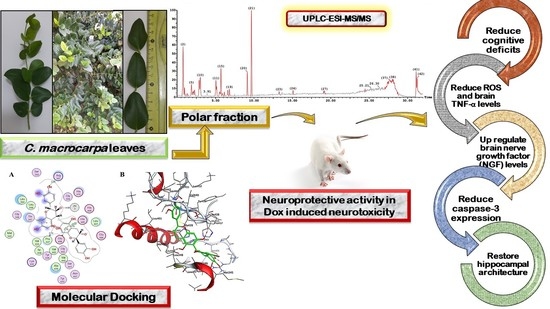
Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers
Abstract
:1. Introduction
2. Results
2.1. In Vivo Neuroprotective Activity of C. macrocarpa Leaves Polar Fraction against Dox-Induced Neurotoxicity
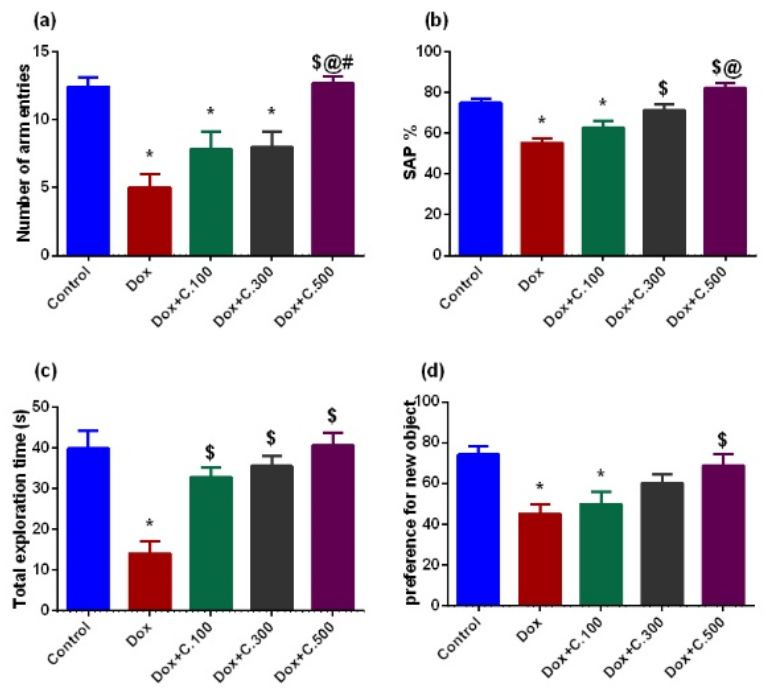
2.1.1. Behavioural Parameters
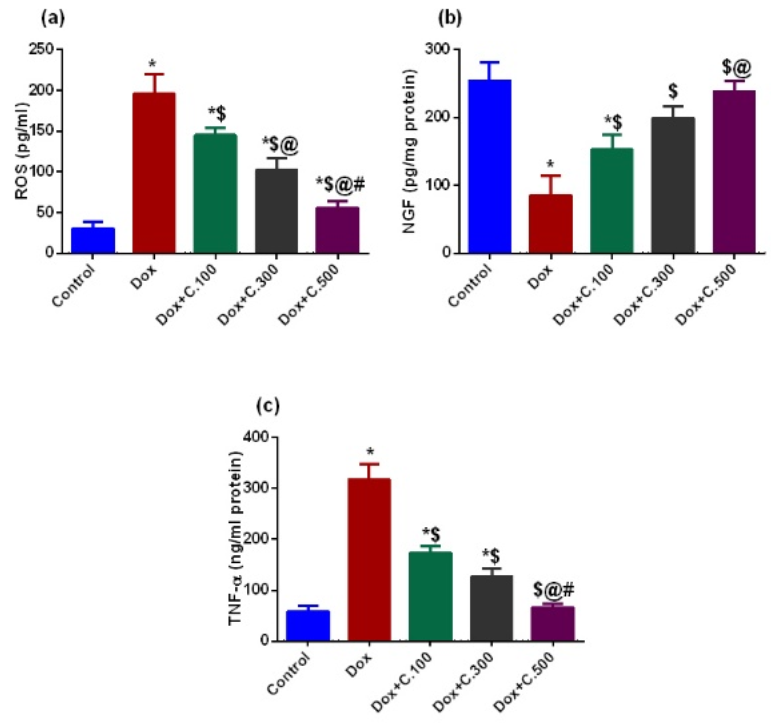
2.1.2. Biochemical Parameters
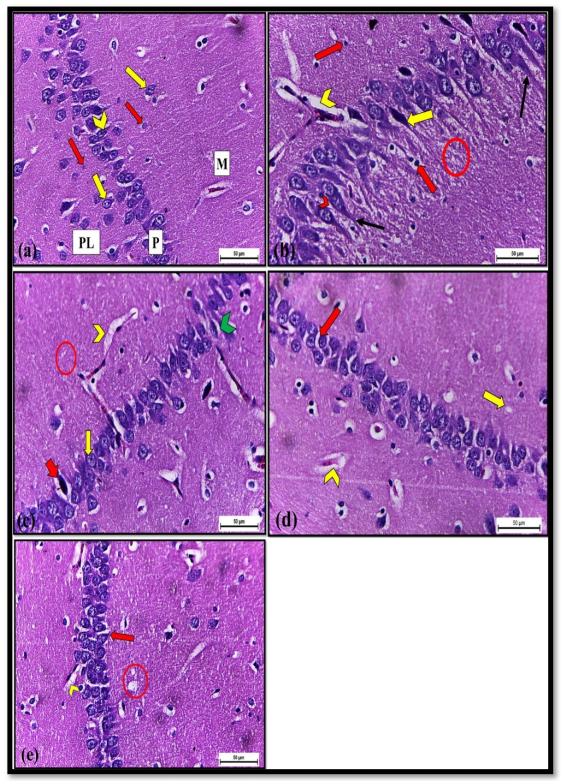
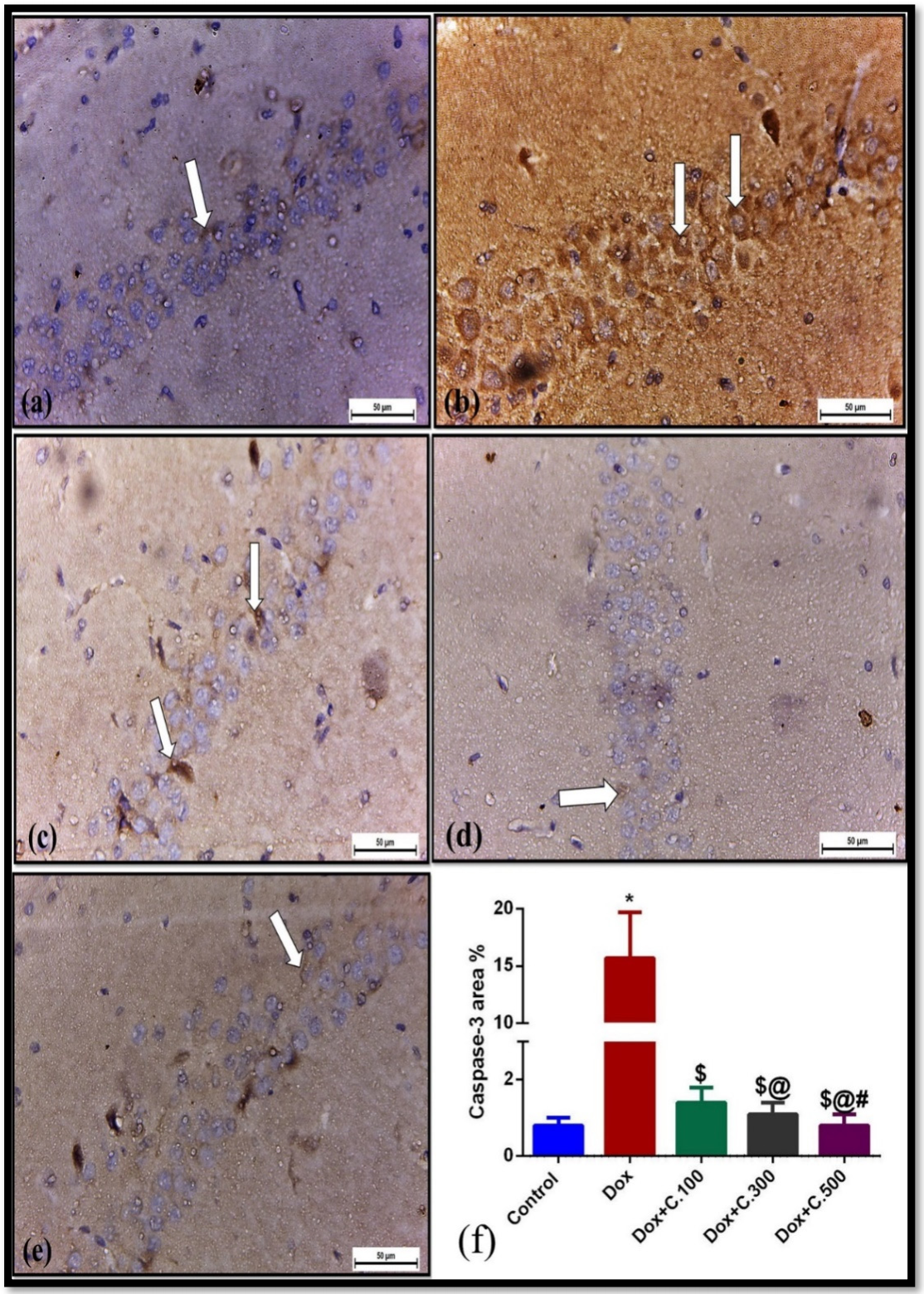
2.1.3. Histopathological and Immunohistochemical Investigations
Histological Examination
Immunohistochemical Examination for Caspase-3
2.2. UPLC-ESI-MS-MS Metabolites Characterization
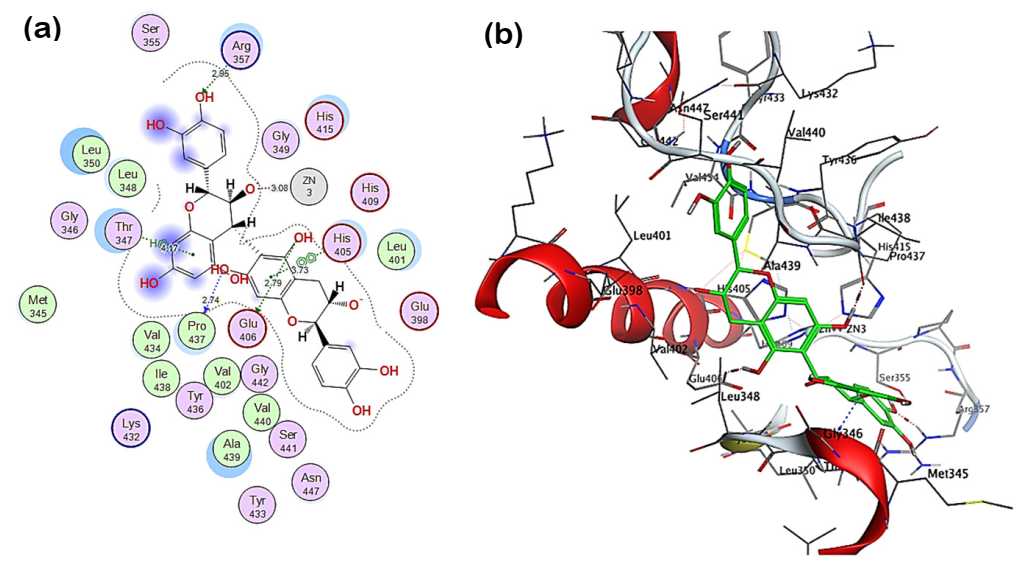
2.3. Molecular Docking of Polyphenolic Compounds against TACE
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Fractionation
4.3. In Vivo Evaluation of the C. macrocarpa Leaves Polar Fraction against Dox-Induced Neurotoxicity
4.3.1. Animals
4.3.2. Acute Toxicity Study
4.3.3. Experimental Design
4.3.4. Behavioural Tasks
4.3.5. Euthanasia and Sample Collection
4.3.6. Biochemical Parameters
Examination of Serum Levels of Reactive Oxygen Species (ROS)
Examination of TNF-α Pro-Inflammatory Mediator in the Brain Tissue
Estimation of Nerve Growth Factor (NGF) Levels in the Brain Tissue
4.3.7. Histopathological and Immunohistochemical Investigations
Histological Examination
Immunohistochemical Examination for Caspase-3
4.4. UPLC-ESI-MS/MS Analysis of C. macrocarpa Leaves Polar Fraction
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5874321/ (accessed on 7 July 2021). [CrossRef] [PubMed]
- Mohamed, R.H.; Karam, R.A.; Amer, M.G. Epicatechin attenuates doxorubicin-induced brain toxicity: Critical role of TNF-α, iNOS and NF-κB. Brain Res. Bull. 2011, 86, 22–28. [Google Scholar] [CrossRef]
- Liao, D.; Xiang, D.; Dang, R.; Xu, P.; Wang, J.; Han, W.; Fu, Y.; Yao, D.; Cao, L.; Jiang, P. Neuroprotective Effects of dl-3-n-Butylphthalide against Doxorubicin-Induced Neuroinflammation, Oxidative Stress, Endoplasmic Reticulum Stress, and Behavioral Changes. Oxid. Med. Cell. Longev. 2018, 2018, e9125601. [Google Scholar] [CrossRef] [Green Version]
- Rizk, H.A.; Masoud, M.A.; Maher, O.W. Prophylactic effects of ellagic acid and rosmarinic acid on doxorubicin-induced neurotoxicity in rats. J. Biochem. Mol. Toxicol. 2017, 31, e21977. [Google Scholar] [CrossRef]
