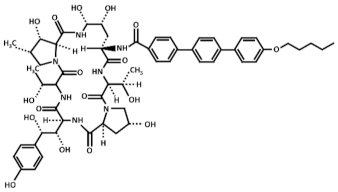
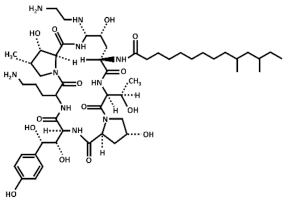
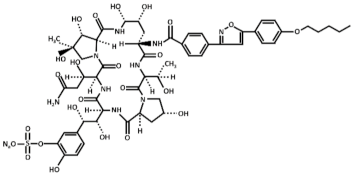
Abstract
Fission yeast contains three essential β(1,3)-D-glucan synthases (GSs), Bgs1, Bgs3, and Bgs4, with non-overlapping roles in cell integrity and morphogenesis. Only the bgs4+ mutants pbr1-8 and pbr1-6 exhibit resistance to GS inhibitors, even in the presence of the wild-type (WT) sequences of bgs1+ and bgs3+. Thus, Bgs1 and Bgs3 functions seem to be unaffected by those GS inhibitors. To learn more about echinocandins’ mechanism of action and resistance, cytokinesis progression and cell death were examined by time-lapse fluorescence microscopy in WT and pbr1-8 cells at the start of treatment with sublethal and lethal concentrations of anidulafungin, caspofungin, and micafungin. In WT, sublethal concentrations of the three drugs caused abundant cell death that was either suppressed (anidulafungin and micafungin) or greatly reduced (caspofungin) in pbr1-8 cells. Interestingly, the lethal concentrations induced differential phenotypes depending on the echinocandin used. Anidulafungin and caspofungin were mostly fungistatic, heavily impairing cytokinesis progression in both WT and pbr1-8. As with sublethal concentrations, lethal concentrations of micafungin were primarily fungicidal in WT cells, causing cell lysis without impairing cytokinesis. The lytic phenotype was suppressed again in pbr1-8 cells. Our results suggest that micafungin always exerts its fungicidal effect by solely inhibiting Bgs4. In contrast, lethal concentrations of anidulafungin and caspofungin cause an early cytokinesis arrest, probably by the combined inhibition of several GSs.
1. Introduction
Owing to the increasing population of immunocompromised patients, life-threatening systemic fungal infections have become a major risk for public health. A weakened immune system because of illnesses, such as cancer, diabetes, or HIV/AIDS syndrome, together with the practice of treatments or medicines, such as chemotherapy, organ transplantation, or corticoids, might predispose the afflicted to secondary fungal infections [1,2,3]. Thus, it has been estimated that over 1.6 million people die each year because of severe fungal infections [1,4]. Only four classes of antifungal drugs (echinocandins, polyenes, pyrimidine analogues, and triazoles) are used for treating systemic fungal infections [5,6,7]. Thus, the problem of systemic fungal infections is also worsened by the emergence of fungi resistant to one or several classes of the available antifungals [8,9].
The cell wall is a structure external to the plasma membrane present in all fungal cells. Its integrity is crucial for fungal survival, making the boundary between the cytoplasm and the external milieu and maintaining the internal turgor pressure [10,11]. Because the cell wall is not present in the infected animal hosts, molecules that inhibit its synthesis are appealing as potential antifungal drugs [12,13]. Structural microfibrils of the β(1,3)-D-glucan polysaccharide are the most abundant components in the cell wall framework [14,15,16]. The fission yeast Schizosaccharomyces pombe is an attractive model for exploring the role of the β(1,3)-D-glucan in cell wall synthesis, morphogenesis, and cell integrity. The S. pombe cell wall has no detectable amounts of chitin [16,17,18]; instead, it contains three different and essential β-glucans: a major branched β(1,3)-D-glucan that is the main responsible for the cell wall structure and integrity; a minor linear β(1,3)-D-glucan, which is responsible for the primary septum structure; and a minor branched β(1,6)-D-glucan [19,20].
In all the studied fungi, the catalytic subunit of the β(1,3)-D-glucan synthase (GS), the glycosyltransferase in charge of the β(1,3)-D-glucan synthesis, is constituted by the family of integral membrane proteins Bgs/Fks [19,21,22]. S. pombe contains four essential GS catalytic subunits: Bgs1 synthesizes the linear β(1,3)-D-glucan of the primary septum [19] and is required for septum ingression [19,23,24,25]; Bgs2 is essential for spore wall maturation [26,27]; Bgs3 is essential, although its function remains unknown [28]; and Bgs4 is the only subunit that has been shown to form part of the GS enzyme. It synthesizes the branched β(1,3)-D-glucan and is responsible for most GS activity [19,29]. The branched β(1,3)-D-glucan produced by Bgs4 is vital for maintaining cell shape and septum formation and completion. Differently from Bgs1 and Bgs3, the function of Bgs4 is essential for cell integrity, and thus, Bgs4 depletion causes cell lysis and cytoplasm leakage in the growing poles and mainly in the medial region at the onset of septum degradation and cell separation [30].
Treatment with inhibitors of the GS blocks the incorporation of novel β(1,3)-D-glucan to the wall surrounding the sites of active growth and causes osmotic fragility and cell lysis in exponentially growing cells. Thus, treated fungal cells die by the focalized rupture of the underneath plasma membrane and the subsequent cytoplasm leakage [31,32,33,34]. Three families of antifungals, lipopeptides (echinocandins), acidic terpenoids (enfumafungin), or glycolipids (papulacandins), have been described to alter the integrity of the cell wall by targeting the GS [10,13,35]. From all of them, only the echinocandins anidulafungin, caspofungin, and micafungin have been authorized as drugs for treating systemic fungal infections (Table 1) [5,6,13,35]. Thus, because of their fungicidal effect in yeasts, echinocandins are proposed as the forefront treatment for Candida albicans infections [36,37].

Table 1.
Chemical structure of the echinocandins derivatives approved for therapeutic use (table adapted from [10]).
Resistance to GS inhibitors is well conserved in fungi and is generally associated with point mutations located in highly conserved short regions (hot spots) of the Bgs/Fks proteins [29,38,39,40]. In S. pombe, pbr1-8 and pbr1-6 are the only isolated mutants exhibiting resistance to specific GS inhibitors [29]. These two mutants are exclusively due to point mutations in the bgs4+ sequence. A single mutation within bgs4+ confers resistance to specific GS inhibitors in the presence of the wild-type (WT) sequences of bgs1+ and bgs3+, suggesting that the function of the two encoding synthases, Bgs1 and Bgs3, is not inhibited by the available GS inhibitors [29]. The mode of action of echinocandins inhibits the synthesis of the β(1,3)-D-glucan through a noncompetitive inhibition of the GS activity [41,42,43]. However, the integral membrane GS catalytic subunit has not been homogenously purified, and, thus, neither the biochemistry and structure of the GS complex, nor the molecular mechanism of echinocandin inhibition of the GS activity, have been entirely elucidated [22,44,45,46].
