D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid
Abstract
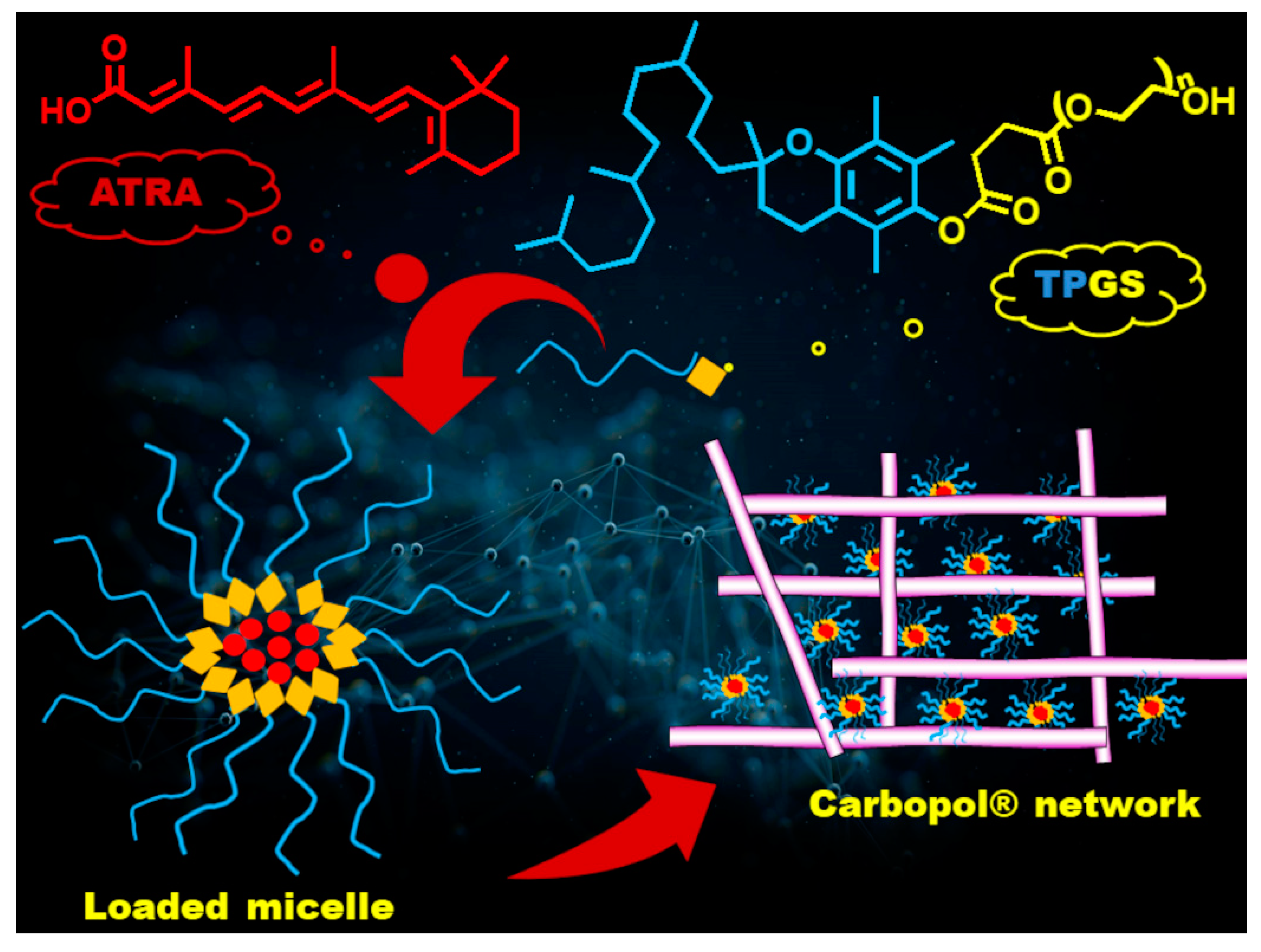
1. Introduction
2. Results
2.1. Effect of TPGS on ATRA Solubility
2.2. Preparation and Characterization of ATRA-Loaded TPGS Micelles (ATRA-TPGSs)
2.3. FTIR Analysis Assisted by PCA
2.3.1. FTIR Spectra
2.3.2. Principal Component Analysis (PCA)
2.4. Thermal Analysis by Differential Scanning Calorimetry (DSC)
2.5. Stability of ATRA-TPGSs
2.6. Nanocarrier-Loaded Hydrogel Preparation and Characterization
Rheological Studies
2.7. Ex Vivo Permeation Study
2.8. Cytotoxicity Studies Performed on Melanoma Cell Lines
3. Materials and Methods
3.1. Materials
3.2. Solubility Studies
3.3. Preparation of ATRA-TPGS Micelles
3.4. Determination of Micelles Size, Polydispersity Index (PDI), and Zeta (ƺ) Potential
3.5. Principal Component Analysis (PCA)-Assisted FTIR Analysis
3.5.1. FTIR Spectra Acquisition
3.5.2. Chemometric Analysis: PCA
3.6. Differential Scanning Calorimetry (DSC)
3.7. Stability Studies
3.8. Incorporation of Micelles in Hydrogels
3.9. Rheological Studies
3.10. Skin Permeation Experiments
3.11. Cell Viability Studies
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddikuzzaman; Guruvayoorappan, C.; Grace, V.B. All Trans Retinoic Acid and Cancer. Immunopharmacol. Immunotoxicol. 2010, 33, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Bergamante, V.; Carosio, R.; Gotti, R.; Montaldo, P.; Orienti, I. Micellar complexes of all-trans retinoic acid with polyvinylalcohol-nicotinoyl esters as new parenteral formulations in neuroblastoma. Drug Deliv. 2009, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Song, Y.; Liu, Q.; Wu, Y.; He, R. Topical treatment of all-trans retinoic acid inhibits murine melanoma partly by promoting CD8+ T-cell immunity. Immunology 2017, 152, 287–297. [Google Scholar] [CrossRef]
- Song, Y.; Yin, W.; Dan, Y.; Sheng, J.; Zeng, Y.; He, R. Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR 1-dependent natural killer cell recruitment. Immunology 2019, 157, 248–256. [Google Scholar] [CrossRef]
- Ferreira, R.; Napoli, J.; Enver, T.; Bernardino, L.; Ferreira, L. Advances and challenges in retinoid delivery systems in regenerative and therapeutic medicine. Nat. Commun. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Khalil, S.; Bardawil, T.; Stephan, C.; Darwiche, N.; Abbas, O.; Kibbi, A.G.; Nemer, G.; Kurban, M. Retinoids: A journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J. Dermatol. Treat. 2017, 28, 684–696. [Google Scholar] [CrossRef]
- Baldwin, H.E.; Nighland, M.; Kendall, C.; Mays, D.A.; Grossman, R.; Newburger, J. 40 years of topical tretinoin use in review. J. Drugs Dermatol. 2013, 12, 638–642. [Google Scholar] [PubMed]