- Yang, M.; Moon, C. Neurotoxicity of cancer chemotherapy. Neural Regen. Res. 2013, 8, 1606–1614. [Google Scholar]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [Green Version]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Amantagula Fruit (Carissa macrocarpa (Eckl.) A.DC.): Nutritional and Phytochemical Characterization. Plant Foods Hum. Nutr. 2019, 74, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Izhar, S.; Ahmed, D. Carissa opaca: A plant with great potential for future drugs for degenerative and infectious diseases. ChemistrySelect 2016, 1, 3005–3011. [Google Scholar] [CrossRef]
- Kaunda, J.S.; Zhang, Y.-J. The genus Carissa: An ethnopharmacological, phytochemical and pharmacological review. Nat. Prod. Bioprospect. 2017, 7, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Patel, S. Emerging Bioresources with Nutraceutical and Pharmaceutical Prospects; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Pewlong, W.; Sajjabut, S.; Eamsiri, J.; Chookaew, S. Evaluation of antioxidant activities, anthocyanins, total phenolic content, vitamin C content and cytotoxicity of Carissa carandas Linn. Chiang Mai Univ. J. Nat. Sci. Spec. Issue Food Appl. Biosci. 2014, 13, 509–517. [Google Scholar] [CrossRef]
- David, M.; Karekalammanavar, G. Spectrographic analysis and in vitro study of antibacterial, anticancer activity of aqueous ethanolic fruit extract of Carissa carandas. J. Adv. Sci. Res. 2015, 6, 10–13. [Google Scholar]
- Fanta, A.S.Y. Neuroprotective effect of Carissa edulis against d-galactose-induced neurotoxicity and memory impairment in rats. IBRO Rep. 2019, 7, 2. [Google Scholar] [CrossRef]
- Kareti, S.R.; Subash, P. In Silico Molecular Docking Analysis of Potential Anti-Alzheimer’s Compounds Present in Chloroform Extract of Carissa carandas Leaf Using Gas Chromatography MS/MS. Curr. Ther. Res. 2020, 93, 100615. [Google Scholar] [CrossRef]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimers Dis. 2020, 2020, e6372059. [Google Scholar] [CrossRef]
- Moodley, R.; Chenia, H.; Jonnalagadda, S.B.; Koorbanally, N. Antibacterial and anti-adhesion activity of the pentacyclic triterpenoids isolated from the leaves and edible fruits of Carissa macrocarpa. J. Med. Plants Res. 2011, 5, 4851–4858. [Google Scholar]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Phenolic profile and bioactive properties of carissa macrocarpa (Eckl.) A.DC.: An in vitro comparative study between leaves, stems, and flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef] [Green Version]
- Patel, S. Food, pharmaceutical and industrial potential of Carissa genus: An overview. Rev. Environ. Sci. Biotechnol. 2013, 12, 201–208. [Google Scholar] [CrossRef]
- Alakolanga, A.; Siriwardane, A.; Kumar, N.S.; Jayasinghe, L.; Jaiswal, R.; Kuhnert, N. LC-MSn identification and characterization of the phenolic compounds from the fruits of Flacourtia indica (Burm. F.) Merr. and Flacourtia inermis Roxb. Food Res. Int. 2014, 62, 388–396. [Google Scholar] [CrossRef]
