahepatic metastasis) patients with preserved liver function and absence of physical impairment (ECOG PS 0) [4,98]. These patients, classified as BCLC stage B, represent a heterogeneous population with different tumour burdens and, consequently, different survival rates after TACE. The median OS expected in the best responders is around 36–45 months versus 11 months in the patients who do not show a response to treatment. The American and European guidelines do not provide precise recommendations about the number of TACE cycles to use in this setting before switching to systemic therapy [4,98,99,100].
In this context, the subclassification of the intermediate stage defining specific treatment for each subgroup and the identification of prognostic scores of patients’ stratification after TACE are topics of great interest, and much work is being done about them. In 2012, Bolondi et al. [100] proposed a sub-classification of stage B, which included four stages (B1, B2, B3, B4) and, in addition, another stage defined “quasi C”. These stages were identified according to four parameters: Child–Pugh score, “Beyond Milan” and “within up to seven” criteria (defined as HCC with 7 as the sum of the diameter of the largest tumour (in cm) and the number of tumours), ECOG performance status and presence (or absence) of portal vein tumour thrombosis. For each stage, the authors matched a first treatment option, an alternative option and a median survival time.
For the B1 substage, TACE was indicated as the first option, reserving the transplantation for patients who met the up-to-seven criteria; for B2 patients, TACE or Y-radioembolization (TARE) in the first instance and sorafenib as an alternative option; for B3 patients, clinical trial or TACE/sorafenib; for B4 patients, liver transplantation if they met the up-to-seven-criteria and sorafenib for stage “Quasi C”.
The median survivals of these subgroups were different: 41.0 months in stage B1, 22.1 months in stage B2 and 14.1 months in stage B3, confirming the assumed prognostic value of sub-classification in relation to treatment choice. Later, Kudo et al. proposed the “kinki criteria”, distinguishing the patients in the already mentioned three stages (B1-B2-B3) [101,102] but considering only the Child–Pugh score (5–7 or 8–9) and the “Beyond Milan” + “within up to seven” criteria (yes or no), in a more useful way for clinical practice.
Although these systems need external validation in terms of prognostic ability, they highlighted that patients with substage B2 HCC, with bilobar multiple HCC, beyond the up-to-seven criteria, are those that could be considered for a first line with sorafenib, as they could easily become TACE-refractory.
The other attractive aspect of the intermediate stage is the re-treatment algorithm in patients with TACE failure. In this regard, the Assessment for Retreatment with TACE (ART) score was externally validated [103], and higher ART score values (≥2.5 prior to the second TACE) were associated with major adverse events after the second TACE (p = 0.011), identifying patients who may not benefit from further TACE. However, to date, only hepatoma arterial-embolization prognostic (HAP) score [104] has been validated in a prospective trial; it performed better than other scoring systems in differentiating high- and low-risk groups. The HAP score is another predictor of outcomes of patients with HCC undergoing TACE/TAE.
In the elderly population, TACE confirms the clinical outcome and safety profile already shown in the younger (<70 years old). In 1994, Mondazzi et al. showed that disease stage (tumour stage, alphafetoprotein value, hepatic functional reserve) was the main independent prognostic factor of survival in elderly patients, whereas there was no correlation with the chronological age [105]. Then, Yau et al., in a large Korean study, showed an increase in survival in patients older than 70 years compared to younger patients (median OS 14 months versus 8 months), even if the two populations were unbalanced by baseline characteristics [106]. Additionally, TACE was well tolerated in both groups (elderly and non-elderly patients) in retrospective studies and meta-analysis, including observational cohorts [6]. Although there is a lack of randomized controlled trials, based on the available data in the literature, the cumulative risk of both liver-related death and PFS after TACE is comparable in “elderly” and “younger” patients. Therefore, TACE remains a preferred choice in elderly patients even when they meet criteria for resection, due to the safety of TACE and the higher risk of surgical complications in this population [107,108]. In this regard, Liu et al. empathized this aspect, reporting that 67% of old patients underwent surgical resection, compared with 74% of younger patients [109].
Regarding the use of subsequent TACE in elderly patients after disease recurrence, international guidelines do not recommend the procedure due to the high risk of AEs, especially gastrointestinal ones (nausea, vomiting, diarrhea) [4,98]. Therefore, the use of systemic therapy might be considered after the first TACE failure with a median OS ranging between 11 and 14 months in this population [110,111].
In conclusion, the choice of the optimal treatment strategy in elderly patients with intermediate HCC stage depends on the cancer stage and—most importantly—on the assessment of general clinical conditions in a multidisciplinary context. Based on the data available in the literature, there is no difference in the optimal treatment according to age. However, larger and further prospective studies are needed to confirm these results in this heterogeneous population.
3.3. Advanced Stage HCC (BCLC Stage C)
According to current international clinical guidelines, advanced-stage HCC patients, which include subjects showing vascular involvement/extrahepatic spread (N1 and/or M1) or physical impairment (ECOG PS 1–2), are candidates for systemic therapy [4,98].
Table 1 summarizes the most important data in the literature regarding systemic treatment in elderly patients with advanced HCC, in both the first- and second-line settings.

Table 1.
Landmark trials for advanced HCC treatment in the first- and second-line settings with a focus on the elderly patients.
3.3.1. First-Line Treatment
To date, FDA has approved sorafenib, lenvatinib and atezolizumab plus bevacizumab as a first-line therapy, whilst European Medical Agency (EMA) has approved only the first two drugs [120,121].
Sorafenib was the first drug approved for advanced HCC patients. It is an oral multikinase inhibitor of serine/threonine-kinases CRAF and BRAF, but also of transmembrane receptors such as vascular endothelial growth factor receptor 2 (VEGFR2) and 3 (VEGFR3), platelet-derived growth factor receptor (PDGFR), c-KIT (or CD117), FLT3 (or CD135) and RET [122]. Concerning pharmacokinetics, sorafenib tablets are administered 400 mg bis in die, with a discrete bioavailability (<50%); it is metabolized by hepatic enzymes such as CYP3A4 and UGT1A9, and then its glucuronide conjugate is mainly excreted via the biliary/fecal route [123]. The main AEs are diarrhea, fatigue, hand–foot skin reaction (HFSR) and hypertension [124]. Sorafenib was approved by the FDA in 2007 on the basis of the results of two phase III clinical trials: SHARP, conducted in western countries, and the Sorafenib Asia-Pacific both demonstrated a meaningful prolongation in OS by using sorafenib versus placebo [112,113]. In a combined analysis of the two trials, there was no difference in OS between patients younger or older than 75 years (p = 0.6304) [125].
A recent international cohort study on 5598 HCC patients treated with sorafenib, including 792 patients aged ≥75 years, tried to answer several issues concerning elderly patients [126]. The trial confirmed similar efficacy of treatment in both patient groups as well as a similar rate in grade 2–4 AEs (63.5 versus 56.7%, p = 0.11); however, elderly patients showed a slightly higher rate of discontinuation due to AEs (27.0 versus 21.6%, p < 0.01). Additionally, in this retrospective analysis, a lower starting dose of sorafenib (200 or 400 mg die), which is a consolidated but not formally recommended clinical practice, did not affect survival in patients over 75 years [127]. Therefore, this dosage could be considered as an option in those patients, especially if they have had some AEs, in order to proceed with the therapy.
