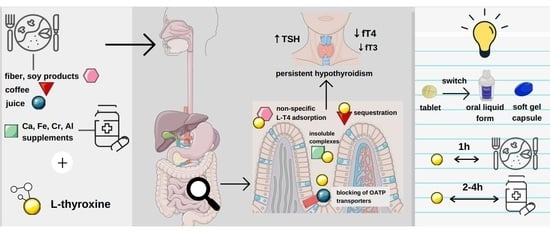
Levothyroxine Interactions with Food and Dietary Supplements–A Systematic Review
Abstract
1. Introduction
2. Results and Discussion
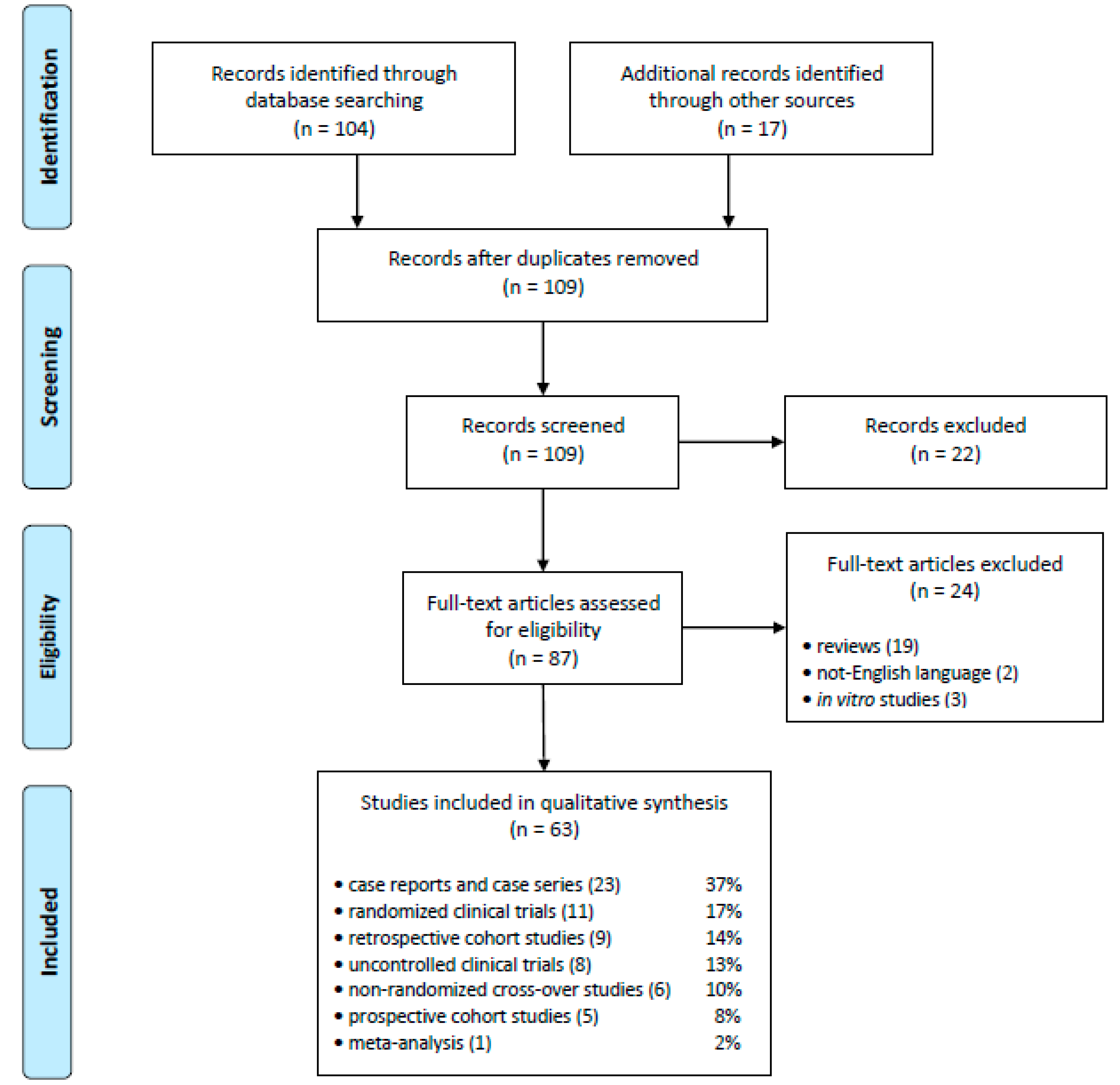
2.1. Eligible Studies
2.2. Aspects of Levothyroxine Pharmacokinetics
2.3. Schedules of Levothyroxine Administration
2.4. Impact of Food Intake on Pharmacokinetics and Pharmacodynamics of Levothyroxine
2.4.1. Tablets
2.4.2. Liquid Form
2.4.3. Soft Gel Capsule
2.5. Levothyroxine–Fiber Interaction
2.6. Levothyroxine-Soy Products Interaction
2.7. Levothyroxine–Milk Interaction
2.8. Levothyroxine–Coffee Interaction
2.9. Levothyroxine-Fruit Interaction
2.9.1. Fruit Juices
2.9.2. Papaya
2.10. Interaction of Levothyroxine with Essential and Trace Elements
2.10.1. Calcium
2.10.2. Iron
2.10.3. Aluminium
2.10.4. Chromium
2.11. Levothyroxine—Vitamin C Interaction
2.12. Levothyroxine—Enteral Nutrition Interaction
2.13. Limitations of Studies
2.14. Recommendations
3. Materials and Methods
3.1. Search Strategy
3.2. Inclusion and Exclusion Criteria
3.3. Data Extraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EN | Enteral nutrition |
| fT3 | Free T3 |
| fT4 | Free T4 |
| L-T4 | Levothyroxine (L-thyroxine) |
| MCT | Monocarboxylate transporter family |
| NTCP | Sodium-taurocholate co-transporting polypeptide transporters |
| OATP | Organic anion-transporting polypeptide family |
| PDA | Prolonged Dose Adjustment |
| PPI | Pomp proton inhibitors |
| RCT | Randomized control trial |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TFT | Thyroid function tests |
| TSH | Thyroid-stimulating hormone |
| TT4 | Serum total thyroxine level |
References
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef]
- Kane, S. The Top 200 of 2020. Available online: https://clincalc.com/DrugStats/Top300Drugs.aspx (accessed on 19 October 2020).