- Jaiswal, R.; Matei, M.F.; Subedi, P.; Kuhnert, N. Does roasted coffee contain chlorogenic acid lactones or/and cinnamoylshikimate esters? Food Res. Int. 2014, 61, 214–227. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Verde, S.C.; Barros, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Effects of gamma irradiation on cytotoxicity and phenolic compounds of Thymus vulgaris L. and Mentha × piperita L. LWT Food Sci. Technol. 2016, 71, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Yuzuak, S.; Ballington, J.; Xie, D.-Y. HPLC-qTOF-MS/MS-based profiling of flavan-3-ols and dimeric proanthocyanidins in berries of two muscadine grape hybrids FLH 13-11 and FLH 17-66. Metabolites 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.A.; Al-Gendy, A.A.; Hamdan, D.I.; El-Shazly, A.M. Phytoconstituents, LC-ESI-MS Profile, Antioxidant and Antimicrobial Activities of Citrus x limon L. Burm. f. Cultivar Variegated Pink Lemon. J. Pharm. Sci. Res. 2017, 9, 375. [Google Scholar]
- Jiao, Q.-S.; Xu, L.-L.; Zhang, J.-Y.; Wang, Z.-J.; Jiang, Y.-Y.; Liu, B. Rapid characterization and identification of non-diterpenoid constituents in Tinospora sinensis by HPLC-LTQ-orbitrap MSn. Molecules 2018, 23, 274. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed. Res. Int. 2015, 2015, 1–11. [Google Scholar]
- Lin, L.-Z.; Chen, P.; Harnly, J.M. New phenolic components and chromatographic profiles of green and fermented teas. J. Agric. Food Chem. 2008, 56, 8130–8140. [Google Scholar] [CrossRef] [Green Version]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-De La Vega, H.; Hernández De La Cruz, O.N.; López-Camarillo, C. Dietary compounds as epigenetic modulating agents in cancer. Front. Genet. 2019, 10, 79. Available online: https://internal-journal.frontiersin.org/articles/10.3389/fgene.2019.00079/full (accessed on 8 May 2021). [CrossRef] [Green Version]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H. Bin Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kanemoto, E.; Sugimoto, M.; Machida, A.; Nakamura, Y.; Naito, N.; Kanzaki, H.; Miyazaki, I.; Asanuma, M.; Sendo, T. Influence of nicotine on doxorubicin and cyclophosphamide combination treatment-induced spatial cognitive impairment and anxiety-like behavior in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2017, 390, 369–378. [Google Scholar] [CrossRef]