Lenvatinib is an oral multikinase inhibitor of VEGFR 1–3, fibroblast growth factor receptors 1-4 (FGFR1-4), PDGFRα, RET and c-KIT, thus showing a similar pharmacodynamics profile to sorafenib [128]. Lenvatinib’s daily dose differs according to bodyweight—8 mg if <60 kg, 16 mg if ≥60 kg; it is metabolized by CYP3A4 and excreted in both stool and urine [129]. Lenvatinib was approved by FDA in 2018, relying on results of the non-inferiority phase III REFLECT trial, which shows similar efficacy data compared with the standard of care sorafenib, but there was a different safety profile between the two drugs: less frequent diarrhea and HFSR for lenvatinib, but more frequent hypertension, proteinuria and fatigue than the control arm [111]. In this trial, ~30% of patients were aged 65–75 years and ~13% were aged ≥75 years; no difference between patients aged <65 and ≥65 years was reported in terms of both progression-free survival (PFS) (HR: 0.67 versus 0.61) and OS (HR: 0.94 versus 0.84), according to non-preplanned subgroup analysis. Then, a propensity score matching analysis from a cohort of patients who received lenvatinib as a first-line treatment showed that patients aged ≥75 years had similar efficacy but different rates of AEs compared to those aged <70 years, with HFSR being less frequent in the elderly (22% versus 42%, p = 0.053). However, HFSR occurrence was associated with an increased OS [130].
The recently approved association of atezolizumab—a monoclonal antibody directed against the programmed death ligand 1 (PD-L1)—and bevacizumab—a monoclonal antibody directed against VEGF—has been tested versus sorafenib in a phase III trial as a first-line treatment for advanced HCC, showing a statistically significant improvement in OS (HR: 0.59; p < 0.001) [110]. An exploratory subgroup analysis from this study pointed out a similar survival and AEs rate in patients younger or older than 65 years, with a slightly higher incidence of AEs leading to dose interruption [131].
In conclusion, to date, all these therapeutic options appear to be effective and safe in elderly people affected by advanced HCC, although a careful geriatric assessment should be performed before starting treatment in order to identify points of potential weakness, i.e., expected impact of specific adverse events on everyday life activities.
3.3.2. Second-Line Treatment
To date, the FDA has approved regorafenib, cabozantinib, ramucirumab, nivolumab and pembrolizumab as second-line therapy for advanced HCC patients, whilst EMA has not yet approved the last two drugs [132,133,134,135,136,137,138,139].
Regorafenib is an oral multikinase inhibitor of VEGFR 1-3, TIE-2, PDGFR, FGFR, c-KIT, RET and BRAF [140] administered at a daily dose of 160 mg; it is converted by CYP3A4 and UGT1A9 in its pharmacologically active metabolites M-2 and M-5, which undergoes enterohepatic circulation [141,142]. The phase III RESORCE trial randomized HCC patients who progressed after sorafenib to regorafenib once daily for 21 consecutive days of each 28-day cycle or placebo. Patients receiving regorafenb had a meaningful benefit in OS (HR: 0.63, p < 0.0001), and the efficacy of regorafenib in patients ≥ 65 years was comparable to that observed in the overall population, with an HR of 0.74 in the subgroup analysis [116]. In this trial, the main AEs were HFSR, diarrhea, fatigue, hypertension and increased blood bilirubin. No further data about elderly HCC patients treated with regorafenib have been reported to date.
Cabozantinib is another multikinase inhibitor, targeting VEGFR2, RET, KIT, AXL, TIE2, ROS1, TRKB, and FLT3, which shows a pharmacokinetic profile similar to lenvatinib [143]. In a phase III CELESTIAL trial, cabozantinib was compared with placebo in HCC patients who progressed after at least one systemic treatment; half of the enrolled population was ≥65 years old [117]. Diarrhea, decreased appetite, HFSR, fatigue, nausea and hypertension are major AEs. A sub-analysis of this trial in patients >65 years showed that patients receiving cabozantinib had a benefit in both OS and PFS compared to younger ones (HR: 0.74 versus 0.81 and HR: 0.46 versus 0.45, respectively). Adverse events, good quality of life—based on similar rate of dose reductions—and patients receiving subsequent therapies were similar in elderly and younger patients [144].
Ramucirumab is a monoclonal antibody-blocking VEGFR2 activity, which demonstrated activity in HCC patients who progressed to sorafenib, when α-fetoprotein concentrations were ≥ 400 ng/mL, according to REACH-2 trial results [119]. Among AEs, peculiar ones are bleeding or hemorrhage events, proteinuria, hypertension and epistaxis. A recent pooled analysis of REACH and REACH-2 trials showed no difference in both efficacy and safety between patients <65 years, 65–75 years and >75 years treated with ramucirumab, with a remarkable median relative dose intensity greater than 97% [145].
Nivolumab and pembrolizumab are both anti-PD-1 monoclonal antibodies; they inhibit the block due to PD-1/PD-L1 cooperation, resulting in an increased T-cell cytotoxic activity. They have been tested as second-line treatment in HCC patients in two phase II clinical trials, showing a manageable safety profile [146,147]. However, specific data about elderly patients treated with immune-checkpoint inhibitors have not been reported yet.
3.3.3. Comorbidities and Contraindications
Treatment of advanced HCC in elderly patients could be challenging because of comorbidities, which affect anticancer drug delivery. It must be noted that the above-mentioned clinical trials did not include patients with comorbidities that are indeed frequent in the elderly as well as patients with poor ECOG PS (ECOG PS 2). In fact, a history of cardiac disease was an exclusion criterion in SHARP and REFLECT trials, as well as a history of bleeding or thrombotic disorders or use of anticoagulants, which are major contraindications for anti-angiogenetic multikinase inhibitors [110,114]. Renal impairment is not a contraindication for sorafenib administration except if it is severe (requiring hemo- or peritoneal dialysis). For oral drugs, malabsorption should also be considered [148].
Special attention should be paid to the concomitant medications, given the high frequency of comorbidities requiring drug intake in the elderly. Concomitant medications could affect oral multikinase inhibitor efficacy at several levels, i.e., by reducing their absorption or by favoring their elimination; however, major concerns refer to metabolic interaction via CYP enzymes: CYP inducers or inhibitors could hamper metabolic activation or inactivation of several anticancer drugs. Additionally, CYP expression seems to be decreased in HCC tissue; this fact, associated with a physiologic reduction due to ageing, could severely reverberate on the efficacy of multikinase inhibitors in the elderly or on AEs onset in HCC patients [149,150,151].
Concerning hepatic impairment in treatment-naïve HCC patients who are candidates for multikinase inhibitors, Child–Pugh B was an exclusion criterion in SHARP, Asia-Pacific and REFLECT trials, but some patients were included as a result of protocol violation (<3%) [110,111,112]. In the second and further lines of treatment, multikinase inhibitors have been tested only in Child–Pugh A patients, meaning that HCC patients who switch from Child–Pugh A to B due to progressive disease have a loss of therapeutic chances. Immunotherapy trials in the second-line setting excluded Child–Pugh B patients. However, also in these trials, some patients were improperly included; a recent Korean real-world cohort study of nivolumab in HCC patients pointed out that Child–Pugh B is an independent negative predictor for objective response, with worse OS if compared to Child–Pugh A [152].
Child–Pugh C is an assured exclusion criterion for all drugs: patients belonging to this class must receive best supportive care only. A retrospective analysis of 992 Korean HCC patients showed no difference in percentage of Child–Pugh classes between patients older and younger than 70 years, and a similar result has been reported in extremely elderly patients (>80 years) in a Japanese cohort [153,154].
Even if data specifically on elderly HCC patients are lacking, immunotherapy seems to be feasible in this population without major contraindications (except for active autoimmune diseases), paying attention to steroid administration, which could reduce its efficacy.