- Caviglioli, G.; Parodi, B.; Posocco, V.; Cafaggi, S.; Bignardi, G. Stability Study of Hard Gelatin Capsules Containing Retinoic Acid. Drug Dev. Ind. Pharm. 2000, 26, 995–1001. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Shah, M.K.; Khatri, P.; Vora, N. Recent Advances in Lipid-Based Vesicles and Particulate Carriers for Topical and Transdermal Application. J. Pharm. Sci. 2017, 106, 423–445. [Google Scholar] [CrossRef]
- Sinico, C.; Manconi, M.; Peppi, M.; Lai, F.; Valenti, D.; Fadda, A.M. Liposomes as carriers for dermal delivery of tretinoin: In vitro evaluation of drug permeation and vesicle–skin interaction. J. Control. Release 2005, 103, 123–136. [Google Scholar] [CrossRef]
- Manca, M.L.; Manconi, M.; Nácher, A.; Carbone, C.; Valenti, D.; Maccioni, A.M.; Sinico, C.; Fadda, A.M. Development of novel diolein–niosomes for cutaneous delivery of tretinoin: Influence of formulation and in vitro assessment. Int. J. Pharm. 2014, 477, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Singh, B.; Lohan, S.; Sharma, G.; Negi, P.; Yachha, Y.; Katare, O.P. Nano-lipoidal carriers of tretinoin with enhanced percutaneous absorption, photostability, biocompatibility and anti-psoriatic activity. Int. J. Pharm. 2013, 456, 65–72. [Google Scholar] [CrossRef]
- Charoenputtakhun, P.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. All-trans retinoic acid-loaded lipid nanoparticles as a transdermal drug delivery carrier. Pharm. Dev. Technol. 2013, 19, 164–172. [Google Scholar] [CrossRef]
- Miura, T.; Takada, A.; Ooe, M. Tretinoin Cyclodextrin Complex (RA/CyD) Causes Less Irritation with an Equal Antiwrinkle Effect Compared with Conventional Tretinoin: Clinical and Histologic Studies of Photoaged Skin. Aesthetic Plast. Surg. 2012, 36, 971–981. [Google Scholar] [CrossRef]
- Lapteva, M.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale 2015, 7, 18651–18662. [Google Scholar] [CrossRef]
- Chavoshy, F.; Makhmalzade, B.S. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef] [PubMed]
- Youk, H.-J.; Lee, E.; Choi, M.-K.; Lee, Y.-J.; Chung, J.H.; Kim, S.-H.; Lee, C.-H.; Lim, S.-J. Enhanced anticancer efficacy of α-tocopheryl succinate by conjugation with polyethylene glycol. J. Control. Release 2005, 107, 43–52. [Google Scholar] [CrossRef]
- Duhem, N.; Danhier, F.; Préat, V. Vitamin E-based nanomedicines for anti-cancer drug delivery. J. Control. Release 2014, 182, 33–44. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Constantinou, C.; Papageorgis, P.; Constantinou, A.I. d-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Survivin-overexpressing breast cancer cells. Biochem. Pharmacol. 2014, 89, 31–42. [Google Scholar] [CrossRef]