- McMillan, M.; Rotenberg, K.S.; Vora, K.; Sterman, A.B.; Thevathasan, L.; Ryan, M.F.; Mehra, M.; Sandulli, W. Comorbidities, Concomitant Medications, and Diet as Factors Affecting Levothyroxine Therapy: Results of the CONTROL Surveillance Project. Drugs RD 2016, 16, 53–68. [Google Scholar] [CrossRef]
- Michel, R.; Neafsey, P. Self Medication Practices among Patients taking Levothyroxine. Internet J. Adv. Nurs. Pract. 2012, 6, 2–9. [Google Scholar] [CrossRef]
- Colucci, P.; Yue, C.S.; Ducharme, M.; Benvenga, S. A review of the pharmacokinetics of levothyroxine for the treatment of hypothyroidism. Eur. Endocrinol. 2013, 9, 40–47. [Google Scholar] [CrossRef]
- Virili, C.; Antonelli, A.; Santaguida, M.G.; Benvenga, S.; Centanni, M. Gastrointestinal malabsorption of thyroxine. Endocr. Rev. 2018, 40, 118–136. [Google Scholar] [CrossRef]
- Ward, L.S. The difficult patient: Drug interaction and the influence of concomitant diseases on the treatment of hypothyroidism. Arq. Bras. Endocrinol. Metabol. 2010, 54, 435–442. [Google Scholar] [CrossRef]
- Centanni, M. Thyroxine treatment: Absorption, malabsorption, and novel therapeutic approaches. Endocrine 2013, 43, 8–9. [Google Scholar]
- Ianiro, G.; Mangiola, F.; Di Rienzo, T.A.; Bibbò, S.; Franceschi, F.; Greco, A.V.; Gasbarrini, A. Levothyroxine absorption in health and disease, and new therapeutic perspectives. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 451–456. [Google Scholar]
- Sachmechi, I.; Reich, D.M.; Aninyei, M.; Wibowo, F.; Gupta, G.; Kim, P.J. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr. Pract. 2007, 13, 345–349. [Google Scholar] [CrossRef]
- Irving, S.A.; Vadiveloo, T.; Leese, G.P. Drugs that interact with levothyroxine: An observational study from the Thyroid Epidemiology, Audit and Research Study (TEARS). Clin. Endocrinol. 2015, 82, 136–141. [Google Scholar] [CrossRef]
- Paśko, P.; Wołtosz, A.; Zwolińska-Wcisło, M.; Zachwieja, Z. Influence of proton pump inhibitors on calcium and iron homeostasis. Bromatol. Chem. Toksykol. 2015, 48, 484–489. [Google Scholar]
- Dietrich, J.W.; Boehm, B.O. Thyroxine in goiter, H. pylori infection, and gastritis. N. Engl. J. Med. 2006, 355, 1177. [Google Scholar] [CrossRef]
- Ananthakrishnan, S.; Braverman, L.E.; Levin, R.M.; Magnani, B.; Pearce, E.N. The effect of famotidine, esomeprazole, and ezetimibe on levothyroxine absorption. Thyroid 2008, 18, 493–498. [Google Scholar] [CrossRef]
- Yue, C.S.; Scarsi, C.; Ducharme, M.P. Pharmacokinetics and potential advantages of a new oral solution of levothyroxine vsother available dosage forms. Arzneim. Forsch. Drug Res. 2012, 62, 631–636. [Google Scholar] [CrossRef]
- Fallahi, P.; Ferrari, S.M.; Antonelli, A. Oral L-thyroxine liquid versus tablet in patients with hypothyroidism without malabsorption: A prospective study. Endocrine 2016, 52, 597–601. [Google Scholar] [CrossRef]
- Laurent, I.; Tang, S.; Astère, M.; Wang, K.R.; Deng, S.; Xiao, L.; Li, Q.F. Liquid L-thyroxine versus tablet L-thyroxine in patients on L- thyroxine replacement or suppressive therapy: A meta-analysis. Endocrine 2018, 61, 28–35. [Google Scholar] [CrossRef]
- Virili, C.; Giovanella, L.; Fallahi, P.; Antonelli, A.; Santaguida, M.G.; Centanni, M.; Trimboli, P. Levothyroxine therapy: Changes of TSH levels by switching patients from tablet to liquid formulation. A systematic review and meta-analysis. Front. Endocrinol. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Vita, R.; Saraceno, G.; Trimarchi, F.; Benvenga, S. Switching levothyroxine from the tablet to the oral solution formulation corrects the impaired absorption of levothyroxine induced by proton-pump inhibitors. J. Clin. Endocrinol. Metab. 2014, 99, 4481–4486. [Google Scholar] [CrossRef]
- Vita, R.; Di Bari, F.; Benvenga, S. Oral liquid levothyroxine solves the problem of tablet levothyroxine malabsorption due to concomitant intake of multiple drugs. Expert Opin. Drug Deliv. 2017, 14, 467–472. [Google Scholar] [CrossRef]
- Pabla, D.; Akhlaghi, F.; Zia, H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur. J. Pharm. Biopharm. 2009, 72, 105–110. [Google Scholar] [CrossRef]
- Santaguida, M.G.; Virili, C.; Duca, S.C.D.; Cellini, M.; Gatto, I.; Brusca, N.; De Vito, C.; Gargano, L.; Centanni, M. Thyroxine softgel capsule in patients with gastric-related T4 malabsorption. Endocrine 2015, 49, 51–57. [Google Scholar] [CrossRef]
- Trimboli, P.; Virili, C.; Centanni, M.; Giovanella, L. Thyroxine treatment with softgel capsule formulation: Usefulness in hypothyroid patients without malabsorption. Front. Endocrinol. 2018, 9, 118. [Google Scholar] [CrossRef]
- Philippe, J.; Dibner, C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J. Biol. Rhythms 2015, 30, 76–83. [Google Scholar] [CrossRef]