3.3.4. A Focus on Sarcopenia in Elderly HCC Patients
One of the most important assessments in elderly patients is the nutritional one due to the higher rate of sarcopenia in this population. In particular, sarcopenia consists of a progressive skeletal muscle wasting and weakness due to due to loss of muscle quantity and quality, typically associated with age and often being part of cancer cachexia syndrome [155]. Concerning HCC patients, a Japanese retrospective analysis showed a 35% prevalence of sarcopenia in elderly patients (>70 years), with lower serum albumin levels and lower nutrition index, conferring a poor prognosis [156]. Sarcopenic patients undergoing sorafenib treatment had a significantly decreased OS (39 weeks compared to 61 weeks in non-sarcopenic patients) in an Italian retrospective analysis; sarcopenia might be enhanced by sorafenib administration through a reduction in carnitine absorption, suggesting that patients could benefit from carnitine integration [157,158]. Carnitine deficiency has been also observed in patients who developed fatigue during lenvatinib treatment [159].
Overall, these data highlight the role of muscle metabolism in HCC patients and especially in older ones, showing that improving the muscular status of these patients could be advantageous in their clinical management, particularly in advanced stages where supportive therapy plays a fundamental role [160].
4. Conclusions
HCC is a complex illness to manage, especially in the elderly, and even today there is a lack of prospective evidence in the literature or treatment guidelines in this population. The increase in life expectance and the peak of HCC incidence in older age enhanced the number of elderly HCC patients. Therefore, the management of elderly patients with HCC is a largely unexplored field worth investigating in clinical trials. In general, these patients should be referred to dedicated, high-volume centers with specialized tasks.
HCC patients require a comprehensive multidisciplinary—including geriatric—assessment, in order to receive correct tumour staging and a careful evaluation of the abilities and comorbidities. Finally, elderly patients should be distinguished into “frail” and “fit” patients. Even if specific data do not exist in the literature to date, the evidence summarized in this review suggests the use of geriatric scales in everyday clinical practice. In particular, fit patients could receive the best standard treatment for HCC according to BCLC stage, regardless the chronological age [4,98], which should not represent one of the criteria to select patients for treatments. Additionally, also in the geriatric population, the novel concept of stage migration is becoming an important issue to consider in order to give them the opportunity to receive a treatment different from that indicated by BCLC because of its clinical characteristics. Thus, an in-depth evaluation of the risk/benefit of different treatments should be especially mandatory in this population.
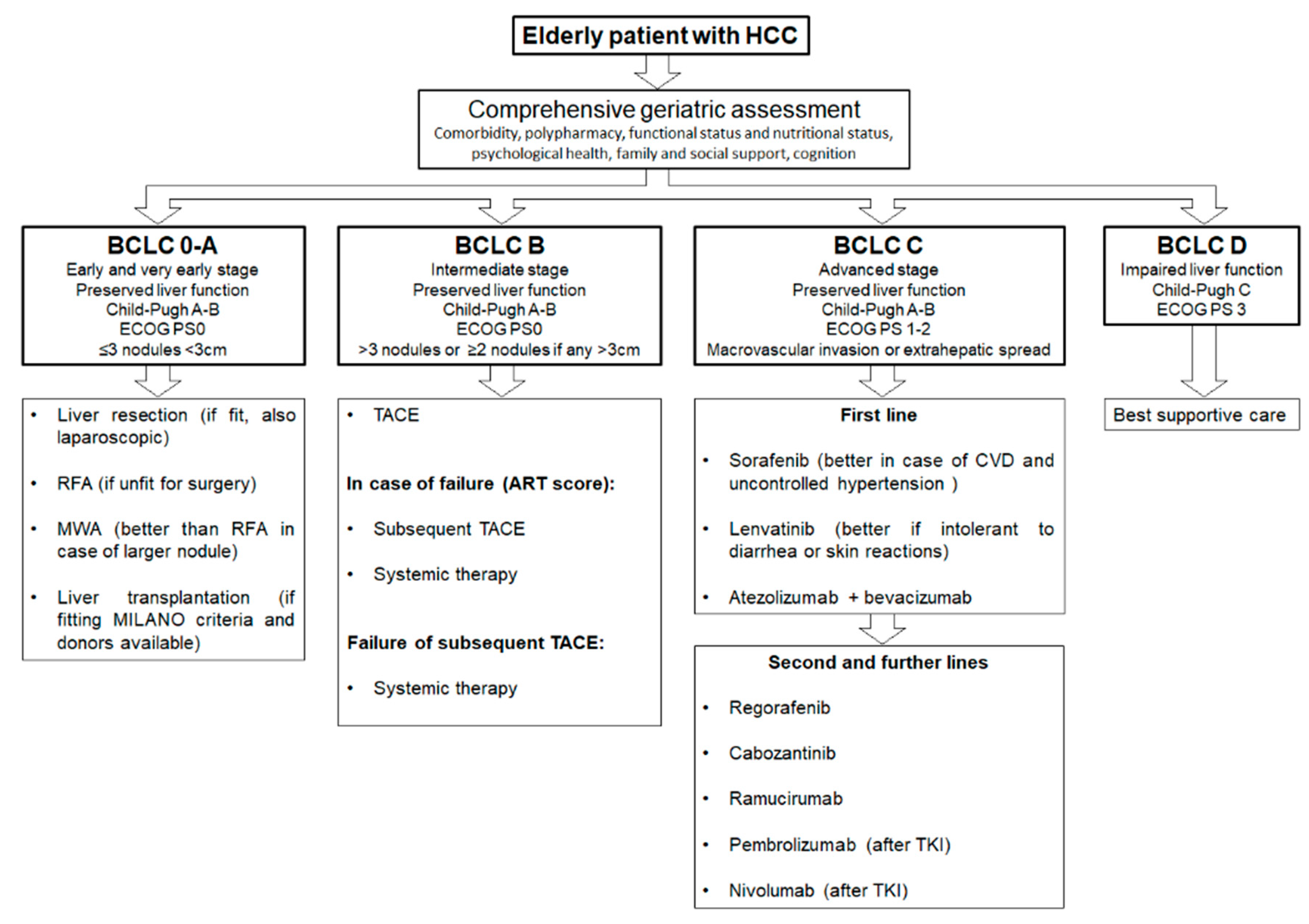
Finally, in the case of frail elderly patients, they should be candidates for the best supportive care and eventually referred to the palliative care unit. However, more specific and prospective data are needed in this field, and further investigations are required in order to standardize the treatment for HCC elderly patients. In Figure 2, we propose a summary algorithm for treating HCC in elderly patients.
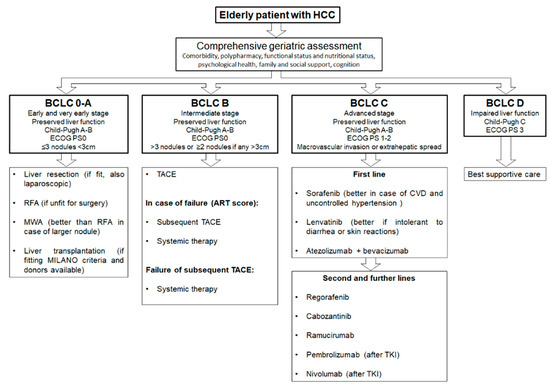
Figure 2.
Proposed algorithm for treating elderly hepatocellular carcinoma (HCC) patients according to Barcelona Clinic Liver Cancer (BCLC) stages.