- Abraham, J.; Salama, N.N.; Azab, A.K. The role of P-glycoprotein in drug resistance in multiple myeloma. Leuk. Lymphoma 2014, 56, 26–33. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Xiong, H.; Xu, C.; Yao, J.; Zhu, X.; Zhou, J.; Yao, J. Mechanisms of TPGS and its derivatives inhibiting P-glycoprotein efflux pump and application for reversing multidrug resistance in hepatocellular carcinoma. Polym. Chem. 2018, 9, 1827–1839. [Google Scholar] [CrossRef]
- Di Cagno, M.P.; Stein, P.C.; Stýskala, J.; Hlaváč, J.; Skalko-Basnet, N.; Bauer-Brandl, A. Overcoming instability and low solubility of new cytostatic compounds: A comparison of two approaches. Eur. J. Pharm. Biopharm. 2012, 80, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Sheu, M.-T.; Wu, A.-B.; Lin, K.-P.; Ho, H.-O. Simultaneous effects of tocopheryl polyethylene glycol succinate (TPGS) on local hair growth promotion and systemic absorption of topically applied minoxidil in a mouse model. Int. J. Pharm. 2005, 306, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.-T.; Chen, S.-Y.; Chen, L.-C.; Ho, H.-O. Influence of micelle solubilization by tocopheryl polyethylene glycol succinate (TPGS) on solubility enhancement and percutaneous penetration of estradiol. J. Control. Release 2003, 88, 355–368. [Google Scholar] [CrossRef]
- Aggarwal, N.; Goindi, S.; Mehta, S.D. Preparation and Evaluation of Dermal Delivery System of Griseofulvin Containing Vitamin E-TPGS as Penetration Enhancer. AAPS PharmSciTech 2011, 13, 67–74. [Google Scholar] [CrossRef]
- Giuli, M.V.; Hanieh, P.N.; Giuliani, E.; Rinaldi, F.; Marianecci, C.; Screpanti, I.; Checquolo, S.; Carafa, M. Current Trends in ATRA Delivery for Cancer Therapy. Pharmaceutics 2020, 12, 707. [Google Scholar] [CrossRef]
- Pitorre, M.; Gondé, H.; Haury, C.; Messous, M.; Poilane, J.; Boudaud, D.; Kanber, E.; Ndombina, G.A.R.; Benoit, J.-P.; Bastiat, G. Recent advances in nanocarrier-loaded gels: Which drug delivery technologies against which diseases? J. Control. Release 2017, 266, 140–155. [Google Scholar] [CrossRef]
- Qi, Z.H.; Shieh, W.J. Aqueous Media for Effective Delivery of Tretinoin. J. Incl. Phenom. Macrocycl. Chem. 2002, 44, 133–136. [Google Scholar] [CrossRef]