- Skelin, M.; Lucijanić, T.; Liberati-Čizmek, A.M.; Klobučar, S.M.; Lucijanić, M.; Jakupović, L.; Bakula, M.; Lončar, J.V.; Marušić, S.; Matić, T.; et al. Effect of timing of levothyroxine administration on the treatment of hypothyroidism: A three-period crossover randomized study. Endocrine 2018, 432–439. [Google Scholar] [CrossRef]
- Bolk, N.; Visser, T.J.; Nijman, J.; Jongste, I.J.; Tijssen, J.G.P.; Berghout, A. Effects of evening vs. morning levothyroxine intake: A randomized double-blind crossover trial. Arch. Intern. Med. 2010, 170, 1996–2003. [Google Scholar] [CrossRef]
- Rajput, R.; Chatterjee, S.; Rajput, M. Can levothyroxine be taken as evening dose? Comparative evaluation of morning versus evening dose of levothyroxine in treatment of hypothyroidism. J. Thyroid Res. 2011, 2011. [Google Scholar] [CrossRef]
- Bolk, N.; Visser, T.J.; Kalsbeek, A.; Van Domburg, R.T.; Berghout, A. Effects of evening vs. morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin. Endocrinol. 2007, 66, 43–48. [Google Scholar] [CrossRef]
- Ala, S.; Akha, O.; Kashi, Z.; Asgari, H.; Bahar, A.; Sasanpour, N. Dose administration time from before breakfast to before dinner affect thyroid hormone levels? Casp. J. Intern. Med. 2015, 6, 134–140. [Google Scholar]
- Bach-Huynh, T.G.; Nayak, B.; Loh, J.; Soldin, S.; Jonklaas, J. Timing of levothyroxine administration affects serum thyrotropin concentration. J. Clin. Endocrinol. Metab. 2009, 94, 3905–3912. [Google Scholar] [CrossRef]
- Olejniczak-Rabinek, M. Factors influencing bioavailability of levothyroxine. Farm. Współ. 2016, 9, 194–201. [Google Scholar]
- Pang, X.; Pu, T.; Xu, L.; Sun, R. Effect of L-thyroxine administration before breakfast vs. at bedtime on hypothyroidism: A meta-analysis. Clin. Endocrinol. 2020, 92, 475–481. [Google Scholar] [CrossRef]
- Benvenga, S.; Bartolone, L.; Squadrito, S.; Lo Giudice, F.; Trimarchi, F. Delayed intestinal absorption of levothyroxine. Thyroid 1995, 5, 249–253. [Google Scholar] [CrossRef]
- Wenzel, K.W.; Kirschsieper, H.E. Aspects of the absorption of oral L-thyroxine in normal man. Metabolism 1977, 26, 1–8. [Google Scholar] [CrossRef]
- Lamson, M.J.; Pamplin, C.L.; Rolleri, R.L.; Klein, I. Quantitation of a substantial reduction in levothyroxine (T4) absorption by food. Thyroid 2004, 14, 876. [Google Scholar]
- Seechurn, S.; Sharma, S.; Oyibo, S. Administration of Levothyroxine 45—60 Minutes before Breakfast Improves Biochemical Availability as Evidenced by Reduced Thyrotropin Levels. Open J. Endocr. Metab. Dis. 2012, 2, 36–39. [Google Scholar] [CrossRef]
- Mastroianni, P.D.C.; Forgerini, M. Ajustes da administração de medicamentos para pacientes idosos com disfagia: Um relato de caso. Dement. Neuropsychol. 2018, 12, 97–100. [Google Scholar] [CrossRef]
- Perez, C.L.S.; Araki, F.S.; Graf, H.; De Carvalho, G.A. Serum thyrotropin levels following levothyroxine administration at breakfast. Thyroid 2013, 23, 779–784. [Google Scholar] [CrossRef]
- Bernareggi, A.; Grata, E.; Pinorini, M.T.; Conti, A. Oral liquid formulation of levothyroxine is stable in breakfast beverages and may improve thyroid patient compliance. Pharmaceutics 2013, 5, 621–633. [Google Scholar] [CrossRef]
- Marina, M.; Ceda, G.P.; Aloe, R.; Gnocchi, C.; Ceresini, G. Circulating concentrations of free thyroxine after an oral intake of liquid levothyroxine taken either during fasting conditions or at breakfast. Acta Bio-Med. Atenei Parm. 2016, 87, 247–252. [Google Scholar]
- Morelli, S.; Reboldi, G.; Moretti, S.; Menicali, E.; Avenia, N.; Puxeddu, E. Timing of breakfast does not influence therapeutic efficacy of liquid levothyroxine formulation. Endocrine 2016, 52, 571–578. [Google Scholar] [CrossRef]
- Cappelli, C.; Pirola, I.; Daffini, L.; Formenti, A.; Iacobello, C.; Cristiano, A.; Gandossi, E.; Agabiti Rosei, E.; Castellano, M. A double-blind placebo-controlled trial of liquid thyroxine ingested at breakfast: Results of the TICO study. Thyroid 2016, 26, 197–202. [Google Scholar] [CrossRef]
- Pirola, I.; Gandossi, E.; Brancato, D.; Marini, F.; Cristiano, A.; Delbarba, A.; Agosti, B.; Castellano, M.; Cappelli, C. TSH evaluation in hypothyroid patients assuming liquid levothyroxine at breakfast or 30 min before breakfast. J. Endocrinol. Invest. 2018, 41, 1301–1306. [Google Scholar] [CrossRef]
- De Luca, F. The absorption of liquid levothyroxine is not significantly impaired by food intake. Acta Bio-Med. Atenei Parm. 2016, 87, 231–232. [Google Scholar]
- Guglielmi, R.; Grimaldi, F.; Negro, R.; Frasoldati, A.; Misischi, I.; Graziano, F.; Cipri, C.; Guastamacchia, E.; Triggiani, V.; Papini, E. Shift from Levothyroxine Tablets to Liquid Formulation at Breakfast Improves Quality of Life of Hypothyroid Patients. Endocrine Metab. Immune Disord. Drug Targets 2018, 18, 235–240. [Google Scholar] [CrossRef]
- Cappelli, C.; Pirola, I.; Castellano, M. Liquid Levothyroxine Formulation Taken during Lunch in Italy: A Case Report and Review of the Literature. Case Rep. Endocrinol. 2020, 2020, 4–7. [Google Scholar]