The absence of cell wall chitin and the presence of three essential GS catalytic subunits exhibiting both differential antifungal susceptibility and non-redundant roles in β(1,3)-D-glucan synthesis render S. pombe an ideal tool for studying echinocandins mechanism of action and resistance [19,29]. Bgs proteins are essential for different aspects of cytokinesis and cell integrity. Thus, we have compared the effect of several echinocandins in these cellular processes. For this purpose, S. pombe WT and pbr1-8 strains were imaged through time-lapse fluorescence microscopy in the presence of the drugs. In trying to minimize the effects of cell wall compensatory mechanisms that might alter the echinocandins-derived phenotypes [47,48], time-lapses were performed at very early times of treatment (up to 3 h) with both sublethal (non-inhibitory) and lethal (inhibitory) concentrations of anidulafungin, caspofungin, and micafungin. Our study shows that, depending on the strain and the used concentration, the echinocandin drugs differentially affected the cytokinesis progression and cell integrity. Sublethal and lethal concentrations of micafungin selectively inhibited Bgs4, suggesting that Bgs1 and Bgs3 are not susceptible to this antifungal. Similarly, sublethal concentrations of anidulafungin and caspofungin primarily affected Bgs4, confirming that Bgs4 is the GS subunit most susceptible to echinocandins. Remarkably, lethal concentrations of anidulafungin and caspofungin severely affected cytokinesis progression, showing that besides their effect on Bgs4 activity, they also likely affect the function of Bgs1 and/or Bgs3.
2. Results and Discussion
2.1. Susceptibilities of WT and pbr1-8 Strains to Echinocandins
The susceptibility of yeasts to echinocandins depends on the number of inoculated cells. Thus, greater cell densities require higher drug concentrations to inhibit organism growth [41]. Because the imaging and preparation of yeast cells for time-lapse fluorescence microscopy requires cultures containing a higher density of cells than cultures used for visualizing the presence or absence of growth in susceptibility assays, we compared the susceptibilities to echinocandins of the WT and pbr1-8 strains in cultures inoculated either with a typical lower cell density (5 × 105 cells/mL) or with a higher cell density (5 × 106 cells/mL) that will later be used in time-lapse fluorescence microscopy experiments (Table 2). The minimal inhibitory concentration (MIC) for the WT strain varied between 1 and 10 µg/mL at lower cell densities (Table 2, left column). As expected, the MIC for the WT increased to 10–20 µg/mL at higher cell densities (Table 2, right column). Thus, based on the obtained MICs with a higher number of cells, sublethal (non-inhibitory) and lethal (inhibitory) concentrations for the WT of 2 and 20 µg/mL, respectively, were chosen for performing the comparative time-lapse experiments (see next section). The pbr1-8 strain exhibited a complete resistance to micafungin that was independent of the density of cells in the culture (a MIC of more than 80 µg/mL). In contrast, this strain presented some susceptibility to caspofungin and anidulafungin, with the MICs varying from 2–10 (lower cell density, Table 2, left column) to 20–40 µg/mL (higher cell density, Table 2, right column). Finally, micro-cultures starting at a higher cell density were used to examine the cell morphology by phase-contrast microscopy after 24 h of growth in the presence of increasing concentrations of the echinocandins. The three echinocandins led to the WT cells becoming aggregated, rounded, and swollen. This phenotype started to be observed in cells treated with sublethal concentrations of 1 µg/mL for caspofungin and 4 µg/mL for both anidulafungin and micafungin (Figure 1, WT). None of the applied concentrations of the micafungin was sufficient to induce the phenotype of swollen and rounded cells in the pbr1-8 resistant strain; however, lethal concentrations of anidulafungin and caspofungin were able to induce it. (Figure 1, pbr1-8). These results show that micafungin selectively inhibits the function of Bgs4, whereas Bgs1 and Bgs3 are not affected by this antifungal. The same results were previously described for the other two classes of GS inhibitors, the glycopeptide papulacandin B and the acidic terpenoid enfumafungin that also selectively inhibited Bgs4 [29].

Table 2.
Susceptibilities of S. pombe wild-type and pbr1-8 strains to echinocandins 1.
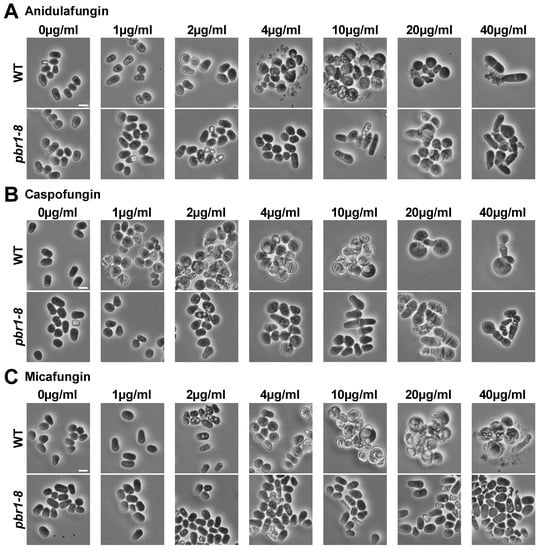
Figure 1.
Morphology of WT and pbr1-8 cells after 24 h of growth in the presence of increasing concentrations of anidulafungin (A), caspofungin (B), and micafungin (C) drugs. Early logarithmic-phase cells of the WT and pbr1-8 strains growing in YES liquid medium at 28 °C were diluted to a high cell density of 5 × 106 in micro-cultures of YES liquid medium containing either DMSO (0.8%, control) or increasing concentrations (1, 2, 4, 10, 20, and 40 µg/mL) of the drugs, grown with shaking for 24 h and imaged by phase-contrast microscopy. The data of this figure are developed in Table 2. Scale bars, 10 µm.
2.2. Differential Effects of the Echinocandins in Cytokinesis Progression
Cytokinesis is the last event of the cell cycle, where a cleavage furrow partitions the cell giving rise to two independent cells. In fungi, the ingression of the cleavage furrow is tightly coupled to the synthesis of a specialized cross wall named division septum [19,25,30,49]. In contrast to the filamentous fungi, yeast cytokinesis also requires the separation of daughter cells through the enzymatic degradation of the septum. To avoid cell lysis during cell separation, strict coordination between degradation and synthesis of the cell wall surrounding the septum is needed [30,50]. Thus, defects in the synthesis of the main cell wall glucans frequently lead to excessive wall degradation and cell lysis at the onset of cell separation [30]. Here, time-lapse fluorescence microscopy was used to examine the dynamics of septation and cell separation in the WT and pbr1-8 strains during the first 3 h of echinocandin treatment. To follow the cytokinesis progression, cells were stained with very low doses of calcofluor white (CW), a fluorochrome that specifically and with high affinity binds to the linear β(1,3)-D-glucan of the primary septum, thus allowing the monitoring of cytokinesis since the very early steps of primary septum synthesis [49]. As described above, cells of the two strains were treated with both sublethal (2 µg/mL) and lethal (20 µg/mL) concentrations of the three echinocandins. As a control for the experiments, we first compared the cytokinesis progression in the WT and pbr1-8 cells growing in the absence of echinocandins (control, Figure 2), observing that septum progression (double arrow depicted in green) and cell separation onset (double arrow depicted in orange) were similar in both strains. Table 3 compiles the data from the performed time-lapse microscopy experiments, showing the elapsed minutes from the beginning to the end of septum formation (septation) and from the end of septum formation to the start of cell separation (cell separation) in the two strains (WT and pbr1-8) growing in the absence (Figure 2, control) or the presence of sublethal and lethal concentrations of the three echinocandins (Figure 3, Figure 4 and Figure 5). Depending on the strain and concentration of the echinocandin, different effects in the cytokinesis were induced: (1) cytokinesis was normal, with the elapsed times of septation and separation being similar to those of control cells (Table 3, boxes depicted in green); (2) cytokinesis was not blocked, but cell death occurred at the onset of cell separation (Table 3, boxes depicted in red); and (3) cytokinesis was blocked or delayed in the septum progression, and interrupted (or greatly delayed) the cell separation onset, and, thus, cell death did not occur (Table 3, boxes depicted in other colors). Thus, we analyzed how each echinocandin affects the cytokinesis progression (see below in the following subsection) and cell integrity (see below in the last section).