Author Contributions
Conceptualization, P.F.; resources, P.F., A.P. (Angelica Petrillo), A.T., E.F.G., A.P. (Annalisa Pappalardo); writing—original draft preparation: all authors (P.F., E.F.G., A.P. (Annalisa Pappalardo), A.T.; G.M., L.A., A.F., A.P. (Angelica Petrillo), B.D.; writing of particular sections: all authors; writing—review and editing, P.F., E.F.G., A.P. (Angelica Petrillo), B.D.; supervision, B.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would thank K. El Bairi (Cancer Biomarkers Working Group, Oujda, Morocco) for the editorial assistance.
Conflicts of Interest
A.P. (Angelica Petrillo) received a personal fee from Eli-Lilly, Servier and MSD; B.D. received a personal fee from Ipsen, Eisai, Eli Lilly, Astra Zeneca, Sanofi, MSD, Bayer, Roche, Amgen. No fees are connected with the submitted paper. The other authors declare no conflict of interest. The funders had no role in the design, writing of the manuscript, or in the decision to publish the paper.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- AIOM; AIRTUM Working Group. I Numeri del Cancro in Italia 2020; Intermedia Editore: Attigliano, Italy, 2020. [Google Scholar]
- Federico, P.; Petrillo, A.; Giordano, P.; Bosso, D.; Fabbrocini, A.; Ottaviano, M.; Rosanova, M.; Silvestri, A.; Tufo, A.; Cozzolino, A.; et al. Immune Checkpoint Inhibitors in Hepatocellular Carcinoma: Current Status and Novel Perspectives. Cancers 2020, 12, 3025. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, J.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.; Neumann, U.; Ricke, C.; Sangro, B.; et al. Hepatocellular Carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef]
- Llovet, J.M.; Fuster, J.; Bruix, J.; Barcelona-Clínic Liver Cancer Group. The Barcelona approach: Diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transplant. 2004, 10, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.K.; Guy, J. Hepatocellular carcinoma in the elderly: Meta-analysis and systematic literature review. World J. Gastroenterol. 2015, 21, 12197–12210. [Google Scholar] [CrossRef]
- Yancik, R.; Ganz, P.A.; Varricchio, C.G.; Conley, B. Perspectives on comorbidity and cancer in older patient: Approaches to expend the knowledge base. J. Clin. Oncol. 2001, 19, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Decoster, L.; van Puyvelde, S.K.; Mohile, U.; Wedding, U.; Basso, G.; Colloca, S.; Rostoft, J.; Overcash, H.; Wildiers, C.; Steer, G.; et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancerpatients: An update on SIOG recommendations. Ann. Oncol. 2015, 26, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Choi, H.C.; Jeong, S.H.; Lee, K.H.; Chung, J.I.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Kim, N.; Lee, N.H.; et al. Hepatocellular carcinoma in older adults: Clinical features, treatments, and survival. J. Am. Geriatr. Soc. 2011, 59, 241–250. [Google Scholar] [CrossRef]
- Berger, N.A.; Savvides, P.; Koroukian, S.M.; Kahana, E.F.; Deimling, G.T.; Rose, J.H.; Bowman, K.F.; Miller, R.H. Cancer in the elderly. Trans. Am. Clin. Climatol. Assoc. 2006, 117, 147–155. [Google Scholar] [PubMed]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J.; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Schonberg, M.A.; Boyd, C.M.; Burhen, P.S.; Canin, B.; Cohen, H.J.; Holmes, H.M.; Hopkins, J.O.; et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J. Clin. Oncol. 2018, 36, 2326–2347. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pelissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Extermann, M.; Green, T.; Tiffenberg, G.; Rich, C.J. Validation of the senior adultoncology program (SAOP) 2 screening questionnaire. Crit. Rev. Oncol. Hematol. 2009, 69, 183–185. [Google Scholar]
- Dubianski, R.; Wildes, T.M.; Wildiers, H. SIOG guidelines-essential for good clinical practice in geriatric oncology. J. Geriatr. Oncol. 2019, 10, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Stokoe, J.M.; Pearce, J.; Sinha, R.; Ring, A. G8 and VES-13 scores predict chemotherapytoxicity in older patients with cancer. J. Geriatr. Oncol. 2012, 3, S81. [Google Scholar] [CrossRef]
- Kenis, C.; Decoster, L.; van Puyvelde, K.; De Greve, J.; Conings, G.; Milisen, K.; Flamaing, J.; Lobelle, J.P.; Wildiers, H. Performance of two geriatric screening tools in older patients with cancer. J. Clin. Oncol. 2014, 32, 19–26. [Google Scholar] [CrossRef]
- Saliba, D.; Elliot, M.; Rubenstein, L.Z.; Solomon, D.H.; Young, R.T.; Kamberg, C.J.; Roth, C.; MacLean, C.H.; Shekelle, P.G.; Sloss, M.E.; et al. The Vulnetable Elders Survey: A tool for identifying vulnerable older people in the community. J. Am. Geriatr. Soc. 2001, 49, 1691–1699. [Google Scholar] [CrossRef]
- Soubeyran, P.; Bellera, C.; Goyard, J.; Heitz, D.; Cure, H.; Roussellot, H.; Albrand, G.; Servant, V.; Saint Jane, O.; Roy, C.; et al. Validation of the G8 screening tool in geriatriconcology: The ONCODAGE project. J. Clin. Oncol. 2011, 29. [Google Scholar] [CrossRef]
- Pottel, L.; Boterberg, T.; Pottel, H.; Goethals, L.; van Den Noortgate, N.; Duprez, F.; De Neve, F.; Rottey, S.; Geldhof, K.; van Eygen, K.; et al. Determination of an adequate screening tool for the identification of vulnerable elderly head and neck cancer patients treatedwith radio(chemo)therapy. J. Geriatr. Oncol. 2012, 3, 24–32. [Google Scholar] [CrossRef]
- Bednarsch, J.; Czigany, Z.; Heise, D.; Joechle, K.; Luedde, T.; Heij, L.; Bruners, P.; Ulmer, T.F.; Neumann, U.P.; Lang, S.A. Prognostic evaluation of HCC patients undergoing surgical resection: An analysis of 8 different staging systems. Langenbecks Arch. Surg. 2021, 406, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, S.; Sprinzl, M.; Schmidtmann, I.; Heil, E.; Koch, S.; Czauderna, C.; Heinrich, B.; Diggs, L.P.P.; Wörns, M.; Kloeckner, R.; et al. Validation of prognostic accuracy of MESH, HKLC, and BCLC classifications in a large German cohort of hepatocellular carcinoma patients. United Eur. Gastroenterol. J. 2020, 8, 444–452. [Google Scholar] [CrossRef]
- Gómez-Rodríguez, R.; Romero-Gutiérrez, M.; Artaza-Varasa, T.; González-Frutos, C.; Ciampi-Dopazo, J.J.; de-la-Cruz-Pérez, G.; Sánchez-Ruano, J.J. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev. Esp. Enferm. Dig. 2012, 104, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Darnell, A.; Forner, A.; Rimola, J.; Ayuso, C.; Bruix, J. Systemic therapy for hepatocellular carcinoma: The issue of treatment stage migration and registration of progression using the BCLC-refined RECIST. Semin. Liver Dis. 2014, 34, 444–455. [Google Scholar] [CrossRef]
- Wehling, C.; Dill, M.T.; Olkus, A.; Springfeld, C.; Chang, D.H.; Naumann, P.; Longerich, T.; Kratochwil, C.; Mehrabi, A.; Merle, U.; et al. Treatment stage migration and treatment sequences in patients with hepatocellular carcinoma: Drawbacks and opportunities. J. Cancer Res. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawala, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [PubMed]
- Raccomandazioni Multisocietarie Italiane (AISF, AIOM, IT-IHPBA, SIC, SIRM, SITO) per la Gestione Clinica Integrata del Paziente con Epatocarcinoma. Available online: https://www.webaisf.org/aisf-guidelines-e-position-papers/page/3/ (accessed on 1 December 2020).