- Cappelli, C.; Pirola, I.; Gandossi, E.; Cristiano, A.; Daffini, L.; Agosti, B.; Casella, C.; Castellano, M. Thyroid Hormone Profile in Patients Ingesting Soft Gel Capsule or Liquid Levothyroxine Formulations with Breakfast. Int. J. Endocrinol. 2016, 2016. [Google Scholar] [CrossRef][Green Version]
- Liwanpo, L.; Hershman, J.M. Conditions and drugs interfering with thyroxine absorption. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 781–792. [Google Scholar] [CrossRef]
- Liel, Y.; Harman-Boehm, I.; Shany, S. Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J. Clin. Endocrinol. Metab. 1996, 81, 857–859. [Google Scholar] [CrossRef]
- Chiu, A.C.; Sherman, S.I. Effects of pharmacological fiber supplements on levothyroxine absorption. Thyroid 1998, 8, 667–671. [Google Scholar] [CrossRef]
- Otun, J.; Sahebkar, A.; Östlundh, L.; Atkin, S.L.; Sathyapalan, T. Systematic Review and Meta-analysis on the Effect of Soy on Thyroid Function. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Pinchera, A.; Macgillivray, M.H.; Crawford, J.D.; Freeman, A.G. Thyroid Refractoriness in an Athyreotic Cretin Fed Soybean Formula. N. Engl. J. Med. 1965, 273, 83–87. [Google Scholar] [CrossRef]
- Chorazy, P.A.; Himelhoch, S.; Hopwood, N.J.; Greger, N.G.; Postellon, D.C. Persistent hypothyroidism in an infant receiving a soy formula: Case report and review of the literature. Pediatrics 1995, 96, 148–149. [Google Scholar]
- Jabbar, M.A.; Larrea, J.; Shaw, R.A. Abnormal thyroid function tests in infants with congenital hypothyroidism: The influence of soy-based formula. J. Am. Coll. Nutr. 1997, 16, 280–282. [Google Scholar] [CrossRef]
- Fruzza, A.G.; Demeterco-Berggren, C.; Jones, K.L. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics 2012, 130, 22908106. [Google Scholar] [CrossRef]
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch. Dis. Child. 2004, 89, 37–40. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Ovalle, F. Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr. Pract. 2001, 7, 193–194. [Google Scholar] [CrossRef]
- Persiani, S.; Sala, F.; Manzotti, C.; Colovic, M.; Zangarini, M.; Donazzolo, Y.; Barbetta, B.; Vitalini, C.; Giacovelli, G.; Benvenuti, C.; et al. Evaluation of Levothyroxine Bioavailability after Oral Administration of a Fixed Combination of Soy Isoflavones in Post-menopausal Female Volunteers. Drug Res. 2015, 66, 136–140. [Google Scholar] [CrossRef]
- Chon, D.A.; Reisman, T.; Weinreb, J.E.; Hershman, J.M.; Leung, A.M. Concurrent Milk Ingestion Decreases Absorption of Levothyroxine. Thyroid 2018, 28, 454–457. [Google Scholar] [CrossRef]
- Sharif, K.; Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Shoenfeld, Y. Coffee and autoimmunity: More than a mere hot beverage! Autoimmun. Rev. 2017, 16, 712–721. [Google Scholar] [CrossRef]
- Benvenga, S.; Bartolone, L.; Pappalardo, M.A.; Russo, A.; Lapa, D.; Giorgianni, G.; Saraceno, G.; Trimarchi, F. Altered intestinal absorption of L-thyroxine caused by coffee. Thyroid 2008, 18, 293–301. [Google Scholar] [CrossRef]
- Sindoni, A.; Vita, R.; Fusco, S.; Saraceno, G.; Pappalardo, M.A.; Cotta, O.; Trimarchi, F.; Benvenga, S.; Sperimentale, C.; Benvenga, S. Coffee impairs intestinal absorption of levothyroxine: Report of additional cases. Hot Thyroidol. 2009, 05/09, 1–7. [Google Scholar]
- Wegrzyn, N.M. Malabsorption of L-T4 Due to Drip Coffee: A Case Report Using Predictors of Causation. J. Acad. Nutr. Diet. 2016, 116, 1073–1075. [Google Scholar] [CrossRef]
- Vita, R.; Saraceno, G.; Trimarchi, F.; Benvenga, S. A novel formulation of L-thyroxine (L-T4) reduces the problem of l-T4 malabsorption by coffee observed with traditional tablet formulations. Endocrine 2013, 43, 154–160. [Google Scholar] [CrossRef]
- Cappelli, C.; Pirola, I.; Gandossi, E.; Formenti, A.; Castellano, M. Oral liquid levothyroxine treatment at breakfast: A mistake? Eur. J. Endocrinol. 2014, 170, 95–99. [Google Scholar] [CrossRef]
- Kinne, A.; Schülein, R.; Krause, G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011, 4, S7. [Google Scholar] [CrossRef]
- Bailey, D.G. Fruit juice inhibition of uptake transport: A new type of food-drug interaction. Br. J. Clin. Pharmacol. 2010, 70, 645–655. [Google Scholar] [CrossRef]
- Paśko, P.; Rodacki, T.; Domagała-Rodacka, R.; Palimonka, K.; Marcinkowska, M.; Owczarek, D. Second generation H1—Antihistamines interaction with food and alcohol—A systematic review. Biomed. Pharmacother. 2017, 93, 27–39. [Google Scholar] [CrossRef]
- Lilja, J.J.; Laitinen, K.; Neuvonen, P.J. Effects of grapefruit juice on the absorption of levothyroxine. Br. J. Clin. Pharmacol. 2005, 60, 337–341. [Google Scholar] [CrossRef]