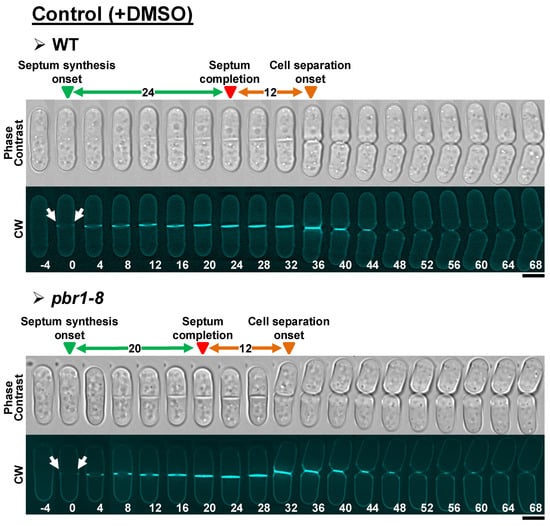
Figure 2.
Normal cytokinesis (septum synthesis and cell separation) in WT and pbr1-8 cells growing in the absence of the three echinocandin drugs. Early logarithmic-phase cells of the indicated strains growing in YES liquid medium at 28 °C were diluted to a cell density of 5 × 106 in liquid YES medium containing both calcofluor white (CW, 1.25 µg/mL) and DMSO (0.8%), and then imaged by time-lapse fluorescence microscopy (1 medial z slice, 4 min elapsed time) for 3 h, as described in the Materials and Methods section. The data of this figure are developed in Table 3, Table 4 and Table 5. White arrow: first CW-stained septum synthesis. Arrowheads: blue, septum synthesis onset (time 0 for the elapsed time until septum synthesis completion); red, septum completion onset (time 0 for the elapsed time until cell separation onset); orange, cell separation onset. Scale bars, 5 µm.

Table 3.
Cytokinesis phenotypes and elapsed times for septation and cell separation periods in S. pombe cells growing in culture chamber slides containing sublethal (2 µg/mL) or lethal (20 µg/mL) concentrations of the three echinocandins.
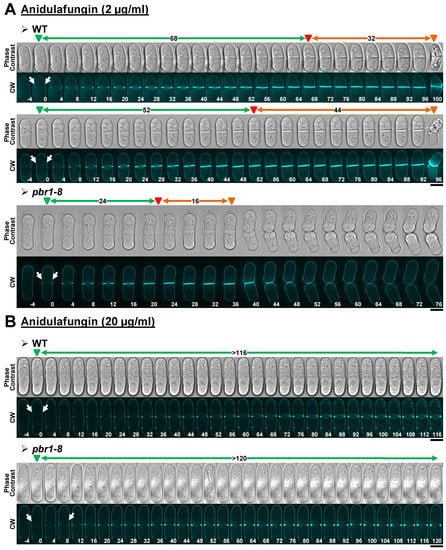
Figure 3.
Cytokinesis phenotypes in WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of anidulafungin. The indicated strains were grown and imaged as in Figure 2 in the presence of either sublethal ((A), 2 µg/mL) or lethal ((B), 20 µg/mL) concentrations of the drug. The data of this figure are developed in Table 3, Table 4 and Table 5. Arrows and arrowheads are as in Figure 2. Scale bars, 5 µm.
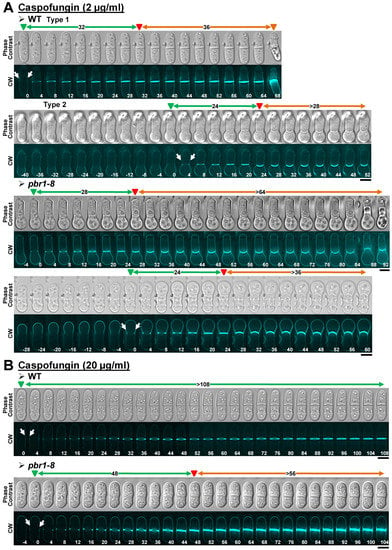
Figure 4.
Cytokinesis phenotypes in WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of caspofungin. The indicated strains were grown and imaged as in Figure 2 in the presence of sublethal ((A), 2 µg/mL) or lethal ((B), 20 µg/mL) concentrations of the drug. The data of this figure are developed in Table 3, Table 4 and Table 5. Arrows and arrowheads are as in Figure 2. Scale bars, 5 µm.
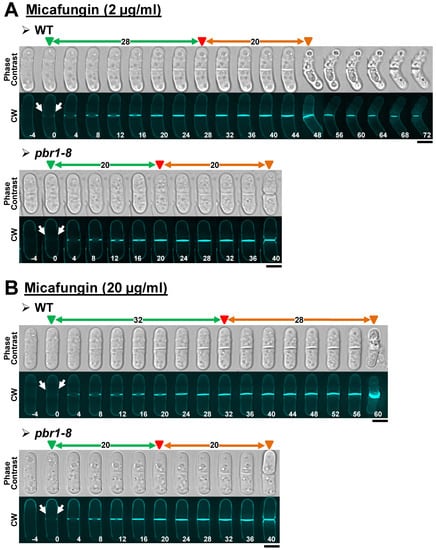
Figure 5.
Cytokinesis phenotypes in WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of micafungin. The indicated strains were grown and imaged as in Figure 2 in the presence of sublethal ((A), 2 µg/mL) or lethal ((B), 20 µg/mL) concentrations of the drug. The data of this figure are developed in Table 3, Table 4 and Table 5. Arrows and arrowheads are as in Figure 2. Scale bars, 5 µm.
2.2.1. Anidulafungin
Treatment of the WT strain with sublethal concentrations of anidulafungin for 3 h mainly induced an increase in the elapsed times of both septum formation and the onset of cell separation, without blocking cytokinesis progression (Table 3, Figure 3A, and Figure S1A). In contrast, the elapsed times for septation and cell separation onset in the pbr1-8 mutant cells treated with sublethal concentrations of the drug were similar to those observed in the non-treated control cells (Table 3, Figure 3A, and Figure S1A). Interestingly, in both strains, lethal concentrations of anidulafungin arrested septum formation and consequently impeded the cell-separation onset (Table 3, Figure 3B, and Figure S1B). These results show that anidulafungin induces defects in the synthesis of β(1,3)-D-glucan that affect the progression of septation. These defects are also proportional to the antifungal concentration: low concentrations slow down septation, whereas higher concentrations completely block it. Thus, sublethal concentrations of the drug primarily induced two phenotypes in the WT strain; slower cytokinesis progression and cell death at the start of cell separation, which were similar to those described for Bgs4 depletion [30]. Additionally, the fact that both phenotypes were absent in the pbr1-8 strain (Figure 3A and Figure S1A) indicates that lower concentrations of anidulafungin cause the sole inhibition of Bgs4. In contrast, the septation arrest observed in the presence of lethal concentrations of the drug was not corrected in the pbr1-8 strain, indicating that these concentrations of the drug lead to the combined inhibition of Bgs4 together with Bgs1 and/or Bgs3 (Figure 3B). In agreement, septum ingression is similarly impaired when the function of Bgs1 is depleted or reduced in some mutant alleles [19,23,24,25].