- Sadoqi, M.; Lau-Cam, C.; Wu, S. Investigation of the micellar properties of the tocopheryl polyethylene glycol succinate surfactants TPGS 400 and TPGS 1000 by steady state fluorometry. J. Colloid Interface Sci. 2009, 333, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Abouzeid, A.H.; Patel, N.R.; Torchilin, V.P. Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer. Int. J. Pharm. 2014, 464, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.K.; Kaddoumi, A.; Nazzal, S. Mixed micelles of PEG2000-DSPE and vitamin-E TPGS for concurrent delivery of paclitaxel and parthenolide: Enhanced chemosenstization and antitumor efficacy against non-small cell lung cancer (NSCLC) cell lines. Eur. J. Pharm. Sci. 2012, 46, 64–71. [Google Scholar] [CrossRef]
- Muthu, M.S.; Sahu, A.K.; Sonali; Abdulla, A.; Kaklotar, D.; Rajesh, C.V.; Singh, S.; Pandey, B.L. Solubilized delivery of paliperidone palmitate by d-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv. 2014, 23, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.V.; Cho, C.-W. Application of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in transdermal and topical drug delivery systems (TDDS). J. Pharm. Investig. 2017, 47, 111–121. [Google Scholar] [CrossRef]
- Zuccari, G.; Carosio, R.; Fini, A.; Montaldo, P.; Orienti, I. Modified polyvinylalcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. J. Control. Release 2005, 103, 369–380. [Google Scholar] [CrossRef]
- Li, X.; Uppala, V.V.S.; Cooksey, T.J.; Robertson, M.L.; Madsen, L.A. Quantifying Drug Cargo Partitioning in Block Copolymer Micelle Solutions. ACS Appl. Polym. Mater. 2020, 2, 3749–3755. [Google Scholar] [CrossRef]
- Bachhav, Y.; Mondon, K.; Kalia, Y.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef]
- Orienti, I.; Zuccari, G.; Carosio, R.; Montaldo, P.G. Preparation and Evaluation of Polyvinyl alcohol-co-oleylvinyl ether Derivatives as Tumor-Specific Cytotoxic Systems. Biomacromolecules 2005, 6, 2875–2880. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J. Vis. Exp. 2013, 51015. [Google Scholar] [CrossRef]
- Mahajan, H.S.; Patil, P.H. Central composite design-based optimization of lopinavir vitamin E-TPGS micelle: In vitro characterization and in vivo pharmacokinetic study. Colloids Surf. B Biointerfaces 2020, 194, 111149. [Google Scholar] [CrossRef]
- Di Tommaso, C.; Como, C.; Gurny, R.; Möller, M. Investigations on the lyophilisation of MPEG–hexPLA micelle based pharmaceutical formulations. Eur. J. Pharm. Sci. 2010, 40, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Carradori, D.; Labrak, Y.; Miron, V.E.; Saulnier, P.; Eyer, J.; Préat, V.; Rieux, A.D. Retinoic acid-loaded NFL-lipid nanocapsules promote oligodendrogenesis in focal white matter lesion. Biomaterials 2020, 230, 119653. [Google Scholar] [CrossRef]
- Foss. Available online: https://www.fossanalytics.com/it-it/news-articles/technologies/a-short-intro-to-ftir-analysis (accessed on 22 October 2020).