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Chan, J.A.; Aloia, T.A.; Chun, Y.S.; Kaseb, A.O.; Passot, G.; Yamashita, S.; Vauthey, J.N.; Conrad, C. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017, 123, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Gil, E.; Kim, J.M.; Jeon, K.; Park, H.; Danbee, K.; Cho, J.; Suh, G.Y.; Park, J. Recipient Age and Mortality After Liver Transplantation: A Population-based Cohort Study. Transplantation 2018, 102, 2025–2032. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, S.R.; Feng, S.; Hameed, B.; Yao, F.; Lai, J.C. Combined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomes. Transplantation 2014, 98, 557–562. [Google Scholar] [CrossRef]
- Wilson, G.C.; Quillin, R.C.; Wima, K.; Sutton, J.M.; Hoehn, R.S.; Hanseman, D.J.; Paquette, I.M.; Paterno, F.; Woodle, E.S.; Abbott, D.E.; et al. Is liver transplantation safe and effective in elderly (≥70 years) recipients? A case-controlled analysis. HPB 2014, 16, 1088–1094. [Google Scholar] [CrossRef]
- Chu, K.K.W.; Chok, K.S.H. Is the treatment outcome of hepatocellular carcinoma inferior in elderly patients? World J. Gastroenterol. 2019, 25, 3563–3571. [Google Scholar] [CrossRef]
- Cescon, M.; Colecchia, A.; Cucchetti, A.; Peri, E.; Montrone, L.; Ercolani, G.; Festi, D.; Pinna, A.D. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann. Surg. 2012, 256, 706–713. [Google Scholar] [CrossRef]
- Imamura, H.; Seyama, Y.; Kokudo, N.; Maema, A.; Sugawara, A.; Sano, K.; Takayama, T.; Makuuchi, M. One Thousand Fifty-Six Hepatectomies without Mortality in 8 Years. Arch. Surg. 2003, 138, 1198–1206. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Koike, K.; Nishino, H. Clinical features and prognosis of elderly patients with hepatocellular carcinoma not indicated for surgical resection. Geriatr. Gerontol. Int. 2017, 17, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Miki, D.; Aikata, H.; Uka, K.; Saneto, H.; Kawaoka, T.; Azakami, T.; Takaki, S.; Jeong, S.C.; Imamura, M.; Kawakami, Y.; et al. Clinicopathological features of elderly patients with hepatitis C virus-related hepatocellular carcinoma. J. Gastroenterol. 2008, 43, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Treatment for hepatocellular carcinoma in elderly patients: A literature review. J. Cancer 2013, 4, 635–643. [Google Scholar] [CrossRef]
- Shirabe, K.; Kajiyama, K.; Harimoto, N.; Gion, T.; Tsujita, E.; Abe, T.; Wakiyama, S.; Nagaie, T.; Maehara, Y. Early outcome following hepatic resection in patients older than 80 years of age. World J. Surg. 2009, 33, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Randall, H.B.; Cao, S.; de Vera, M.E. Transplantation in elderly patients. Arch. Surg. 2003, 138, 1089–1092. [Google Scholar] [CrossRef]
- Orcutt, S.T.; Artinyan, A.; Li, L.T.; Silberfein, E.J.; Berger, D.H.; Albo, D.; Anaya, D.A. Postoperative mortality and need for transitional care following liver resection for metastatic disease in elderly patients: A population-level analysis of 4026 patients. HPB 2012, 14, 863–870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hutchins, L.F.; Unger, J.M.; Crowley, J.J.; Coltman, C.A.; Albain, K.S. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N. Engl. J. Med. 1999, 341, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Itamoto, T.; Kohashi, T.; Matsugu, Y.; Nakahara, H.; Kitamoto, M. Safety of hepatectomy for elderly patients with hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 15028–15036. [Google Scholar] [CrossRef] [PubMed]
- Phan, K.; An, V.V.G.; Ha, H.; Phan, S.; Lam, V.; Pleass, H. Hepatic resection for malignant liver tumours in the elderly: A systematic review and meta-analysis. ANZ J. Surg. 2015, 85, 815–822. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, S.G.; Bennett, K.M.; Scarborough, J.E. Functional status determines postoperative outcomes in elderly patients undergoing hepatic resections. J. Surg. Oncol. 2013, 107, 865–870. [Google Scholar] [CrossRef]
- Cieslak, K.P.; Baur, O.; Verheij, J.; Bennink, R.J.; van Gulik, T.M. Liver function declines with increased age. HPB 2016, 18, 691–696. [Google Scholar] [CrossRef]
- Tufo, A.; Dunne, D.F.; Manu, N.; Lacasia, C.; Jones, L.; de Liguori Carino, N.; Malik, A.Z.; Poston, G.J.; Fenwick, S.W. Changing outlook for colorectal liver metastasis resection in the elderly. Eur. J. Surg. Oncol. 2019, 45, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Tufo, A.; Dunne, D.F.J.; Manu, N.; Joshi, H.; Lacasia, C.; Jones, L.; Malik, H.Z.; Poston, G.J.; Fenwick, S.W. Hepatectomy for octogenarians with colorectal liver metastasis in the era of enhanced recovery. Eur. J. Surg. Oncol. 2018, 44, 1040–1047. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. The Short- and Long-Term Outcomes in Elderly Patients with Hepatocellular Carcinoma after Curative Surgery: A Case-Controlled Study with Propensity Score Matching. Eur. Surg. Res. 2019, 59, 380–390. [Google Scholar] [CrossRef]
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A review of hepatocellular carcinoma in elderly patients focused on management and outcomes. Vivo 2019, 33, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Barbas, A.S.; Turley, R.S.; Gamblin, T.C.; Geller, D.A.; Marsh, J.W.; Tsung, A.; Clary, B.M.; Lagoo-Deenadayalan, S. Major liver resection in elderly patients: A multi-institutional analysis. J. Am. Coll. Surg. 2011, 212, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Wang, K.; Zhang, R.; Dai, Q.; Zou, S. The enhanced recovery after surgery (ERAS) program in liver surgery: A meta-analysis of randomized controlled trials. SpringerPlus 2016, 5, 207. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Cherqui, D.; Geller, D.A.; Buell, J.F.; Kaneko, H.; Han, H.S.; Asbun, O.; O’Rourche, N.; Tanabe, M.; Koffron, A.J.; et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015, 261, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hilal, M.A.; Aldrighetti, L.; Dagher, I.; Edwin, B.; Troisi, R.I.; Alikhanov, R.; Aroori, S.; Belli, G.; Besselink, M.; Briceno, J.; et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018, 268, 11–18. [Google Scholar] [CrossRef]
- Cheek, S.M.; Sucandy, I.; Tsung, A.; Marsh, J.W.; Geller, D.A. Evidence supporting laparoscopic major hepatectomy. J. Hepato-Biliary-Pancreatic Sci. 2016, 23, 257–259. [Google Scholar] [CrossRef]
- Morise, Z.; Ciria, R.; Cherqui, D.; Chen, K.H.; Belli, G.; Wakabayashi, G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepato-Biliary-Pancreatic Sci. 2015, 22, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Sposito, C.; Battiston, C.; Facciorusso, A.; Mazzola, M.; Muscarà, C.; Scotti, M.; Romito, R.; Mariani, L.; Mazzafferro, V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br. J. Surg 2016, 103, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, Z.; Guo, B.; Liu, S.; Xiao, W.; Liang, J. Short- and long-term outcomes of laparoscopic hepatectomy in elderly patients with hepatocellular carcinoma. J. BUON 2018, 23, 971–978. [Google Scholar]