2.2.2. Caspofungin
Next, cytokinesis progression in the presence of caspofungin was examined. Sublethal concentrations of the drug resulted in a slight slowdown of the cytokinesis progression, with many WT cells dying at the onset of cell separation (Table 3, Figure 4A, WT, Type 1, and Figure S2A). After approximately 1.5 h of growth in the presence of the antifungal, many WT cells started to exhibit a progressive swelling of the growing pole, giving rise to cells with a bulb or drumstick appearance. Interestingly, due to a blockage of the onset of cell separation, most of these drumstick-like cells were still alive by the end of the 3 h treatment (Table 3, Figure 4A, WT, Type 2, and Figure S2A). The same drumstick-like cells with blocked separation were also observed in the presence of sublethal concentrations of both anidulafungin and micafungin (Table 3, Figures S1A and S3A, yellow arrows), as previously described for the echinocandin lipopeptide aculeacin A [32]. The pbr1-8 mutation partially suppressed the phenotype of cell death at the start of cell separation, with many cells at the earliest treatment times exhibiting normal cytokinesis (Table 3, data not shown). However, most of the resistant cells that entered cytokinesis at later treatment times acquired the same drumstick appearance of the WT strain (Table 3, Figure 4A, and Figure S2A). Although lethal concentrations of caspofungin did not block the cytokinesis progression like anidulafungin (Figure 3B), the septum completion was extremely slowed down, and the cytokinesis was still incomplete by the end of the 3 h treatment (Table 3 and Figure 4A). The pbr1-8 mutation partially suppressed the septum progression defects of cells exposed to higher doses of caspofungin (≥121.6 ± 26.5 min in the WT compared to 51.4 ± 8.4 min in the resistant strain), although, because of the blocked cell separation, cytokinesis progression was still very delayed and incomplete by the end of the 3 h treatment (Table 3 and Figure 4B). The fact that the pbr1-8 mutation did not completely suppress the phenotypes observed in the WT strain suggests that caspofungin also acted against Bgs4 and Bgs1 and/or Bgs3, like anidulafungin. In agreement and similarly to anidulafungin, lethal concentrations of caspofungin led to an extremely slow cytokinesis development.
2.2.3. Micafungin
In contrast to the other two echinocandins, the studies performed with micafungin showed that this drug solely inhibits the activity of Bgs4 at both sublethal and lethal concentrations (Table 3 and Figure 5). Thus, lower micafungin concentrations did not significatively affect cytokinesis progression, inducing a phenotype of cell death at the onset of cell separation (Table 3, Figure 5A and Figure S3A). At later treatment times, a few drumstick-like cells with blocked separation were also observed (Table 3 and Figure S3A, yellow arrows). Interestingly, both phenotypes were entirely suppressed in the resistant pbr1-8 strain (Table 3, Figure 5A and Figure S3A). Similarly, lethal concentrations of the drug only caused a slight slowdown of the cytokinesis progression, with all the cells in cytokinesis dying at the cell separation onset. In these conditions, no drumstick-like cells were observed within the 3 h treatment (Table 3, Figure 5B and Figure S3B), suggesting that those drumstick-like cells may be the germ for the survivors observed after 24 h of treatment with sublethal concentrations of the three drugs. Again, non-cell death was observed in the treated cells of the pbr1-8 strain, which also exhibited a normal cytokinesis progression (Table 3, Figure 5B and Figure S3B). It is noteworthy to mention that the cytokinesis phenotypes observed in the WT strain with lethal concentrations of micafungin were the opposite of those with anidulafungin and caspofungin. Additionally, while the pbr1-8 mutation suppressed all the phenotypes caused by both sublethal and lethal concentrations of the micafungin, the mutation did not totally suppress the phenotypes caused by the other two echinocandins. All these results show that both sublethal and lethal concentrations of micafungin selectively inhibit Bgs4. In contrast, the other two echinocandins, Bgs1 and Bgs3, seem to not be affected by micafungin. This observation also might suggest that these two enzymes are not the targets for micafungin.
Currently, it is unknown why Bgs4 is susceptible to all the examined GS inhibitors, while Bgs1 and Bgs3 are susceptible to some inhibitors (anidulafungin and caspofungin) but not susceptible to others (micafungin, papulacandin B, and enfumafungin). Although Bgs1, Bgs3, and Bgs4 show high degrees of conservation (approximately 55% identity), they might still acquire different tertiary structures, making Bgs1 and Bgs3 less accessible to some echinocandins and more accessible to others. Another explanation might be due to changes in the lipid composition of the microdomains containing the GS subunits. Lipid composition impacts the structural and functional properties of the cell membranes, thus influencing the function of integral membrane proteins, such as the GS enzymes. Besides this, specific lipids may distribute asymmetrically between leaflets, originating different nanoscale domains along the plasma membrane [51]. Echinocandins are cyclic hexapeptides with a lipid side chain (Table 1) that is essential for their antibiotic activity and toxicity [52]. The lipid side chain is thought to interact with the outer leaflet of the plasma membrane containing the GS enzymes [45]. Thus, the differential susceptibility of the Bgs proteins to the echinocandins might be caused by differences in the organization and/or composition of the Bgs-containing lipid microenvironment. Consequently, it has been proposed that changes in the membrane lipids’ composition might modulate the interaction of echinocandins with the plasma membrane depending on the structure of the lipid side chain [53,54]. Alternatively, caspofungin and anidulafungin might affect the function of Bgs1 and/or Bgs3 through a secondary mechanism. Caspofungin and anidulafungin treatments induce a reduction in the levels of Bgs1 in the cleavage furrow (our unpublished results). In the budding yeast, the echinocandin B inhibits the localization of Spa2 [55], a cell polarity protein that regulates the localization of Chs2, which is responsible for the chitin synthesis of the primary septum [56]. Similarly, S. pombe Spa2 interacts with the F-BAR protein Cdc15 that is essential for the localization of Bgs1 to the division site [19,56].
2.3. Differential Effects of the Echinocandins in Cell Integrity
Next, the phenotypes of cell death, as well as the percentages corresponding to each phenotype, were analyzed in the time-lapse microscopy experiments described in the previous section. Depending on the effect of echinocandins in cytokinesis progression, differential levels of cell death were observed. Sublethal concentrations of any of the three antifungals did not block cytokinesis (Table 3), inducing death in 80–90% of the cells in the examined fields (Table 4). The cell death occurred either at the start of cell separation (Figure 3A, Figure 4A and Figure 5A) or during polarized growth in interphase (Figures S1A–S3A, and S4). Interestingly, these high percentages of initial death were not enough for arresting the cell growth (Table 2), probably because of the emergence of drumstick-like survivors at the later times of the 3 h treatment (Figures S1A–S3A, yellow arrows). Something similar is observed at longer times of Bgs4 depletion, where a small population of drumstick-like cells is also able to survive ([30], and our unpublished results). As expected, lethal concentrations also induced differential effects in cell integrity (Table 4). Both anidulafungin and caspofungin were primarily fungistatic and caused low percentages of cell death (22–27%) because they blocked the cell separation. In contrast, micafungin did not block cell separation, mainly being a fungicide and inducing high percentages of cell death (97%) (Table 4).