- Alharbi, I.; Alharbi, H.; Almogbel, Y.; Alalwan, A.; Alhowail, A. Effect of Metformin on Doxorubicin-Induced Memory Dysfunction. Brain Sci. 2020, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Keeney, J.T.R.; Ren, X.; Warrier, G.; Noel, T.; Powell, D.K.; Brelsfoard, J.M.; Sultana, R.; Saatman, K.E.; St. Clair, D.K.; Butterfield, D.A. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: Protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget 2018, 9, 30324–30339. [Google Scholar] [CrossRef] [Green Version]
- Leung, W.S.; Kuo, W.W.; Ju, D.T.; Wang, T.D.; Shao-Tsu Chen, W.; Ho, T.J.; Lin, Y.M.; Mahalakshmi, B.; Lin, J.Y.; Huang, C.Y. Protective effects of diallyl trisulfide (DATS) against doxorubicin-induced inflammation and oxidative stress in the brain of rats. Free Radic. Biol. Med. 2020, 160, 141–148. [Google Scholar] [CrossRef]
- Tangpong, J.; Cole, M.P.; Sultana, R.; Joshi, G.; Estus, S.; Vore, M.; Clair, W.S.; Ratanachaiyavong, S.; Clair, D.K.S.; Butterfield, D.A. Adriamycin-induced, TNF-α-mediated central nervous system toxicity. Neurobiol. Dis. 2006, 23, 127–139. [Google Scholar] [CrossRef]
- Mohammed, W.I.; Radwan, R.A.; Elsayed, H.M. Prophylactic and Ameliorative Effect of N-Acetylcysteine on Doxorubicin-Induced Neurotoxicity in Wister Rats. Egypt. J. Basic Clin. Pharmacol. 2019, 9, 16. Available online: http://www.kenzpub.com/journals/ejbcp/2019/101396/ (accessed on 9 March 2021). [CrossRef]
- Galal, M.K.; Elleithy, E.M.M.; Abdrabou, M.I.; Yasin, N.A.E.; Shaheen, Y.M. Modulation of caspase-3 gene expression and protective effects of garlic and spirulina against CNS neurotoxicity induced by lead exposure in male rats. NeuroToxicology 2019, 72, 15–28. [Google Scholar] [CrossRef]
- Alkreathy, H.; Damanhouri, Z.A.; Ahmed, N.; Slevin, M.; Ali, S.S.; Osman, A.-M.M. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food Chem. Toxicol. 2010, 48, 951–956. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Mohammed, H.S. Protective effect of nanocurcumin against neurotoxicity induced by doxorubicin in rat’s brain. NeuroToxicology 2021, 85, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aluise, C.D.; Sultana, R.; Tangpong, J.; Vore, M.; Clair, D.S.; Moscow, J.A.; Butterfield, D.A. Chemo Brain (Chemo Fog) as a Potential Side Effect of Doxorubicin Administration: Role of Cytokine-Induced, Oxidative/Nitrosative Stress in Cognitive Dysfunction. In Chemo Fog: Cancer Chemotherapy-Related Cognitive Impairment; Advances in Experimental Medicine and Biology; Raffa, R.B., Tallarida, R.J., Eds.; Springer: New York, NY, USA, 2010; pp. 147–156. [Google Scholar] [CrossRef]