- Meyer Zu Schwabedissen, H.E.; Ferreira, C.; Schaefer, A.M.; Oufir, M.; Seibert, I.; Hamburger, M.; Tirona, R.G. Thyroid hormones are transport substrates and transcriptional regulators of organic anion transporting polypeptide 2B1. Mol. Pharmacol. 2018, 94, 700–712. [Google Scholar] [CrossRef]
- Tesic, D.; Stokic, E.; Medic/Stojanoska, M.; Mitrovic, M.; Tomic-Naglic, D.; Icin, T.; Bajkin, I.; Dejanovic, B.; Tomic, M. Tea and juice as a causes of levothyroxine malabsorption and intermittent hypothyreoidism: A case report. Endocr. Abstr. 2018, 3947, 3–4. [Google Scholar] [CrossRef]
- Deiana, L.; Marini, S.; Mariotti, S. Ingestion of large amounts of papaya fruit and impaired effectiveness of levothyroxine therapy. Endocr. Pract. 2012, 18, 98–100. [Google Scholar] [CrossRef]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85. [Google Scholar] [CrossRef]
- Levy, I.; Attias, S.; Ben-Arye, E.; Goldstein, L.; Schiff, E. Adverse events associated with interactions with dietary and herbal supplements among inpatients. Br. J. Clin. Pharmacol. 2017, 83, 836–845. [Google Scholar] [CrossRef]
- Liel, Y.; Sperber, A.D.; Shany, S. Nonspecific intestinal adsorption of levothyroxine by aluminum hydroxide. Am. J. Med. 1994, 97, 363–365. [Google Scholar] [CrossRef]
- Schneider, R.; Reiners, C. The Effect of Levothyroxine Therapy on Bone Mineral Density: A Systematic Review of the Literature. Exp. Clin. Endocrinol. Diabetes 2003, 111, 455–470. [Google Scholar] [CrossRef]
- Singh, N.; Singh, P.N.; Hershman, J.M. Effect of calcium carbonate on the absorption of levothyroxine. JAMA 2000, 283, 2822–2825. [Google Scholar] [CrossRef]
- Kung, A.W.; Yeung, S.S. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: The effect of calcium and calcitonin. J. Clin. Endocrinol. Metab. 1996, 81, 1232–1236. [Google Scholar] [CrossRef]
- Singh, N.; Weisler, S.L.; Hershman, J.M. The acute effect of calcium carbonate on the intestinal absorption of levothyroxine. Thyroid 2001, 11, 967–971. [Google Scholar] [CrossRef]
- Schneyer, C.R. Calcium Carbonate and Reduction of Levothyroxine Efficacy. JAMA. 1998, 279, 750. [Google Scholar] [CrossRef]
- Csako, G.; McGriff, N.J.; Rotman-Pikielny, P.; Sarlis, N.J.; Pucino, F. Exaggerated levothyroxine malabsorption due to calcium carbonate supplementation in gastrointestinal disorders. Ann. Pharmacother. 2001, 35, 1578–1583. [Google Scholar] [CrossRef]
- Butner, L.E.; Fulco, P.P.; Feldman, G. Calcium Carbonate–Induced Hypothyroidism. Ann. Intern. Med. 2000, 132, 595. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Giannakopoulos, T.G.; Starakis, I.K. Interaction between levothyroxine and calcium carbonate. Can. Fam. Physician 2008, 54, 39. [Google Scholar]
- Diskin, C.J.; Stokes, T.J.; Dansby, L.M.; Radcliff, L.; Carter, T.B. Effect of phosphate binders upon TSH and L-thyroxine dose in patients on thyroid replacement. Int. Urol. Nephrol. 2007, 39, 599–602. [Google Scholar] [CrossRef]
- Zamfirescu, I.; Carlson, H.E. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid 2011, 21, 483–486. [Google Scholar] [CrossRef]
- Morini, E.; Catalano, A.; Lasco, A.; Morabito, N.; Benvenga, S. L-Thyroxine Malabsorption Due To Calcium Carbonate Impairs Blood Pressure, Total Cholesterolemia, and Fasting Glycemia. Endocrine 2019, 64, 284–292. [Google Scholar] [CrossRef]
- Benvenga, S.; Di Bari, F.; Vita, R. Undertreated hypothyroidism due to calcium or iron supplementation corrected by oral liquid levothyroxine. Endocrine 2017, 56, 138–145. [Google Scholar] [CrossRef]
- Mazokopakis, E.E. Counseling Patients Receiving Levothyroxine (L-T4) and Calcium Carbonate. Mil. Med. 2006, 171, 1094. [Google Scholar]
- Campbell, N.R.C.; Hasinoff, B.B.; Stalts, H.; Rao, B.; Wong, N.C.W. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann. Intern. Med. 1992. [Google Scholar] [CrossRef]
- Shakir, K.M.M.; Chute, J.P.; Aprill, B.S.; Lazarus, A.A. Ferrous sulfate-induced increase in requirement for thyroxine in a patient with primary hypothyroidism. South. Med. J. 1997, 90, 637–639. [Google Scholar] [CrossRef]
- Fiaux, E.; Kadri, K.; Levasseur, C.; Le Guillou, C.; Chassagne, P. Hypothyroïdie secondaire à une interaction médicamenteuse entre lévothyroxine et sels de fer. Rev. Med. Interne 2010, 31, 3–5. [Google Scholar] [CrossRef]
- Leger, C.S.; Ooi, T.C. Ferrous fumarate-induced malabsorption of thyroxine. Endocrinologist 1999, 9, 493–495. [Google Scholar] [CrossRef]
- Atruktsang, T.S.; Zaborek, N.A.; Imbus, J.R.; Long, K.; Pitt, S.C.; Sippel, R.S.; Schneider, D.F. Identifying Predictors of Prolonged Levothyroxine Dose Adjustment After Thyroidectomy. J. Surg. Res. 2019, 242, 166–171. [Google Scholar] [CrossRef]
- Sperber, A.D.; Liel, Y. Evidence for Interference with the Intestinal Absorption of Levothyroxine Sodium by Aluminum Hydroxide. Arch. Intern. Med. 1992, 152, 183–184. [Google Scholar] [CrossRef]