Table 4.
Percentages and types of cell death observed in the time-lapses of WT and pbr1-8 cells growing in the presence of sublethal (2 µg/mL) or lethal (20 µg/mL) concentrations of the three echinocandin drugs.
Table 4.
Percentages and types of cell death observed in the time-lapses of WT and pbr1-8 cells growing in the presence of sublethal (2 µg/mL) or lethal (20 µg/mL) concentrations of the three echinocandin drugs.
| Drug (Concentration) | Strain | Number of Cells | Cell Death 1 | Cell Lysis 2 | Non-Cell Lysis 3 |
|---|---|---|---|---|---|
| Control (+DMSO) | WT | n = 30 | 0 4 (0) 5 | 0 4 (0) 5 | 0 4 (0) 5 |
| pbr1-8 | n = 33 | 0 (0) | 0 (0) | 0 (0) | |
| Anidulafungin (2 µg/mL) | WT | n = 79 | 89.9 (100) | 65.2 (72.5) | 24.7 (27.5) |
| pbr1-8 | n = 61 | 0 (0) | 0 (0) | 0 (0) | |
| Anidulafungin (20 µg/mL) | WT | n = 60 | 21.7 (100) | 13.3 (61.5) | 8.4 (38.5) |
| pbr1-8 | n = 45 | 8.9 (100) | 2.2 (25) | 6.7 (75) | |
| Caspofungin (2 µg/mL) | WT | n = 96 | 82.3 (100) | 58.3 (70.9) | 24 (29.1) |
| pbr1-8 | n = 85 | 22.4 (100) | 5.9 (26.3) | 16.5 (73.7) | |
| Caspofungin (20 µg/mL) | WT | n = 33 | 27.3 (100) | 12.1 (44.4) | 15.2 (55.6) |
| pbr1-8 | n = 64 | 22.7 (100) | 0 (0) | 22.7 (100) | |
| Micafungin (2 µg/mL) | WT | n = 99 | 80.3 (100) | 13.6 (17) | 69.7 (83) |
| pbr1-8 | n = 36 | 0 (0) | 0 (0) | 0 (0) | |
| Micafungin (20 µg/mL) | WT | n = 61 | 97.5 (100) | 97.5 (100) | 0 (0) |
| pbr1-8 | n = 46 | 0 (0) | 0 (0) | 0 (0) |
1 Cell death is the sum of the percentages of cell lysis and non-cell lysis. 2 Cells shrunk and died with cell breakage and cytoplasm leakage. 3 Cells shrunk and died without cell breakage and cytoplasm leakage. 4 Value is the percentage of dead cells with respect to the total number of visualized cells (n). 5 Value is the percentage of cell lysis or non-cell lysis with respect to the total number of dead cells.
As expected, the pbr1-8 resistant mutation entirely suppressed the cell death caused by both sublethal and lethal concentrations of micafungin, reinforcing the finding that micafungin only inhibits Bgs4 activity (Table 4 and Table 5). Similarly, the pbr1-8 mutation either suppressed or reduced the death caused by sublethal and lethal concentrations of anidulafungin, respectively (Table 4 and Table 5). In contrast, the pbr1-8 mutation partially suppressed the death observed at lower concentrations (from 80% to 22%) but was largely ineffective with higher concentrations of caspofungin (22% in both WT and pbr1-8). Again, these results reinforce that caspofungin and anidulafungin inhibit several GS subunits to different degrees.

Table 5.
Percentages of cell lysis either at interphase or at cell separation onset observed in the time-lapses of WT and pbr1-8 cells growing in the presence of sublethal (2 µg/mL) or lethal (20 µg/mL) concentrations of the three echinocandin drugs.
Table 5.
Percentages of cell lysis either at interphase or at cell separation onset observed in the time-lapses of WT and pbr1-8 cells growing in the presence of sublethal (2 µg/mL) or lethal (20 µg/mL) concentrations of the three echinocandin drugs.
| Drug (Concentration) | Strain | Number of Cells | Total Cell Lysis 1 | Lysis at Separation 1 | Lysis at Interphase 1 |
|---|---|---|---|---|---|
| Control (+DMSO) | WT | n = 30 | 0 2 (0) 3 | 0 2 (0) 3 | 0 2 (0) 3 |
| pbr1-8 | n = 33 | 0 (0) | 0 (0) | 0 (0) | |
| Anidulafungin (2 µg/mL) | WT | n = 79 | 65.2 (72.5) | 45.6 (50.7) | 19.6 (21.8) |
| pbr1-8 | n = 61 | 0 (0) | 0 (0) | 0 (0) | |
| Anidulafungin (20 µg/mL) | WT | n = 60 | 13.3 (61.5) | 10.0 (46.1) | 3.3 (15.4) |
| pbr1-8 | n = 45 | 2.2 (25) | 0 (0) | 2.2 (25) | |
| Caspofungin (2 µg/mL) | WT | n = 96 | 58.3 (70.9) | 40.6 (49.4) | 17.7 (21.5) |
| pbr1-8 | n = 85 | 5.9 (26.3) | 2.4 (10.5) | 3.5 (15.8) | |
| Caspofungin (20 µg/mL) | WT | n = 33 | 12.1 (44.4) | 12.1 (44.4) | 0 (0) |
| pbr1-8 | n = 64 | 0 (0) | 0 (0) | 0 (0) | |
| Micafungin (2 µg/mL) | WT | n = 99 | 13.6 (17) | 3.0 (3.8) | 10.6 (13.2) |
| pbr1-8 | n = 36 | 0 (0) | 0 (0) | 0 (0) | |
| Micafungin (20 µg/mL) | WT | n = 61 | 97.5 (100) | 44.3 (45.4) | 53.3 (54.6) |
| pbr1-8 | n = 46 | 0 (0) | 0 (0) | 0 (0) |
Cell lysis is cell death accompanied by cell breakage and cytoplasm leakage. 1 Total cell lysis is the sum of the percentages of both cell lysis at interphase and at cell separation onset. 2 Value is the percentage of lysed cells with respect to the total number of cells (n). 3 Value is the percentage of lysed or non-lysed cells with respect to the total number of dead cells.