- Dumronggittigule, W.; Han, H.S.; Ahn, S.; Yoon, Y.S.; Cho, J.Y.; Choi, Y.R. Laparoscopic versus Open Hepatectomy for Hepatocellular Carcinoma in Elderly Patients: A Single-Institutional Propensity Score Matching Comparison. Dig. Surg. 2020, 37, 495–504. [Google Scholar] [CrossRef]
- Nomi, T.; Hirokawa, F.; Kaibori, M.; Ueno, M.; Tanaka, S.; Hokuto, D.; Noda, T.; Nakai, T.; Ikoma, H.; Iida, H.; et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in elderly patients: A multi-centre propensity score-based analysis. Surg. Endosc. 2020, 34, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Tanaka, K.; Nojiri, K.; Kumamoto, T.; Mori, R.; Taniguchi, K.; Matsuyama, R.; Takeda, K.; Ueda, M.; Akiyama, H.; et al. Hepatic Resection for Hepatocellular Carcinoma in the Elderly: Selecting Hepatectomy Procedures Based on Patient Age. Anticancer Res. 2015, 35, 6855–6860. [Google Scholar] [PubMed]
- Famularo, S.; Di Sandro, S.; Giani, A.; Angrisani, M.; Lauterio, A.; Romano, F.; Gianotti, L.; De Carlis, L. The impact of age and ageing on hepatocarcinoma surgery: Short- and long-term outcomes in a multicentre propensity-matched cohort. Liver Int. 2019, 39, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Takeshita, A.; Shibayama, Y.; Uchiyama, K. Surgical Outcomes and Clinical Characteristics of Elderly Patients Undergoing Curative Hepatectomy for Hepatocellular Carcinoma. J. Gastrointest. Surg. 2013, 17, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Dajti, E.; Ravaioli, F.; Festi, D.; Colecchia, A. Clinical outcomes after treatment with di-rect-acting antivirals: Not all concern hepatocellular carcinoma risk. Hepatobiliary Surg. Nutr. 2020, 9, 505–507. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, F.; Conti, F.; Brillanti, S.; Andreone, P.; Mazzella, G.; Buonfiglioli, F.; Serio, I.; Verrucchi, G.; Reggiani, M.L.B.; Colli, A.; et al. Hepatocellular carcinoma risk assessment by the measurement of liver stiffness variations in HCV cirrhotics treated with direct acting antivirals. Dig. Liver Dis. 2018, 50, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Colecchia, A.; Colli, A.; Ravaioli, F.; Casazza, G.; Reggiani, M.L.B.; Cucchetti, A.; Cescon, M.; Festi, D. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 2019, 70, 440–448. [Google Scholar] [CrossRef]
- Itamoto, T.; Nakahara, H.; Amano, H.; Kohashi, T.; Ohdan, H.; Tashiro, H.; Asahara, T. Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 2007, 141, 589–597. [Google Scholar] [CrossRef]
- Zhu, G.Q.; Sun, M.; Liao, W.T.; Yu, W.; Zhou, S.; Zhou, Z.; Shi, T.; Fan, J.; Zhou, J.; Qiu, L.; et al. Comparative efficacy and safety between ablative therapies or surgery for small hepatocellular carcinoma: A network meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Ding, Y.; Liao, X.; Wang, C.; Wang, B.; Chen, X. Radiofrequency ablation versus surgical resection in elderly patients with early-stage hepatocellular carcinoma in the era of organ shortage. Saudi J. Gastroenterol. 2018, 24, 317–325. [Google Scholar] [CrossRef]
- Kaibori, M.; Yoshii, K.; Hasegawa, K.; Ogawa, A.; Kubo, S.; Tateishi, R.; Izumi, N.; Kadoya, M.; Kudo, M.; Kumada, T.; et al. Treatment Optimization for Hepatocellular Carcinoma in Elderly Patients in a Japanese Nationwide Cohort. Ann. Surg. 2019, 270, 121–130. [Google Scholar] [CrossRef]
- Cucchetti, A.; Piscaglia, F.; Cescon, M.; Colecchia, A.; Bolondi, L.; Pinna, A.D. 265 Cost-effectiveness of hepatic resection versus percutaneous ablation for hepatocellular carcinoma within the Milan criteria. J. Hepatol. 2013, 58, S63–S227. [Google Scholar] [CrossRef]
- Jiang, Y.Q.; Wang, Z.X.; Deng, Y.N.; Yang, Y.; Wang, G.Y.; Chen, G.H. Efficacy of hepatic resection vs. radiofrequency ablation for patients with very-early-stage or early-stage hepatocellular carcinoma: A population-based study with stratification by age and tumor size. Front. Oncol. 2019, 9, 113. [Google Scholar] [CrossRef]
- Sala, M.; Fuster, J.; Llovet, J.M.; Navasa, M.; Solè, M.; Varela, M.; Pons, F.; Rimola, A.; Garcia-Valdecasas, J.C.; Brú, C.; et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: An indication for salvage liver transplantation. Liver Transpl. 2004, 10, 1294–1300. [Google Scholar] [CrossRef]
- Cho, S.W.; Steel, J.; Tsung, A.; Marsh, J.W.; Geller, D.A.; Gamblin, T.C. Safety of liver resection in the elderly: How important is age? Ann. Surg. Oncol. 2011, 18, 1088–1095. [Google Scholar] [CrossRef]
- Fujiwara, N.; Tateishi, R.; Kondo, M.; Minami, T.; Mikami, S.; Sato, M.; Uchino, K.; Enooku, K.; Masuzaki, R.; Nakagawa, H.; et al. Cause-specific mortality associated with aging in patients with hepatocellular carcinoma undergoing percutaneous radiofrequency ablation. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Osaki, Y.; Iguchi, E.; Takeda, H.; Ohara, Y.; Sakamoto, A.; Hatamaru, K.; Henmi, S.; Saito, S.; Nasu, A.; et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma: Clinical outcome and safety in elderly patients. J. Gastrointest. Liver Dis. 2012, 21, 397–405. [Google Scholar]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, J.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hsu, C.Y.; Chu, C.W.; Liu, P.H.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Chiou, Y.Y.; Lin, H.C.; Huo, T.L. Radiofrequency ablation is better than surgical resection in patients with hepatocellular carcinoma within the Milan criteria and preserved liver function: A retrospective study using propensity score analyses. J. Clin. Gastroenterol. 2015, 49, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.A.; Shim, J.H.; Kim, M.J.; Kim, S.Y.; Won, H.J.; Shin, Y.M.; Kim, P.N.; Kim, K.H.; Lee, S.G.; Lee, H.C. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br. J. Surg. 2016, 103, 126–135. [Google Scholar] [CrossRef]
- Miura, J.T.; Johnston, F.M.; Tsai, S.; Eastwood, D.; Banejree, A.; Christians, K.K.; Turaga, K.K.; Gamblin, T.C. Surgical resection versus ablation for hepatocellular carcinoma ≤ 3 cm: A population-based analysis. HPB 2015, 17, 896–901. [Google Scholar] [CrossRef]
- Ng, K.K.; Poon, R.T. Radiofrequency ablation for malignant liver tumor. Surg. Oncol. 2005, 14, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Gong, J.F.; Yu, F.; Tang, W.H.; Yang, K. Long-Term Efficacy of Laparoscopic Radiofrequency Ablation in Early Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fan, Y.C.; Wang, K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant systematic review and meta-analysis. Medicine 2020, 99, e22703. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, M.; Amabile, C.; Cassarino, S.; Tosoratti, N.; Pinto, R.; Lopresto, V. Influence of the target tissue size on the shape of ex vivo microwave ablation zones. Int. J. Hyperth. 2015, 31, 48–57. [Google Scholar] [CrossRef]