- Mersebach, H.; Rasmussen, Å.K.; Kirkegaard, L.; Feldt-Rasmussen, U. Intestinal adsorption of levothyroxine by antacids and laxatives: Case stories and in vitro experiments. Pharmacol. Toxicol. 1999, 84, 107–109. [Google Scholar] [CrossRef]
- Tian, H.; Guo, X.; Wang, X.; He, Z.; Sun, R.; Ge, S.; Zhang, Z. Chromium picolinate supplementation for overweight or obese adults. Cochrane Database Syst. Rev. 2013, 11. [Google Scholar] [CrossRef]
- Tsang, C.; Taghizadeh, M.; Aghabagheri, E.; Asemi, Z.; Jafarnejad, S. A meta-analysis of the effect of chromium supplementation on anthropometric indices of subjects with overweight or obesity. Clin. Obes. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- John-Kalarickal, J.; Pearlman, G.; Carlson, H.E. New medications which decrease levothyroxine absorption. Thyroid 2007, 17, 763–765. [Google Scholar] [CrossRef]
- Jubiz, W.; Ramirez, M. Effect of vitamin c on the absorption of levothyroxine in patients with hypothyroidism and gastritis. J. Clin. Endocrinol. Metab. 2014, 99, 1031–1034. [Google Scholar] [CrossRef]
- Antúnez, P.; Licht, S.D. Vitamin C improves the apparent absorption of levothyroxine in a subset of patients receiving this hormone for primary hypothyroidism. Rev. Argent. Endocrinol. Metab. 2011, 48, 16–24. [Google Scholar]
- Reis, A.M.M.; de Carvalho, R.E.F.L.; de Faria, L.M.P.; de Oliveira, R.C.; Zago, K.S.D.A.; Cavelagna, M.F.; Silva, A.G.; Neto, M.L.; Cassiani, S.H.D.B. Prevalence and clinical significance of interactions drug-enteral nutrition in Intensive Care Units. Rev. Bras. Enferm. 2014, 67, 85–90. [Google Scholar] [CrossRef][Green Version]
- Dickerson, R.N.; Maish, G.O.; Minard, G.; Brown, R.O. Clinical relevancy of the levothyroxine-continuous enteral nutrition interaction. Nutr. Clin. Pract. 2010, 25, 646–652. [Google Scholar] [CrossRef]
- Manessis, A.; Lascher, S.; Bukberg, P.; Darmody, T.; Yen, V.; Sadek, S.; Young, I. Quantifying amount of adsorption of levothyroxine by percutaneous endoscopic gastrostomy tubes. J. Parenter. Enter. Nutr. 2008, 32, 197–200. [Google Scholar] [CrossRef]
- Wohlt, P.D.; Zheng, L.; Gunderson, S.; Balzar, S.A.; Johnson, B.D.; Fish, J.T. Recommendations for the use of medications with continuous enteral nutrition. Am. J. Heal. Pharm. 2009, 66, 1458–1467. [Google Scholar] [CrossRef]
- Pirola, I.; Daffini, L.; Gandossi, E.; Lombardi, D.; Formenti, A.; Castellano, M.; Cappelli, C. Comparison between liquid and tablet levothyroxine formulations in patients treated through enteral feeding tube. J. Endocrinol. Invest. 2014, 37, 583–587. [Google Scholar] [CrossRef][Green Version]
- Heuberger, R. Polypharmacy and Food-Drug Interactions among Older Persons: A Review. J. Nutr. Gerontol. Geriatr. 2012, 31, 325–403. [Google Scholar] [CrossRef]
- Paśko, P.; Rodacki, T.; Domagała-Rodacka, R.; Owczarek, D. Interactions between medications employed in treating benign prostatic hyperplasia and food—A short review. Biomed. Pharmacother. 2016, 83, 1141–1145. [Google Scholar] [CrossRef]
| Study | Participants | L-T4 Dose (µg/Day) | L-T4 Formulation | Type of Food | Observed Effect |
|---|---|---|---|---|---|
| Wenzel et al. [34] | not specified | 100 | Tablets | not specified | ↓ L-T4 absorption (by 15%) |
| Lamson et al. [35] | 48, healthy | 600 | Tablets | breakfast, 950 kcal | ↓ AUC0-48 h (by 38–40%), ↓ Cmax (by 40–49%) |
| Perez et al. [38] | 42, hypothyroid | 98 ± 35 | Tablets | breakfast, 162–381 kcal | ↑ TSH level (by 64%) |
| Marina et al. [40] | 14, hypothyroid | 200 | liquid form | breakfast, 132 kcal | no significant changes in fT4 levels |
| Morelli et al. [41] | 59, hypothyroid | not specified | liquid form | patient’s usual breakfast | no significant changes in TSH levels |
| Cappelli et al. [42] | 77, hypothyroid | 75 | liquid form | patient’s usual breakfast | no significant changes in TSH, fT4 and fT3 levels |
| Pirola et al. [43] | 761, hypothyroid | 75 | liquid form | patient’s usual breakfast | no significant changes in TSH levels |
| Cappelli et al. [46] | 1, hypothyroid | 75 | liquid form | Lunch | no significant changes in thyroid hormonal profiles |
| Cappelli et al. [47] | 60, euthyroid | 106 ± 24 | soft gel capsules | patient’s usual breakfast | no significant changes in TSH levels, ↓ fT4 and fT3 levels (by 7% both) |
| Liel et al. [49] | 13, hypothyroid | 50–470 | Not specified | fiber (whole wheat bread, bran, granola, psyllium) | ↑ TSH level (ranging from 7,4 to > 50 mU/L) |
| Chiu et al. [50] | 8, healthy | 600 | Tablets | fiber (psyllium) | ↓ L-T4 absorption (by 8%) |
| Fruzza et al. [55] | 1, hypothyroid | 50 | Not specified | soy-based infant formula | ↑ TSH level (216 mU/L), ↓ fT4 level (4.0 μg/dL) |
| 1, hypothyroid | 112 | Not specified | soy-based infant formula | ↑ TSH level (248 mU/L), ↓ fT4 level (<0.4ng/dL) | |
| Conrad et al. [56] | 78, hypothyroid | 7.4 per kg | Not specified | soy-based infant formula | 62.5% patients with TSH > 10 mU/L after 4 months |