The three echinocandins induced two death phenotypes: death with cytoplasm leakage (Figures S1–S3, lysis, red arrows) and without cytoplasm leakage (Figures S1–S3, non-lysis, white arrows). Sublethal concentrations of anidulafungin and caspofungin mainly induced a lytic phenotype that mostly occurred at the onset of cell separation and was almost entirely suppressed in the pbr1-8 strain (Table 4 and Table 5). In contrast, the cell death without an apparent leakage of the cytoplasm was still observed in the pbr1-8 cells treated with caspofungin and was residual in the case of anidulafungin (Table 4 and Table 5). Intriguingly, the results of micafungin show a phenotypic difference in cell death that depends on the used concentration (Figure 5 and Figure S3). Sublethal concentrations of this antifungal induced, in the WT strain, a very high percentage of death without lysis (70%), while, with lethal concentrations, the totality of cell death involved the release of the cytoplasmic content (Table 3 and Table 5). Both death phenotypes were completely suppressed in the pbr1-8 strain, indicating that both micafungin effects are due to the Bgs4 function. The phenotype of cell lysis is clearly the result of a localized rupture of the plasma membrane located under the weakened and/or reduced cell wall because of the reduction in the Bgs4-derived branched β(1,3)-D-glucan [19,30]. In contrast, death without lysis does not involve release of cytoplasmic content and might be due to a general alteration of plasma membrane permeability. The same phenotype of death without lysis was also observed in a few cells during Bgs4 depletion (our unpublished results) or when the function of the regulatory subunit of the GS complex, GTPase Rho1, is affected in the mutant allele rho1-596 [57]. Endoplasmic reticulum (ER) stress in the budding yeast induces the permeabilization of the vacuolar membrane, causing cell acidification and death [58]. Interestingly, it has been described in several yeast species that cell wall stress activates an ER stress-like response that requires the entry of calcium and the function of the phosphatase calcineurin [57]. Thus, the cell damage caused by micafungin-inhibition of Bgs4 might activate a calcineurin-dependent ER stress-like response in fission yeast. In agreement, many mutants resistant to the calcineurin inhibitor FK506 exhibit hypersensitivity to micafungin [59]
3. Materials and Methods
3.1. Strains and Culture Conditions
The S. pombe strains examined in this study were isogenic to the WT strain h- 972. The pbr1-8 mutant was isolated by ethyl methane sulfonate mutagenesis and selection in the presence of 20 µg/mL papulacandin B [29]. The standard rich yeast growth medium (YES from “Yeast Extract with Supplements”) has been described previously [60]. Cell growth was monitored by measuring the A600 of early log-phase cell cultures in a Smart-Spec 3000 spectrophotometer (Bio-Rad, Hercules, CA, USA; A600 0.1 = 106 cells/mL).
3.2. Antifungal Drugs and Susceptibility Assays
The echinocandins used in this study were generous gifts from Pfizer, New York, NY, USA (anidulafungin), Merck Sharp and Dohme, Kenilworth, NJ, USA (caspofungin), and Astellas Pharma, Chūō, Tokyo, Japan (micafungin). The echinocandins were kept at −20 °C in stock solution (10 mg/mL in DMSO) and assayed at the final concentrations specified in the text, tables, and figures. For micro-culture assays of a large number of samples, log-phase cultures grown in YES medium were diluted to a cell density of 5 × 105 cells/mL (lower cell density) or 5 × 106 cells/mL (higher cell density) in YES medium containing increasing concentrations of echinocandins (1, 2, 4, 10, 20, 40, and 80 µg/mL) or an equal volume of solvent (0.8% DMSO), which was the control cell culture. The cell cultures were grown in an orbital roller at 28 °C, and turbidity was examined after 24 h of growth. The MIC was the minimal concentration of echinocandin that induced a complete inhibition of the cell growth after 24 h of growth. The values were calculated from at least three independent experiments.
3.3. Microscopy Techniques and Data Analysis
Images of cells after 24 h of growth in the presence of the echinocandins (Figure 1) were directly obtained from the micro-cultures used for the susceptibility assays with a Nikon Eclipse 50i microscope, a Nikon Plan FLUOR 20 ×/0.45 objective, a Nikon Ds-Fi1 digital camera, and a Nikon Digital Sight DS-L2 control unit. Time-lapse imaging was performed as previously described [49]. A volume of 0.3 mL of logarithmic-phase cells grown in YES medium at 28 °C was collected at the cell density of 5 × 106 cells/mL. The cells were gently centrifuged (1 min at 1000g), and the cell pellet was suspended in the same YES medium containing calcofluor white (CW) at a very low final concentration of 1.25 μg/mL together with 0.8% DMSO as control or the corresponding echinocandin at a final concentration of 2 µg/mL (sublethal, non-inhibitory) or 20 µg/mL (lethal, inhibitory), and placed in a well from a μ-Slide 8 well (80,821-Uncoated; Ibidi, Gräfelfing, Germany) previously covered with 5 μL of 1 mg/mL soybean lectin (L1395; Sigma-Aldrich, Burlington, MA, USA). All the time-lapse experiments were performed at 28 °C by acquiring epifluorescence and phase contrast cell images in single planes every 4 min and 1 × 1 binning on an inverted microscope (Olympus IX71) equipped with a PlanApo 100 ×/1.40 IX70 objective and a Personal DeltaVision system (Applied Precision LLC., Issaquah, WA, USA). Images were obtained using CoolSnap HQ2 monochrome camera (Photometrics, Tucson, AZ, USA) and softWoRx 5.5.0 release 6 imaging software (Applied Precision LLC.). Subsequently, CW time-lapse images were restored and corrected by 3D Deconvolution (conservative ratio, 10 iterations, and medium noise filtering) through soft-WoRx imaging software. Finally, images were processed with Image J (National Institutes of Health, Bethesda, MD, USA) and Adobe Photoshop software. All the time-lapse videos were repeated at least twice, and the data were calculated from at least two independent experiments.
4. Conclusions
Here, we have compared the cytokinesis and cell integrity processes in cells treated with sublethal and lethal concentrations of three echinocandin drugs: anidulafungin, caspofungin, and micafungin. We found that micafungin only inhibits activity due to Bgs4. Thus, and similarly to Bgs4 depletion, both sublethal and lethal concentrations of this drug primarily caused a phenotype of cell death in the WT that was entirely suppressed by the bgs4+ mutation in pbr1-8 cells. Our results indicate that all three echinocandins appear to act via Bgs4. In comparison, from the examination of septation and cell separation, it is evident that anidulafungin appears to affect, in some degree, the functions of Bgs1 and/or Bgs3, while caspofungin affects to a lower extent, and micafungin does not affect at all. To our knowledge, this is the first study analyzing, in detail, the progression and dynamics of cytokinesis in the presence of the echinocandin drugs. This experimental approximation has helped us identify that echinocandins induce differential effects in cytokinesis and cell integrity. Globally, these results show that caspofungin and anidulafungin are the most effective echinocandins against the resistant strain pbr1-8, probably because they combinedly affect the function of Bgs4 and other GS catalytic subunits.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ph14121332/s1, Figure S1: Representative time-lapse field of WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of anidulafungin, Figure S2: Representative time-lapse field of WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of caspofungin, Figure S3: Representative time-lapse fields of WT and pbr1-8 cells growing in the presence of sublethal and lethal concentrations of micafungin, Figure S4: Cell lysis and cytoplasm leakage during interphase in WT cells growing in the presence of the echinocandin drugs.
Author Contributions
Conceptualization, J.C.G.C., P.P. and J.C.R.; methodology, N.Y., L.G.-D., M.Á.C., V.S.D.C., M.B.M., P.P., J.C.R. and J.C.G.C.; formal analysis, N.Y., L.G.-D., M.Á.C., V.S.D.C., M.B.M., P.P., J.C.R. and J.C.G.C.; investigation, N.Y.; resources, J.C.R.; writing—original draft preparation, J.C.G.C. and J.C.R.; writing—review and editing, J.C.G.C. and J.C.R.; supervision, J.C.G.C., P.P. and J.C.R.; project administration, P.P. and J.C.R.; funding acquisition, P.P. and J.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants PGC2018-098924-B-I00 (the Spanish Ministry of Science and Innovation, MICINN, Spain; the European Regional Developmental Fund, FEDER, EU), and CSI150P20 and “Escalera de Excelencia” CLU-2017-03 (Regional Government of Castile and Leon, JCYL, Spain; and the European Regional Developmental Fund, FEDER, EU).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Material.