- Kuzu, M.; Kandemir, F.M.; Yildirim, S.; Kucukler, S.; Caglayan, C.; Turk, E. Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2018, 106, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Ginsberg, S.D.; Mahady, L.; Perez, S.E.; Massa, S.M.; Longo, F.M.; Ikonomovic, M.D. Nerve growth factor pathobiology during the progression of Alzheimer’s disease. Front. Neurosci. 2019, 13, 533. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2019.00533/full (accessed on 7 March 2021). [CrossRef] [PubMed]
- Iijima, Y.; Bandow, K.; Amano, S.; Sano, M.; Hino, S.; Kaneko, T.; Horie, N.; Sakagami, H. Protection of bortezomib-induced neurotoxicity by antioxidants. Anticancer Res. 2020, 40, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Udomruk, S.; Kaewmool, C.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Sesamin Promotes Neurite Outgrowth under Insufficient Nerve Growth Factor Condition in PC12 Cells through ERK1/2 Pathway and SIRT1 Modulation. Evid. Based Complement. Alternat. Med. 2020, 2020, e9145458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2013, 2013, 1–18. Available online: https://www.hindawi.com/journals/omcl/2013/891748/ (accessed on 8 November 2021). [CrossRef] [Green Version]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef]
- Menard, C.; Bastianetto, S.; Quirion, R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front. Cell Neurosci. 2013, 7, 281. Available online: https://www.frontiersin.org/articles/10.3389/fncel.2013.00281/full (accessed on 8 May 2021). [CrossRef] [Green Version]
- Sandoval-Avila, S.; Diaz, N.F.; Gómez-Pinedo, U.; Canales-Aguirre, A.A.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; Marquez-Aguirre, A.L.; Díaz-Martínez, N.E. Neuroprotective effects of phytochemicals on dopaminergic neuron cultures. Neurol. Engl. Ed. 2019, 34, 114–124. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. Available online: https://www.ingentaconnect.com/content/ben/cad/2011/00000007/00000002/art00008 (accessed on 12 March 2021). [CrossRef]
- Kumara, H.; Suhas, R.; Vardhan, D.M.S.; Shobha, M.; Gowda, D.C. A correlation study of biological activity and molecular docking of Asp and Glu linked bis-hydrazones of quinazolinones. RSC Adv. 2018, 8, 10644–10653. [Google Scholar] [CrossRef] [Green Version]
- Nawrot-Hadzik, I.; Matkowski, A.; Kubasiewicz-Ross, P.; Hadzik, J. Proanthocyanidins and Flavan-3-ols in the Prevention and Treatment of Periodontitis—Immunomodulatory Effects, Animal and Clinical Studies. Nutrients 2021, 13, 239. [Google Scholar] [CrossRef]
- Monteiro, A.F.M.; De Viana, J.O.; Nayarisseri, A.; Zondegoumba, E.N.; Mendonça Junior, F.J.B.; Scotti, M.T.; Scotti, L. Computational studies applied to flavonoids against Alzheimer’s and Parkinson’s diseases. Oxid. Med. Cell. Longev. 2018, 1–21. Available online: https://www.hindawi.com/journals/omcl/2018/7912765/ (accessed on 11 October 2021).