| Bell et al. [57] | 1, hypothyroid | 200 | Tablets | soy-protein containing cocktail | difficulty in suppressing TSH level |
| Persiani et al. [58] | 12, hypothyroid | 25–125 | Tablets | soy-containing supplement | no significant changes in thyroid hormones levels |
| Chon et al. [59] | 10, healthy | 1000 | Tablets | cow milk | ↓ peak serum TT4 level by 7.8%, ↓ AUC by 8% |
| Benvenga et al. [61] | 6, hypothyroid | 200 | Tablets | espresso | average T4 ↓ 36%, peak T4 ↓ 30%, tmax delayed by 38 min. |
| 9, healthy | 200 | Tablets | espresso | average T4 ↓ 29%, peak T4 ↓ 19%, tmax delayed by 43 min. | |
| Sindoni et al. [62] | 6, hypothyroid | 1.6–2.2 per kg | Tablets | espresso and barley coffee | failure to normalize TSH levels |
| Węgrzyn [63] | 1, hypothyroid | 175 | Tablets | drip coffee | clinical signs of hypothyroidism (TSH level—8.27 mU/L) |
| Vita et al. [64] | 8, hypothyroid | 1.6–2.8 per kg | soft gel capsules | coffee | comparable TSH levels for coffee 5 min. and 1h after L-T4 |
| Cappelli et al. [65] | 54, hypothyroid | 73±14 | liquid form | coffee | comparable TSH, fT3 and fT4 levels for coffee 30 min. before and with L-T4 |
| Lilja et al. [69] | 1, hypothyroid | 100 | Not specified | grapefruit juice | ↑ TSH level (63.7 mU/L), ↓ fT4 level (6.4 pmol/L) |
| 10, healthy | 600 | Not specified | grapefruit juice | ↓ AUC (by 9%), ↓ Cmax (by 11%) | |
| Tesic et al. [71] | 1, hypothyroid | 200–700 | Tablets | juice and mint tea | ↑ TSH level (> 100 mU/L), ↓ fT4 level (5.9 pmol/L), undetectable fT3 level |
| Deiana et al. [72] | 1, hypothyroid | 1.6 per kg | Not specified | papaya | ↑ TSH level (25 mU/L) |
| 1, hypothyroid | 1.6 per kg | Not specified | papaya | ↑ TSH level (from 0.8 to 15 mU/L), ↓fT3 and fT4 levels | |
| Singh et al. [77] | 20, hypothyroid | > 1 per kg | Not specified | calcium carbonate | ↓fT4 level, ↑ TSH level in 20% of patients |
| Singh et al. [79] | 7, healthy | 1000 | Tablets | calcium carbonate | ↓ L-T4 absorption (from 83.7% to 53.7%), tmax delayed (from 2 to 4 h) |
| Schneyer et al. [80] | 3, hypothyroid | 125–325 | Not specified | calcium carbonate | ↑ TSH level (ranging from 7.3 to 13.3 mU/L) |
| Csako et al. [81] | 1, hypothyroid | 175–188 | Not specified | calcium carbonate | ↑ TSH level (41.4 mU/L) |
| Butner et al. [82] | 1, hypothyroid | 150 | Not specified | calcium carbonate | ↑ TSH level (21.85 mU/L) |
| Mazokopakis et al. [83] | 1, hypothyroid | 88 | Not specified | calcium carbonate | ↑ TSH level (9.8 mIU/L),↓fT4 level (0.2 ng/dL) |
| Irving et al. [11] | 450, hypothyroid | not specified | Tablets | calcium carbonate | ↑ TSH level (up to over 5 mU/L) in 4.4% of patients |
| Diskin et al. [84] | 65, hypothyroid | 95–98 | Not specified | calcium carbonate | ↑ TSH level (23.8 ± 19.5 mU/L) |
| Zamfirescu et al. [85] | 8, healthy | 1000 | Tablets | calcium carbonate, calcium citrate, calcium acetate | ↓ L-T4 absorption (by 20–25%) |
| Morini et al. [86] | 50, hypothyroid | 1.47 per kg | Tablets | calcium supplements | ↑ TSH level (3.33 ± 1.93 mU/L) |
| Benvenga et al. [87] | 12, hypothyroid | 1.7 per kg | liquid form and tablets | calcium carbonate | ↓ TSH for liquid form vs. tablet (2.15 ± 1,4 mU/L vs. 8.74 ± 7.2 mU/L) |
| Campbell et al. [89] | 14, hypothyroid | 75-150 | Not specified | ferrous sulfate | ↑ TSH level (from 1.6 to 5.4 mU/L) |
| Shakir et al. [90] | 1, hypothyroid | 150 | Not specified | ferrous sulfate | ↑ TSH level (56 mU/L), ↓ fT4 level (0,48 ng/dL) |
| Leger et al. [92] | 1, hypothyroid | not specified | Not specified | ferrous fumarate | ↑ TSH level (243 mU/L), ↓ fT4 level (<0.52 pmol/L) |
| Irving et al. [11] | 429, hypothyroid | not specified | Tablets | iron supplements | ↑ TSH level in 7.5% of patients |
| Benvenga et al. [87] | 8, hypothyroid | 1.7 per kg | liquid form and tablets | ferrous sulfate | ↓ TSH for liquid form vs. tablet (1.68 ± 0.9 mU/L vs. 8.74 ± 7.2 mU/L) |
| Liel et al. [75] | 5, hypothyroid | not specified | Not specified | aluminium hydroxide | ↑ TSH level (from 2.62 to 7.19 mU/L) |
| John-Kalarickal et al. [98] | 7, hypothyroid | 1000 | Not specified | chromium picolinate | ↓ L-T4 bioavailability (by 17%) |
| Jubiz et al. [99] | 31, hypothyroid | 100 | Tablets | vitamin C | ↓ TSH level (by 69%), normalized TSH in 54.8% of patients |
| Antunez et al. [100] | 28, hypothyroid | >1.7 per kg | Tablets | vitamin C | ↓ TSH level (from 9.01 ± 5.51 mU/L to 2.27±1.61 mU/L) |
| Dickerson et al. [102] | 13, hypothyroid | not specified | not specified | enteral nutrition | hypothyroidism subclinical (TSH—6–10 mU/L) or overt (TSH >10 mU/L) |
| Pirola et al. [105] | 20, euthyroid | 1.6 per kg | liquid form and crushed tablets | enteral nutrition | comparable thyroid hormones profile for both formulations |