Acknowledgments
Vanessa S. D. Carvalho and Laura Gómez-Delgado recognize financial support received through contracts from the Regional Government of Castile and Leon (JCYL, Spain) and the European Regional Developmental Fund (FEDER, EU), and the Spanish Ministry of Science and Innovation (MICINN, Spain), respectively. The authors are grateful to the companies Astellas Pharma, Pfizer, and Merck Sharp and Dohme for the generous gifts of micafungin, caspofungin, and anidulafungin, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A.; ECMM; ISHAM. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef]
- Limper, A.H.; Adenis, A.; Le, T.; Harrison, T.S. Fungal infections in HIV/AIDS. Lancet Infect. Dis. 2017, 17, e334–e343. [Google Scholar] [CrossRef]
- LIFE. Leading International Fungal Education: The Burden of Fungal Disease 2017. Available online: http://www.life-worldwide.org/media-centre/article/the-burden-of-fungal-disease-new-evidence-to-show-the-scale-of-the-problem (accessed on 3 November 2021).
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Ostrosky-Zeichner, L.; Casadevall, A.; Galgiani, J.N.; Odds, F.C.; Rex, J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010, 9, 719–727. [Google Scholar] [CrossRef]
- Denning, D.W.; Bromley, M.J. Infectious Disease. How to bolster the antifungal pipeline. Science 2015, 347, 1414–1416. [Google Scholar] [CrossRef] [Green Version]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Florl, C.; Perlin, D.S. Drug-resistant fungi: An emerging challenge threatening our limited antifungal armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef]
- Ksiezopolska, E.; Schikora-Tamarit, M.A.; Beyer, R.; Nunez-Rodriguez, J.C.; Schuller, C.; Gabaldón, T. Narrow mutational signatures drive acquisition of multidrug resistance in the fungal pathogen Candida glabrata. Curr. Biol. 2021. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Curto, M.A.; Carvalho, V.S.D.; Pérez, P.; Ribas, J.C. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 2019, 37, 107352. [Google Scholar] [CrossRef]
- Hopke, A.; Brown, A.J.P.; Hall, R.A.; Wheeler, R.T. Dynamic Fungal Cell Wall Architecture in Stress Adaptation and Immune Evasion. Trends Microbiol. 2018, 26, 284–295. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Tkacz, J.S. The fungal cell wall as a drug target. Trends Microbiol. 1995, 3, 98–104. [Google Scholar] [CrossRef]
- Curto, M.A.; Butassi, E.; Ribas, J.C.; Svetaz, L.A.; Cortés, J.C.G. Natural products targeting the synthesis of β(1,3)-D-glucan and chitin of the fungal cell wall. Existing drugs and recent findings. Phytomedicine 2021, 88, 153556. [Google Scholar] [CrossRef]
- Cabib, E.; Arroyo, J. How carbohydrates sculpt cells: Chemical control of morphogenesis in the yeast cell wall. Nat. Rev. Microbiol. 2013, 11, 648–655. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, V.S.D.; Gómez-Delgado, L.; Curto, M.A.; Moreno, M.B.; Pérez, P.; Ribas, J.C.; Cortés, J.C.G. Analysis and application of a suite of recombinant endo-β(1,3)-D-glucanases for studying fungal cell walls. Microb. Cell Fact. 2021, 20, 126. [Google Scholar] [CrossRef]
- Horiseberger, M.; Rosset, J. Localization of α-galactomannan on the surface of Schizosaccharomyces pombe cells by scanning electron microscopy. Arch. Microbiol. 1977, 112, 123–126. [Google Scholar] [CrossRef]
- Kreger, D.R. Observations on cell walls of yeasts and some other fungi by x-ray diffraction and solubility tests. Biochim. Biophys. Acta 1954, 13, 1–9. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Ramos, M.; Osumi, M.; Pérez, P.; Ribas, J.C. The Cell Biology of Fission Yeast Septation. Microbiol. Mol. Biol. Rev. 2016, 80, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Humbel, B.M.; Konomi, M.; Takagi, T.; Kamasawa, N.; Ishijima, S.A.; Osumi, M. In situ localization of β-glucans in the cell wall of Schizosaccharomyces pombe. Yeast 2001, 18, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Balasubramanian, M.K. 1,3-β-Glucan synthase: A useful target for antifungal drugs. Curr. Drug Targets-Infect. Disord. 2001, 1, 159–169. [Google Scholar] [CrossRef]
- Latge, J.P. The cell wall: A carbohydrate armour for the fungal cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef]
- Le Goff, X.; Woollard, A.; Simanis, V. Analysis of the cps1 gene provides evidence for a septation checkpoint in Schizosaccharomyces pombe. Mol. Gen. Genet. 1999, 262, 163–172. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Balasubramanian, M.K. A checkpoint that monitors cytokinesis in Schizosaccharomyces pombe. J. Cell Sci. 2000, 113, 1223–1230. [Google Scholar] [CrossRef]
- Ramos, M.; Cortés, J.C.G.; Sato, M.; Rincón, S.A.; Moreno, M.B.; Clemente-Ramos, J.A.; Osumi, M.; Pérez, P.; Ribas, J.C. Two S. pombe septation phases differ in ingression rate, septum structure, and response to F-actin loss. J. Cell Biol. 2019, 218, 4171–4194. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Tang, X.; Wang, H.; Balasubramanian, M. Bgs2p, a 1,3-β-glucan synthase subunit, is essential for maturation of ascospore wall in Schizosaccharomyces pombe. FEBS Lett. 2000, 478, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Roncero, C.; Sánchez, Y. Cell separation and the maintenance of cell integrity during cytokinesis in yeast: The assembly of a septum. Yeast 2010, 27, 521–530. [Google Scholar] [CrossRef]
- Martín, V.; García, B.; Carnero, E.; Durán, A.; Sánchez, Y. Bgs3p, a putative 1,3-β-glucan synthase subunit, is required for cell wall assembly in Schizosaccharomyces pombe. Eukaryot. Cell 2003, 2, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Martins, I.M.; Cortés, J.C.G.; Muñoz, J.; Moreno, M.B.; Ramos, M.; Clemente-Ramos, J.A.; Durán, A.; Ribas, J.C. Differential activities of three families of specific β(1,3)glucan synthase inhibitors in wild-type and resistant strains of fission yeast. J. Biol. Chem. 2011, 286, 3484–3496. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, J.; Cortés, J.C.G.; Sipiczki, M.; Ramos, M.; Clemente-Ramos, J.A.; Moreno, M.B.; Martins, I.M.; Pérez, P.; Ribas, J.C. Extracellular cell wall β(1,3)glucan is required to couple septation to actomyosin ring contraction. J. Cell Biol. 2013, 203, 265–282. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.F. Lysis of yeast cell walls induced by 2-deoxyglucose at their sites of glucan synthesis. J. Bacteriol. 1968, 95, 1169–1172. [Google Scholar] [CrossRef] [Green Version]
- Miyata, M.; Kitamura, J.; Miyata, H. Lysis of growing fissin-yeast cells induced by aculeacin A, a new antifungal antibiotic. Arch. Microbiol. 1980, 127, 11–16. [Google Scholar] [CrossRef]