- Bose, S.; Datta, R.; Kirlin, W.G. Toxicity Studies Related to Medicinal Plants. In Evidence Based Validation of Traditional Medicines: A comprehensive Approach; Mandal, S.C., Chakraborty, R., Sen, S., Eds.; Springer: Singapore, 2021; pp. 621–647. [Google Scholar] [CrossRef]
- Banda, M.; Nyirenda, J.; Muzandu, K.; Sijumbila, G.; Mudenda, S. Antihyperglycemic and Antihyperlipidemic Effects of Aqueous Extracts of Lannea edulis in Alloxan-Induced Diabetic Rats. Front. Pharmacol. 2018, 9, 1099. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2018.01099/full (accessed on 7 January 2021). [CrossRef]
- Ramalingayya, G.V.; Nayak, P.G.; Shenoy, R.R.; Mallik, S.B.; Gourishetti, K.; Hussain, S.M.; Rao, C.M.; Nandakumar, K. Naringin ameliorates doxorubicin-induced neurotoxicity in vitro and cognitive dysfunction in vivo. Pharmacogn. Mag. 2018, 14, 197. [Google Scholar]
- Wu, Y.Q.; Dang, R.L.; Tang, M.M.; Cai, H.L.; De Li, H.; Liao, D.H.; He, X.; Cao, L.J.; Xue, Y.; Jiang, P. Long chain omega-3 polyunsaturated fatty acid supplementation alleviates doxorubicin-induced depressive-like behaviors and neurotoxicity in rats: Involvement of oxidative stress and neuroinflammation. Nutrients 2016, 8, 243. [Google Scholar] [CrossRef] [Green Version]
- Khalil, H.M.A.; Eliwa, H.A.; El-Shiekh, R.A.; Al-Mokaddem, A.K.; Hassan, M.; Tawfek, A.M.; El-Maadawy, W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021, 277, 114141. [Google Scholar] [CrossRef]
- Khalil, H.M.A.; Salama, H.H.; Al-Mokaddem, A.K.; Aljuaydi, S.H.; Edris, A.E. Edible dairy formula fortified with coconut oil for neuroprotection against aluminium chloride-induced Alzheimer’s disease in rats. J. Funct. Foods 2020, 75, 104296. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histology Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; pp. 83–134. [Google Scholar]
- Hsu, L.-K.P.; Haynes, H.W. Effective diffusivity by the gas chromatography technique: Analysis and application to measurements of diffusion of various hydrocarbons in zeolite NaY. AIChE J. 1981, 27, 81–91. [Google Scholar] [CrossRef]
- Hamdan, D.I.; Fayed, M.A.A.; Adel, R. Echinops taeckholmiana Amin: Optimization of a Tissue Culture Protocol, Biological Evaluation, and Chemical Profiling Using GC and LC-MS. ACS Omega 2021, 6, 13105–13115. [Google Scholar] [CrossRef]
- Anwar, S.; Mohammad, T.; Shamsi, A.; Queen, A.; Parveen, S.; Luqman, S.; Hasan, G.M.; Alamry, K.A.; Azum, N.; Asiri, A.M.; et al. Discovery of Hordenine as a Potential Inhibitor of Pyruvate Dehydrogenase Kinase 3: Implication in Lung Cancer Therapy. Biomedicines 2020, 8, 119. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