- Andre, C.M.; Soukoulis, C. Food Quality Assessed by Chemometrics. Foods 2020, 9, 897. [Google Scholar] [CrossRef]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. ChemistrySelect 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a fast, low cost, conservative and eco-friendly method for quantifying gallic acid in polymeric formulations by FTIR spectroscopy in solution. Chem. Sel. 2020, 5, 4381–4388. [Google Scholar]
- Giron, D. Applications of Thermal Analysis and Coupled Techniques in Pharmaceutical Industry. J. Therm. Anal. Calorim. 2002, 68, 335–357. [Google Scholar] [CrossRef]
- Caviglioli, G.; Pani, M.; Gatti, P.; Parodi, B.; Cafaggi, S.; Bignardi, G. Study of retinoic acid polymorphism. J. Pharm. Sci. 2006, 95, 2207–2221. [Google Scholar] [CrossRef]
- Koulouktsi, C.; Nanaki, S.; Barmpalexis, P.; Kostoglou, M.; Bikiaris, D. Preparation and characterization of Alendronate depot microspheres based on novel poly(-ε-caprolactone)/Vitamin E TPGS copolymers. Int. J. Pharm. X 2019, 1, 100014. [Google Scholar] [CrossRef]
- Sharadha, M.; Gowda, D.V.; Gupta, V.N.; Akhila, A.R. An Overview on Topical Drug Delivery System–Updated Review. IJRPS 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Adewale, F.J.; Lucky, A.P.; Abioye, P.; Oluwabunmi, A.P.; Elehinafe, F.; Boluwaji, E.F. Selecting the Most Appropriate Model for Rheological Characterization of Synthetic Based Drilling Mud. Int. J. Appl. Eng. Res. 2017, 12, 7614–7629. [Google Scholar]
- Montenegro, L.; Panico, A.M.; Ventimiglia, A.; Bonina, F.P. In Vitro retinoic acid release and skin permeation from different liposome formulations. Int. J. Pharm. 1996, 133, 89–96. [Google Scholar] [CrossRef]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric Micelle Nanocarriers for the Cutaneous Delivery of Tacrolimus: A Targeted Approach for the Treatment of Psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.; Burgoon, T. Differential effects of retinoic acid on the growth of normal fibroblast-like cells from human, swine and rabbit skin. Cell Biol. Int. Rep. 1982, 6, 163–170. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Constantinou, A.I. Drug Delivery Innovations for Enhancing the Anticancer Potential of Vitamin E Isoforms and Their Derivatives. BioMed Res. Int. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, C.; Pang, X.; Yu, Z.; Qi, Y.; Chen, X.; Liang, H.; Fang, X.; Sha, X. Adding Vitamin E-TPGS to the Formulation of Genexol-PM: Specially Mixed Micelles Improve Drug-Loading Ability and Cytotoxicity against Multidrug-Resistant Tumors Significantly. PLoS ONE 2015, 10, e0120129. [Google Scholar] [CrossRef] [PubMed]
- Dubholkar, R.D.; Sawant, R.M.; Mongayt, D.A.; Devarajan, P.V.; Torchilin, V.P. Polyethylene gly-col-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: Some properties, loading with paclitaxel, and mod-ulation of P-glycoprotein-mediate efflux. Int. J. Pharm. 2006, 315, 148–157. [Google Scholar] [CrossRef]