- Liang, P.; Yu, J.; Lu, M.D.; Dong, B.W.; Yu, X.L.; Zhou, X.D.; Hu, B.; Xie, M.X.; Cheng, W.; He, W.; et al. Practice guidelines for ultrasound-guided percutaneous microwave ablation for hepatic malignancy. World J. Gastroenterol. 2013, 19, 5430–5438. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X.L.; Han, Z.Y.; Cheng, Z.G.; Liu, F.Y.; Zhai, H.Y.; Mu, M.J.; Liu, Y.M.; Liang, P. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: A phase III randomised controlled trial. Gut 2017, 66, 1172–1173. [Google Scholar] [CrossRef]
- Liang, P.; Wang, Y.; Yu, X.L.; Dong, B. Malignant liver tumors: Treatment with percutaneous microwave ablation—Complications among cohort of 1136 patients. Radiology 2009, 251, 933–940. [Google Scholar] [CrossRef]
- Liang, P.; Yu, J.; Yu, X.L.; Wang, X.H.; Wei, Q.; Yu, S.Y.; Li, H.X.; Sun, H.T.; Zhang, Z.Y.; Liu, H.C.; et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: A multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut 2012, 61, 1100–1101. [Google Scholar] [CrossRef]
- Facciorusso, A.; Di Maso, M.; Muscatiello, N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 339–344. [Google Scholar] [CrossRef]
- Shen, X.; Ma, S.; Tang, X.; Wang, T.; Qi, X.; Chi, J.; Wang, Z.; Cui, D.; Zhang, Y.; Li, P.; et al. Clinical outcome in elderly Chinese patients with primary hepatocellular carcinoma treated with percutaneous microwave coagulation therapy (PMCT): A Strobe-compliant observational study. Medicine 2018, 97, e11618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, Z.; Yu, J.; Li, K.; Hao, G.; Liu, F.; Han, Z.; Yu, X.; Liang, P. US-guided percutaneous microwave ablation for early-stage hepatocellular carcinoma in elderly patients is as effective as in younger patients: A 10-year experience. J. Cancer Res. Ther. 2020, 16, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, G.G.; Tortora, R.; D’Adamo, G.; De Luca, M.; Lampasi, F.; Addario, L.; Lanza, A.G.; Picciotto, F.P.; Tartaglione, M.T.; Cordone, G.; et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: A randomized trial. J. Gastroenterol. Hepatol. 2015, 30, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.G.; Bhattacharya, R.; Yeh, M.M.; Padia, S.A. Irreversible electroporation can effectively ablate hepatocellular carcinoma to complete pathologic necrosis. J. Vasc. Interv. Radiol. 2015, 26, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Hepatobiliary Cancers Version 5. 2020. Available online: https://www.nccn.org. (accessed on 4 August 2020).
- Cheng, A.L.; Amarapurkar, D.; Chao, Y.; Chen, P.J.; Geschwind, J.F.; Goh, K.L.; Han, K.H.; Kudo, M.; Lee, H.C.; Lee, R.C.; et al. Re-evaluatingntransarterial chemoembolization for the treatment of hepatocellular carcinoma: Consensus recommendations and review by an International Expert Panel. Liver Int. 2014, 34, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Bolondi, L.; Burroughs, A.; Dufour, J.F.; Galle, P.R.; Mazzaferro, V.; Piscaglia, F.; Raoul, J.L.; Sangro, B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: Proposal for a subclassification to facilitate treatment decisions. Semin. Liver Dis. 2012, 32, 348–359. [Google Scholar] [CrossRef]
- Kudo, M.; Arizumi, T.; Ueshima, K.; Sakurai, T.; Kitano, M.; Nishida, N. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: Proposal of modified Bolondi’s subclassification (Kinki criteria). Dig. Dis. 2015, 33, 751–758. [Google Scholar] [CrossRef]
- Kudo, M. Heterogeneity and subclassification of Barcelona Clinic Liver Cancer Stage B. Liver Cancer 2016, 5, 91–96. [Google Scholar] [CrossRef]
- Raoul, J.L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Bernie, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burrough, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolization for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Mondazzi, L.; Bottelli, R.; Brambilla, G.; Rampoldi, A.; Rezakovic, I.; Zavaglia, C.; Alberti, A.; Idèo, G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: A multivariate analysis of prognostic factors. Hepatology 1994, 19, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Yao, T.J.; Chan, P.; Epstein, R.J.; Ng, K.K.; Chok, S.H.; Cheung, T.T.; Fan, S.T.; Poon, R.T. The outcomes of elderly patients with hepatocellular carcinoma treated with transarterial chemoembolization. Cancer 2009, 115, 5507–5515. [Google Scholar] [CrossRef]
- Cheng, H.M.; Tanaka, T.; Nishiofuku, H.; Chanoki, Y.; Horiuchi, K.; Masada, T.; Tatsumoto, S.; Matsumoto, T.; Marugami, N.; Kichikawa, K. Safety and Prognosis of Transarterial Chemoembolization for Octogenarians with Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2019, 42, 1413–1419. [Google Scholar] [CrossRef]
- Cohen, M.J.; Levy, I.; Barak, O.; Bloom, A.I.; Fernandez-Ruiz, M.; Di Maio, M.; Perrone, F.; Poon, R.T.; Shouval, D.; Yau, T.; et al. Transarterial chemo-embolization is safe and effective for elderly advanced hepatocellular carcinoma patients: Results from an international database. Liver Int. 2014, 34, 1109–1117. [Google Scholar] [CrossRef]
- Liu, P.H.; Hsu, C.Y.; Lee, Y.H.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Chiou, Y.Y.; Lin, H.C.; Huo, T.I. Uncompromised treatment efficacy in elderly patients with hepatocellular carcinoma: A propensity score analysis. Medicine 2014, 93, e264. [Google Scholar] [CrossRef] [PubMed]
- Aizumi, T.; Ueshima, K.; Minami, T.; Kono, M.; Chishina, H.; Takita, M.; Kitai, S.; Inoue, T.; Yada, N.; Hagiwara, S.; et al. Effectiveness of Sorafenib in Patients with Transcatheter Arterial Chemoembolization (TACE) Refractory and Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2015, 4, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Chiba, T.; Ooka, Y.; Kanogawa, N.; Motoyama, T.; Suzuki, E.; Tawada, A.; Kanai, F.; Yoshikawa, M.; Yokosuka, O. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology 2014, 87, 330–341. [Google Scholar] [CrossRef]
- Llover, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.; de Olivera, A.C.; Santoro, A.; Raoul, J.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2015, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.; Chumg, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Lang, L. FDA approves sorafenib for patients with inoperable liver cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/nexavar-epar-product-information_en.pdf (accessed on 5 March 2021).
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Nexavar Epar Scientific Discussion. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/nexavar-epar-scientific-discussion_en.pdf (accessed on 5 March 2021).