| Peak No. | Rt | [M−H]− | [M+H]+ | MS2 | Tentative Identification | References |
|---|---|---|---|---|---|---|
| 1 | 0.74 | 377.16 | 379.25 | 333, 271, 257, 163, 119 | Carinol | [20] |
| 2 | 1.87 | 353.16 | 355.13 | 191,179,161 | 3-O-Caffeolyquinic acid | [9] |
| 3 | 2.03 | 353.17 | - | 191,173,161 | 4-O-Caffeolyquinic acid | |
| 4 | 2.13 | 325.14 | - | 187, 163, 145 | Coumaroyl-β-glucose | [21] |
| 5 | 2.161 | 353.20 | - | 191,179,161 | 5-O-Caffeolyquinic acid | [22] |
| 6 | 2.24 | 577.27 | - | 425, 289 | Type B (epi)catechin dimer | [9] |
| 7 | 2.45 | 865.50 | - | 451, 425, 407, 289 | Type B (epi)catechin trimer | |
| 8 | 2.85 | 319.17 | - | 301, 275, 257, 231, 203,163, 119 | 5-O-p-Coumaroylshikimic acid | [23] |
| 9 | 2.87 | - | 343.18 | 326, 311, 285 | Caffeic acid 3-glucoside | [24] |
| 10 | 2.87 | 451.30 | - | 408, 393, 351, 337, 301, 273, 245 | Catechin-3-O-glucoside | [25] |
| 11 | 2.87 | 319.17 | - | 275, 257, 199, 163, 119 | 4-O-p-Coumaroylshikimic acid | [23] |
| 12 | 2.87 | 451.31 | - | 391, 343, 301, 287, 273, 247 | Epicatechin-3-O-glucoside | [25] |
| 13 | 3.00 | 289.09 | - | 245, 205, 203, 187, 179, 161 | (epi) Catechin | [26] |
| 14 | 5.05 | 755.44 | - | 593, 285 | Kaempferol-7-O-hexoside-3-O-rutinoside | [9] |
| 15 | 5.12 | 755.52 | - | 609, 301 | Quercetin-7-O-deoxyhexoside-3-O-deoxyhexosyl-hexoside | |
| 16 | 5.14 | 451.37 | - | 391, 343, 301, 287, 273, 247 | Epicatechin-3-O-glucoside isomer | [25] |
| 17 | 5.52 | 739.40 | - | 593, 285 | Kaempferol-7-O -deoxyhexoside-3-O -deoxyhexosyl-hexoside isomer 1 | [9] |
| 18 | 5.59 | 739.42 | - | 593, 285 | Kaempferol-7-O-deoxyhexoside-3-O-deoxyhexosyl-hexoside isomer 2 | |
| 19 | 5.59 | 609.27 | - | 301 | Quercetin-3-O-deoxyhexosyl-hexoside isomer 1 | |
| 20 | 5.75 | 609.33 | 611.29 | 465, 303 | Quercetin-3-O-deoxyhexosyl-hexoside isomer 2 | |
| 21 | 5.80 | 577.29 | - | 425, 289 | Type B (epi)catechin dimer | |
| 22 | 5.90 | 449.17 | - | 317, 316 | Myricetin-3-O-xyloside | [23] |
| 23 | 6.04 | - | 302.89 | 275, 257, 229, 215, 153 | Quercetin | |
| 24 | 6.13 | 593.33 | - | 557, 467, 441, 425, 407, 289 | (epi) Gallocatechin-(epi)catechin | [27] |
| 25 | 6.58 | 515.30 | - | 353, 179 | Dicaffeoylquinic acid | [22] |
| 26 | 6.80 | 136.94 | - | 109, 93 | Hydroxy benzoic acid | [28] |
| 27 | 7.01 | 593.33 | - | 557, 467, 441, 425, 407, 289 | (epi) gallocatechin-(epi)catechin | [27] |
| 28 | 8.12 | 196.93 | - | 120, 104, 93, 87 | Syringic acid | [28] |