| Food Interacting with L-thyroxine | Sources of Evidence | Mechanism of Interaction | Effects of Interaction | Recommendations for Health Professionals |
|---|---|---|---|---|
| Fiber (whole wheat bread, bran) | case series [49], non-randomized cross-over study [50] | non-specific adsorption of l-T4 to the fiber | malabsorption of l-T4, impaired efficacy of treatment | advise to separate fiber and l-T4 intake by 1 h monitor thyroid parameters more frequently, adjust L-T4 doses when needed |
| Soy products (soy-protein cocktails, soy-based infant formulas) | case reports [52,53,55,57], case series [54], retrospective cohort study [56] | adsorption of l-T4 on the surface of soy protein | malabsorption of l-T4, impaired efficacy of treatment | advise to separate soy protein and l-T4 intake by 1 h monitor thyroid parameters more frequently, adjust l-T4 doses when needed |
| Cow milk | non-randomized cross-over study [59] | probable adsorption of l-T4 on casein | impaired bioavailability of l-T4 | cannot be made due to the insufficient evidence |
| Coffee (espresso, drip coffee, barley coffee) | case report [63], case series [61,62], uncontrolled clinical study [65], non-randomized cross-over study [64] | the sequestration of l-T4 by coffee | malabsorption of l-T4, impaired efficacy of treatment | advise to delay coffee intake by 1 h after l-T4 administration consider changing formulation from tablets to oral liquid form/gel capsules |
| Juice (grapefruit juice, orange juice, apple juice) | case report [69], randomized clinical trial (against interaction) [69] | blocking of OATP transporters | malabsorption of l-T4, impaired efficacy of treatment | cannot be made due to insufficient evidence advise to avoid excessive intake |
| Fruit (papaya) | case report [72] | unknown | malabsorption of l-T4, impaired efficacy of treatment | cannot be made due to the insufficient evidence advise to avoid excessive intake |
| Calcium (carbonate, acetate, citrate) | case reports [81,82,83], case series [80], retrospective cohort studies [11,84,86], prospective cohort study [77], uncontrolled clinical study [87], non-randomized cross-over study [85] | unspecific adsorption of l-T4, creating insoluble or sparingly soluble complexes in the intestine | malabsorption of l-T4, impaired efficacy of treatment | advise to delay intake by 2–4 h after l-T4 administration consider changing formulation from tablets to oral liquid form/gel capsules monitor thyroid parameters more frequently |
| Iron (ferrous citrate and fumarate) | case reports [89,90,91], retrospective cohort study [11,93], uncontrolled clinical study [88] | unspecific adsorption of l-T4, creating insoluble or sparingly soluble complexes in the intestine | malabsorption of l-T4, impaired efficacy of treatment | advise to delay intake by 2–4 h after l-T4 administration consider changing formulation from tablets to oral liquid form/gel capsules monitor thyroid parameters more frequently |
| Aluminium (hydroxide) | case reports [93,94], uncontrolled clinical study [75] | unspecific adsorption of l-T4, creating insoluble or sparingly soluble complexes in the intestine | malabsorption of l-T4, impaired efficacy of treatment | advise to delay intake by 2–4 h after l-T4 administration consider changing formulation from tablets to oral liquid form/gel capsules monitor thyroid parameters more frequently |
| Chromium (picolinate) | non-randomized cross-over study [98] | unspecific adsorption of l-T4, creating insoluble or sparingly soluble complexes in the intestine | malabsorption of l-T4, impaired efficacy of treatment | advise to delay intake by 3–4 h after l-T4 administration consider changing formulation from tablets to oral liquid form/gel capsules monitor thyroid parameters more frequently |
| Vitamin C | uncontrolled clinical study [100], non-randomized cross-over study [99] | lowering of gastric pH | enhanced absorption of l-T4 | consider advising concomitant ingestion of vitamin C and l-T4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiesner, A.; Gajewska, D.; Paśko, P. Levothyroxine Interactions with Food and Dietary Supplements–A Systematic Review. Pharmaceuticals 2021, 14, 206. https://doi.org/10.3390/ph14030206
Wiesner A, Gajewska D, Paśko P. Levothyroxine Interactions with Food and Dietary Supplements–A Systematic Review. Pharmaceuticals. 2021; 14(3):206. https://doi.org/10.3390/ph14030206
Chicago/Turabian StyleWiesner, Agnieszka, Danuta Gajewska, and Paweł Paśko. 2021. "Levothyroxine Interactions with Food and Dietary Supplements–A Systematic Review" Pharmaceuticals 14, no. 3: 206. https://doi.org/10.3390/ph14030206
APA StyleWiesner, A., Gajewska, D., & Paśko, P. (2021). Levothyroxine Interactions with Food and Dietary Supplements–A Systematic Review. Pharmaceuticals, 14(3), 206. https://doi.org/10.3390/ph14030206