- Ghate, V.M.; Lewis, S.A.; Prabhu, P.; Dubey, A.; Patel, N. Nanostructured lipid carriers for the topical delivery of tretinoin. Eur. J. Pharm. Biopharm. 2016, 108, 253–261. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]












| ATRA:TPGS (w/w) | EE% | DL% |
|---|---|---|
| 1:20 | 35 ± 2 * | 0.76 ± 0.02 * |
| 1:30 | 47 ± 3 | 0.86 ± 0.03 |
| 1:40 | 58.6 ± 0.2 | 0.88 ± 0.01 * |
| 1:50 | 65 ± 3 | 0.83 ± 0.03 |
| 1:60 | 68 ± 2 | 0.78 ± 0.02 |
| 1:70 | 79 ± 8 * | 0.79 ± 0.05 |
| ATRA:TPGS (w/w) | Size (nm) Fresh | PDI Fresh | Size (nm) Lyophilized | PDI Lyophilized | ƺ (mV) mQ Water | ƺ (mV) HEPES |
|---|---|---|---|---|---|---|
| 1:20 | 13.8 ± 0.1 | 0.24 ± 0.03 | 15.0 ± 0.6 | 0.32 ± 0.09 | −7.2 ± 0.5 * | 0 ± 3 * |
| 1:30 | 17 ± 5 * | 0.3 ± 0.1 * | 19 ± 5 | 0.31 ± 0.02 | −9 ± 3 | 5 ± 1 |
| 1:40 | 12.6 ± 0.4 | 0.17 ± 0.06 | 14.1 ± 0.8 | 0.2 ± 0.1 | −13 ± 2 * | 0 ± 2 * |
| 1:50 | 11.9 ± 0.4 | 0.13 ± 0.06 | 14.7 ± 0.1 | 0.212 ± 0.001 | −11 ± 1 | 5 ± 1 |
| 1:60 | 11.8 ± 0.4 | 0.14 ± 0.03 | 21 ± 7 * | 0.4 ± 0.1 * | −13 ± 3 * | 4 ± 2 |
| 1:70 | 11.4 ± 0.1 | 0.11 ± 0.01 | 14.4 ± 0.2 | 0.19 ± 0.03 | −10.1 ± 0.7 | 4 ± 1 |
| Model | Equations | R2 | Slope | Intercept |
|---|---|---|---|---|
| Power Law | y = 0.2877x + 1.9193 1 y = 0.2866x + 2.0967 2 y = 0.3015x + 2.2723 3 | 0.9842 0.9943 0.9971 | 0.2877 0.2866 0.3015 | 1.9193 2.0967 2.2723 |
| Herschel–Bulkley | y = 0.5527x + 1.1508 1 y = 0.5899x + 1.2154 2 y = 0.6090x + 1.38863 | 0.8139 0.8308 0.8226 | 0.5527 0.5899 0.6090 | 1.1508 1.2154 1.3886 |
| Casson | y = 0.4389x + 12.857 1 y = 0.5328x + 15.915 2 y = 0.7189x + 19.668 3 | 0.8980 0.9351 0.9491 | 0.4389 0.5328 0.7189 | 12.857 15.925 19.668 |
| Bingham Plastic | y = 0.5178x + 257.98 1 y = 0.8055x + 378.43 2 y = 1.3961x + 593.18 3 | 0.8350 0.8650 0.8873 | 0.5178 0.8055 1.3961 | 257.98 378.43 593.18 |
| Formulation | Amount Permeated (µg cm−2 ± SD) |
|---|---|
| ATRA-TPGS dispersion | 27 ± 5 |
| ATRA-TPGS-loaded gel | 22 ± 4 |
| ATRA hydroalcholic solution | 32 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. https://doi.org/10.3390/ph14030212
Zuccari G, Baldassari S, Alfei S, Marengo B, Valenti GE, Domenicotti C, Ailuno G, Villa C, Marchitto L, Caviglioli G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals. 2021; 14(3):212. https://doi.org/10.3390/ph14030212
Chicago/Turabian StyleZuccari, Guendalina, Sara Baldassari, Silvana Alfei, Barbara Marengo, Giulia Elda Valenti, Cinzia Domenicotti, Giorgia Ailuno, Carla Villa, Leonardo Marchitto, and Gabriele Caviglioli. 2021. "D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid" Pharmaceuticals 14, no. 3: 212. https://doi.org/10.3390/ph14030212
APA StyleZuccari, G., Baldassari, S., Alfei, S., Marengo, B., Valenti, G. E., Domenicotti, C., Ailuno, G., Villa, C., Marchitto, L., & Caviglioli, G. (2021). D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals, 14(3), 212. https://doi.org/10.3390/ph14030212