- Li, Y.; Gao, Z.H.; Qu, X.J. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin. Pharmacol. Toxicol. 2015, 116, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef]
- Hajiev, S.; Allara, E.; Motedayen-Aval, L.; Arizumi, T.; Bettinger, D.; Pirisi, M.; Rimassa, L.; Pressiani, T.; Personeni, N.; Giordano, L.; et al. Impact of age on sorafenib outcomes in hepatocellular carcinoma: An international cohort study. Br. J. Cancer 2020. [Google Scholar] [CrossRef]
- Reiss, K.A.; Yu, S.; Mamtani, R.; Mehta, R.; D’Addeo, K.; Wileyto, E.P.; Taddei, T.H.; Kaplan, D.E. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: A retrospective, multi-institutional study. J. Clin. Oncol. 2017, 35, 3575–3581. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; Habra, M.A. Lenvatinib: Role in thyroid cancer and other solid tumors. Cancer Treat. Rev. 2016, 42, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Z.; Mizuo, H.; Hayato, S.; Namiki, M.; Shumaker, R. Clinical Pharmacokinetic and Pharmacodynamic Profile of Lenvatinib, an Orally Active, Small-Molecule, Multitargeted Tyrosine Kinase Inhibitor. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 903–914. [Google Scholar] [CrossRef]
- Tada, T.; Kumada, T.; Hiraoka, A.; Michitaka, K.; Atsukawa, M.; Hirooka, M.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: A multicenter analysis with propensity score matching. Hepatol. Res. 2020, 50, 75–83. [Google Scholar] [CrossRef]
- Li, D.; Toh, H.C.; Merle, P.; Tsuchiya, K.; Hernandez, S.; Shao, H.; Mulla, S.; Ding, B.; Kudo, M. Atezolizumab + Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma (HCC): Results from Older Adults Enrolled in IMbrave150, Abstract O-8. In Proceedings of the ESMO World Congress on Gastrointestinal Cancer 2020, Online, 1–4 July 2020. [Google Scholar]
- U.S. Food and Drug. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/regorafenib (accessed on 1 December 2020).
- U.S. Food and Drug. Available online: https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma (accessed on 1 December 2020).
- U.S. Food and Drug. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ramucirumab-hepatocellular-carcinoma (accessed on 1 December 2020).
- U.S. Food and Drug. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib (accessed on 1 December 2020).
- U.S. Food and Drug. Available online: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma (accessed on 1 December 2020).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/variation-report/stivarga-h-c-2573-ii-0020-epar-assessment-report-variation_en.pdf (accessed on 1 December 2020).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/variation-report/cabometyx-h-c-004163-ii-0005-epar-assessment-report-variation_en.pdf (accessed on 1 December 2020).
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/smop/chmppost-authorisation-summary-positive-opinion-cyramza-ii-27_en.pdf (accessed on 1 December 2020).
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Shutz, G.; Thierauch, H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gerisch, M.; Hafner, F.T.; Lang, D.; Radtke, M.; Diefenbach, K.; Cleton, A.; Lettieri, J. Mass balance, metabolic disposition, and pharmacokinetics of a single oral dose of regorafenib in healthy human subjects. Cancer Chemother. Pharmacol. 2018, 81, 195–206. [Google Scholar] [CrossRef]
- Keunecke, A.; Hoefman, S.; Drenth, H.J.; Zisowsky, J.; Cleton, A.; Ploeger, B.A. Population pharmacokinetics of regorafenib in solid tumours: Exposure in clinical practice considering enterohepatic circulation and food intake. Br. J. Clin. Pharmacol. 2020, 86, 2362–2376. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Debaillon Vesque, A.; Decraecker, M.; Blanc, J.F. Profile of Cabozantinib for the Treatment of Hepatocellular Carcinoma: Patient Selection and Special Considerations. J. Hepatocell. Carcinoma 2020, 7, 91–99. [Google Scholar] [CrossRef]
- Kudo, M.; Galle, P.R.; Llovet, J.M.; Finn, R.S.; Vogel, A.; Motomura, K.; Assenat, E.; Merle, P.; Brandi, G.; Daniele, B.; et al. Ramucirumab in elderly patients with hepatocellular carcinoma and elevated alpha-fetoprotein after sorafenib in REACH and REACH-2. Liver Int. 2020, 40, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Holt, P.R. Intestinal malabsorption in the elderly. Dig. Dis. 2007, 25, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.A.; Dip, R.M.; Furlan, M.O.; Rodrigues, S.L. Use of drugs that act on the cytochrome P450 system in the elderly. Clinics 2009, 64, 273–278. [Google Scholar] [CrossRef][Green Version]
- Yan, T.; Lu, L.; Xie, C.; Chen, T.; Peng, X.; Zhu, L.; Wang, L.; Li, Q.; Shi, J.; Zhou, F.; et al. Severely Impaired and Dysregulated Cytochrome P450 Expression and Activities in Hepatocellular Carcinoma: Implications for Personalized Treatment in Patients. Mol. Cancer Ther. 2015, 14, 2874–2886. [Google Scholar] [CrossRef]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009, 41, 67–76. [Google Scholar] [CrossRef]
- Choi, W.M.; Lee, D.; Shim, J.H.; Kim, K.M.; Lim, Y.S.; Lee, H.C.; Yoo, C.; Park, S.R.; Ryu, M.H.; Ryoo, B.Y.; et al. Effectiveness and Safety of Nivolumab in Child—Pugh B Patients with Hepatocellular Carcinoma: A Real-World Cohort Study. Cancers 2020, 12, 1968. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jang, B.K.; Kim, E.S.; Chung, W.J.; Park, K.S.; Cho, B.; Hwang, J.S. Hepatocellular carcinoma in the elderly: Clinical characteristics, treatment, survival analysis in Korean patients older than 70 years. J. Korean Med. Sci. 2012, 27, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Tsukioka, G.; Kakizaki, S.; Sohara, N.; Sato, S.; Takagi, H.; Arai, H.; Abe, T.; Toyoda, M.; Katakai, K.; Kojima, A.; et al. Hepatocellular carcinoma in extremely elderly patients: An analysis of clinical characteristics, prognosis and patient survival. World J. Gastroenterol. 2006, 12, 48–53. [Google Scholar] [CrossRef]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef]
- Harimoto, N.; Yoshizumi, T.; Shimokawa, M.; Sakata, M.; Kimura, K.; Itoh, S.; Ikegami, T.; Ikeda, T.; Shirabe, K.; Maehara, Y. Sarcopenia is a poor prognostic factor following hepatic resection in patients aged 70 years and older with hepatocellular carcinoma. Hepatol. Res. 2016, 46, 1247–1255. [Google Scholar] [CrossRef]
- Antonelli, G.; Gigante, E.; Iavarone, M.; Begini, P.; Sangiovanni, A.; Iannicelli, E.; Biondetti, P.; Pellicelli, A.M.; Miglioresi, L.; Marchetti, P.; et al. Sarcopenia is associated with reduced survival in patients with advanced hepatocellular carcinoma undergoing sorafenib treatment. United Eur. Gastroenterol. J. 2018, 6, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Amanuma, M.; Nagai, H.; Igarashi, Y. Sorafenib Might Induce Sarcopenia in Patients With Hepatocellular Carcinoma by Inhibiting Carnitine Absorption. Anticancer Res. 2020, 40, 4173–4182. [Google Scholar] [CrossRef]
- Okubo, H.; Ando, H.; Ishizuka, K.; Kitagawa, R.; Okubo, S.; Saito, H.; Kokubu, S.; Miyazaki, A.; Ikejima, K.; Shiina, S.; et al. Carnitine insufficiency is associated with fatigue during lenvatinib treatment in patients with hepatocellular carcinoma. PLoS ONE 2020, 15, e0229772. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Serenari, M.; Renzulli, M.; Alemanni, L.V.; Rossini, B.; Pettinari, I.; Dajti, E.; Ravaioli, F.; Golfieri, R.; Cescon, M.; et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J. Gastroenterol. 2020, 55, 927–943. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).