- Cassone, A.; Mason, R.E.; Kerridge, D. Lysis of growing yeast-form cells of Candida albicans by echinocandin: A cytological study. Sabouraudia 1981, 19, 97–110. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hiratani, T.; Baba, M.; Osumi, M. Effect of aculeacin A, a wall-active antibiotic, on synthesis of the yeast cell wall. Microbiol. Immunol. 1985, 29, 609–623. [Google Scholar] [CrossRef]
- Vicente, M.F.; Basilio, A.; Cabello, A.; Peláez, F. Microbial natural products as a source of antifungals. Clin. Microbiol. Infect. 2003, 9, 15–32. [Google Scholar] [CrossRef] [Green Version]
- Suwunnakorn, S.; Wakabayashi, H.; Kordalewska, M.; Perlin, D.S.; Rustchenko, E. FKS2 and FKS3 Genes of Opportunistic Human Pathogen Candida albicans Influence Echinocandin Susceptibility. Antimicrob. Agents Chemother. 2018, 62, e02299-17. [Google Scholar] [CrossRef] [Green Version]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vázquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S612–S617. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Messer, S.A.; Jones, R.N.; Castanheira, M. Antifungal susceptibilities of Candida, Cryptococcus neoformans and Aspergillus fumigatus from the Asia and Western Pacific region: Data from the SENTRY antifungal surveillance program (2010–2012). J. Antibiot. 2015, 68, 556–561. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Katiyar, S.K.; Edlind, T.D. New Fks hot spot for acquired echinocandin resistance in Saccharomyces cerevisiae and its contribution to intrinsic resistance of Scedosporium species. Antimicrob. Agents Chemother. 2011, 55, 3774–3781. [Google Scholar] [CrossRef] [Green Version]
- Sawistowska-Schroder, E.T.; Kerridge, D.; Perry, H. Echinocandin inhibition of 1,3-β-D-glucan synthase from Candida albicans. FEBS Lett. 1984, 173, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Douglas, C.M.; Marrinan, J.A.; Li, W.; Kurtz, M.B. A Saccharomyces cerevisiae mutant with echinocandin-resistant 1,3-β−D-glucan synthase. J. Bacteriol. 1994, 176, 5686–5696. [Google Scholar] [CrossRef] [Green Version]
- Taft, C.S.; Stark, T.; Selitrennikoff, C.P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-β-D-glucan synthase activity. Antimicrob. Agents Chemother. 1988, 32, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D.S. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updates 2007, 10, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Edlind, T.D. Topological and mutational analysis of Saccharomyces cerevisiae Fks1. Eukaryot. Cell 2012, 11, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Ortigosa, C.; Jiang, J.; Chen, M.; Kuang, X.; Healey, K.R.; Castellano, P.; Boparai, N.; Ludtke, S.J.; Perlin, D.S.; Dai, W. Preliminary structural elucidation of β-(1,3)-glucan synthase from Candida glabrata using cryo-electron tomography. J. Fungi 2021, 7, 120. [Google Scholar] [CrossRef]
- García, R.; Itto-Nakama, K.; Rodríguez-Pena, J.M.; Chen, X.; Sanz, A.B.; de Lorenzo, A.; Pavon-Verges, M.; Kubo, K.; Ohnuki, S.; Nombela, C.; et al. Poacic acid, a β-1,3-glucan-binding antifungal agent, inhibits cell-wall remodeling and activates transcriptional responses regulated by the cell-wall integrity and high-osmolarity glycerol pathways in yeast. FASEB J. 2021, 35, e21778. [Google Scholar] [CrossRef]
- Roncero, C.; Celador, R.; Sánchez, N.; García, P.; Sánchez, Y. The role of the cell integrity pathway in septum assembly in yeast. J. Fungi 2021, 7, 729. [Google Scholar] [CrossRef]
- Cortés, J.C.G.; Ramos, M.; Konomi, M.; Barragán, I.; Moreno, M.B.; Alcaide-Gavilán, M.; Moreno, S.; Osumi, M.; Pérez, P.; Ribas, J.C. Specific detection of fission yeast primary septum reveals septum and cleavage furrow ingression during early anaphase independent of mitosis completion. PLoS Genet. 2018, 14, e1007388. [Google Scholar] [CrossRef] [Green Version]
- Sipiczki, M. Splitting of the fission yeast septum. FEMS Yeast Res. 2007, 7, 761–770. [Google Scholar] [CrossRef] [Green Version]
- Makarova, M.; Peter, M.; Balogh, G.; Glatz, A.; MacRae, J.I.; Lopez Mora, N.; Booth, P.; Makeyev, E.; Vigh, L.; Oliferenko, S. Delineating the rules for structural adaptation of membrane-associated proteins to evolutionary changes in membrane lipidome. Curr. Biol. 2020, 30, 367–380.e368. [Google Scholar] [CrossRef] [Green Version]
- Boeck, L.D.; Fukuda, D.S.; Abbott, B.J.; Debono, M. Deacylation of echinocandin B by Actinoplanes utahensis. J. Antibiot. 1989, 42, 382–388. [Google Scholar] [CrossRef]
- Healey, K.R.; Katiyar, S.K.; Raj, S.; Edlind, T.D. CRS-MIS in Candida glabrata: Sphingolipids modulate echinocandin-Fks interaction. Mol. Microbiol. 2012, 86, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Satish, S.; Jiménez-Ortigosa, C.; Zhao, Y.; Lee, M.H.; Dolgov, E.; Kruger, T.; Park, S.; Denning, D.W.; Kniemeyer, O.; Brakhage, A.A.; et al. Stress-induced changes in the lipid microenvironment of β-(1,3)-D-glucan synthase cause clinically important echinocandin resistance in Aspergillus fumigatus. mBio 2019, 10, e00779-19. [Google Scholar] [CrossRef] [Green Version]
- Okada, H.; Ohnuki, S.; Roncero, C.; Konopka, J.B.; Ohya, Y. Distinct roles of cell wall biogenesis in yeast morphogenesis as revealed by multivariate analysis of high-dimensional morphometric data. Mol. Biol. Cell 2014, 25, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Foltman, M.; Filali-Mouncef, Y.; Crespo, D.; Sánchez-Díaz, A. Cell polarity protein Spa2 coordinates Chs2 incorporation at the division site in budding yeast. PLoS Genet. 2018, 14, e1007299. [Google Scholar] [CrossRef]
- Viana, R.A.; Pinar, M.; Soto, T.; Coll, P.M.; Cansado, J.; Pérez, P. Negative functional interaction between cell integrity MAPK pathway and Rho1 GTPase in fission yeast. Genetics 2013, 195, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kim, A.; Cunningham, K.W. Vacuolar H+-ATPase (V-ATPase) promotes vacuolar membrane permeabilization and nonapoptotic death in stressed yeast. J. Biol. Chem. 2012, 287, 19029–19039. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Jiang, W.; Liu, Q.; Ryuko, S.; Kuno, T. Genome-wide screening for genes associated with FK506 sensitivity in fission yeast. PLoS ONE 2011, 6, e23422. [Google Scholar] [CrossRef] [Green Version]
- Alfa, C.; Fantes, P.; Hyams, J.; McLeod, M.; Warbrick, E. (Eds.) Experiments with Fission Yeast: A Laboratory Course Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1993; p. 186. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).