| 29 | 9.14 | 573.70 | - | 397, 223, 173 | Feruloyl-O-sinapoylquinic acid | [22] |
| 30 | 9.94 | 939.06 | - | 778, 735, 732, 717, 571 | Diacetoxy-5-methoxyphenyl) acroyl-O-p-coumaroyl-O-caffeoylquinic acid derivative | [23] |
| 31 | 13.26 | 577.48 | - | 425, 289 | (epi) Catechin dimer | [9] |
| 32 | 13.30 | 543.56 | - | 353, 173 | Dimethoxycinnamoyl-O-caffeoylquinic acid | [22] |
| 33 | 15.66 | 543.33 | - | 353, 173 | Dimethoxycinnamoyl-O-caffeoylquinic acid isomer | |
| 34 | 16.52 | 352.99 | - | 179,161 | 3-O-Caffeoylshikimic acid | |
| 35 | 16.59 | - | 383.25 | 369, 351, 195 | Dimethoxycinnamoylquinic acid | |
| 36 | 18.58 | 455.47 | - | 439, 419, 411, 410, 407, 397 | Ursolic acid | [11,29] |
| 37 | 19.06 | 455.46 | - | 439, 419, 411, 410, 407, 397 | Carissic acid (isomer of ursolic acid) | |
| 38 | 19.16 | 455.50 | 457.43 | 439, 419, 411, 410, 407, 397 | Oleanolic acid | |
| 39 | 25.30 | - | 413.31 | 395, 256, 214 | Stigmasterol | [30] |
| 40 | 27.02 | - | 465.45 | 301, 300, 257, 255, 229, 179. 151 | Hyperoside | [31] |
| 41 | 27.21 | - | 465.42 | 301, 300, 257, 255, 229, 179. 151 | Isoquercetin | |
| 42 | 27.31 | 621.68 | - | 501 | 2(R)-26-([(2E)-3-(4-hydroxy-3-methoxyphenyl)-1-oxo-2- propen-1-yl]oxy)-2,3-dihydroxypropyl ester | [10] |
| 43 | 31.25 | 429. 31 | 430.92 | 205, 191, 177, 149, 121 | α-Tocopherol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabi, M.A.A.; Khalil, H.M.A.; Abouelela, M.E.; Zaafar, D.; Ahmed, Y.H.; Naggar, R.A.; Alyami, H.S.; Abdel-Sattar, E.-S.; Matsunami, K.; Hamdan, D.I. Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers. Pharmaceuticals 2021, 14, 1305. https://doi.org/10.3390/ph14121305
Orabi MAA, Khalil HMA, Abouelela ME, Zaafar D, Ahmed YH, Naggar RA, Alyami HS, Abdel-Sattar E-S, Matsunami K, Hamdan DI. Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers. Pharmaceuticals. 2021; 14(12):1305. https://doi.org/10.3390/ph14121305
Chicago/Turabian StyleOrabi, Mohamed A. A., Heba M. A. Khalil, Mohamed E. Abouelela, Dalia Zaafar, Yasmine H. Ahmed, Reham A. Naggar, Hamad S. Alyami, El-Shaymaa Abdel-Sattar, Katsuyoshi Matsunami, and Dalia I. Hamdan. 2021. "Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers" Pharmaceuticals 14, no. 12: 1305. https://doi.org/10.3390/ph14121305
APA StyleOrabi, M. A. A., Khalil, H. M. A., Abouelela, M. E., Zaafar, D., Ahmed, Y. H., Naggar, R. A., Alyami, H. S., Abdel-Sattar, E.-S., Matsunami, K., & Hamdan, D. I. (2021). Carissa macrocarpa Leaves Polar Fraction Ameliorates Doxorubicin-Induced Neurotoxicity in Rats via Downregulating the Oxidative Stress and Inflammatory Markers. Pharmaceuticals, 14(12), 1305. https://doi.org/10.3390/ph14121305