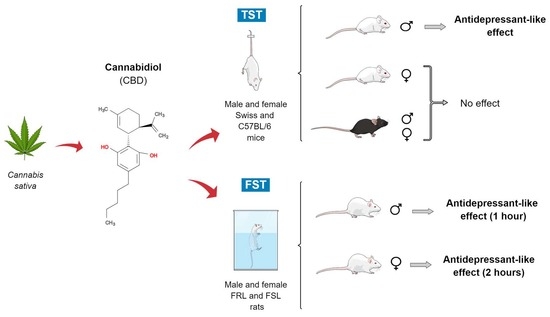
Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol
Abstract
1. Introduction
2. Results
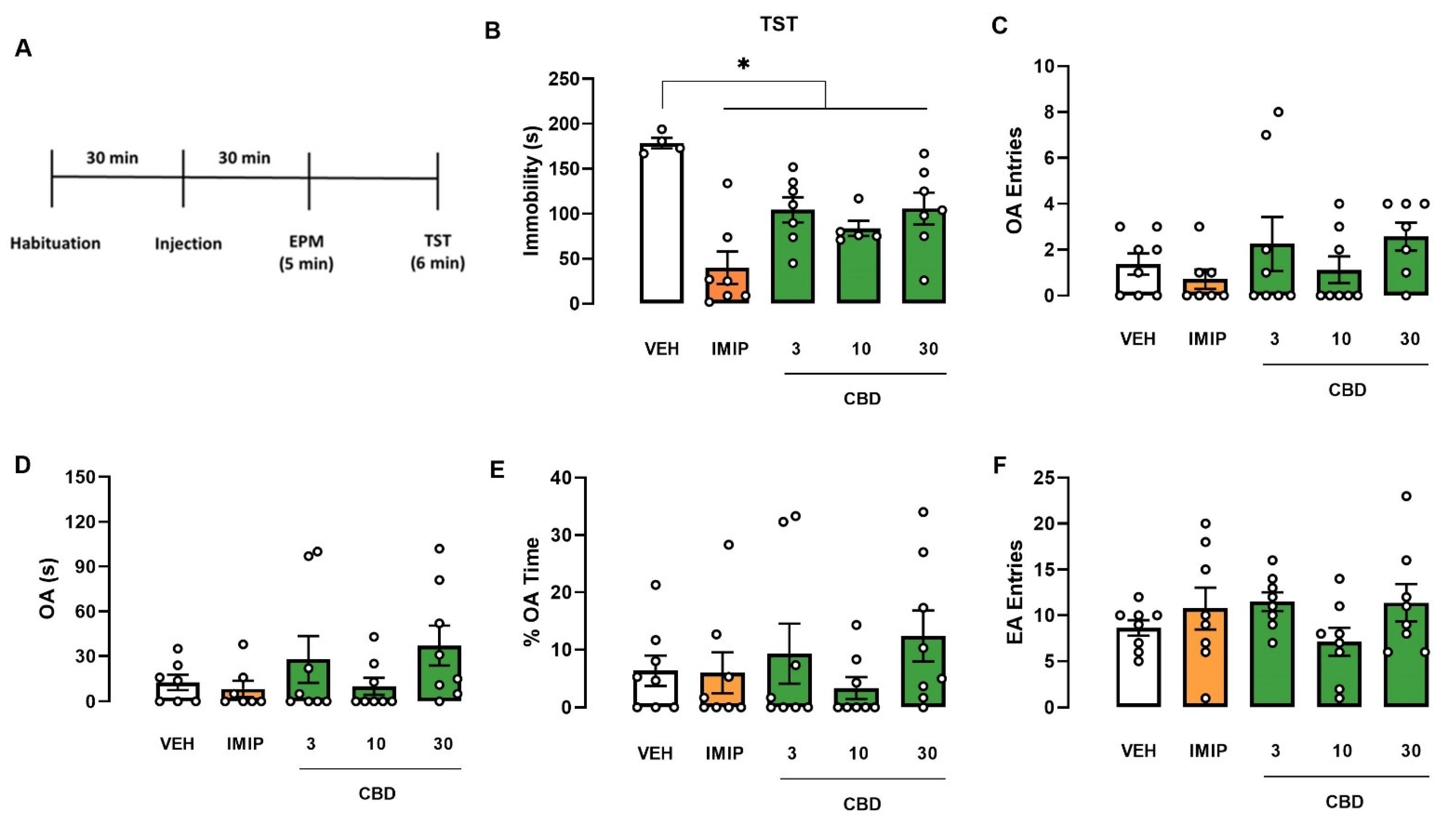
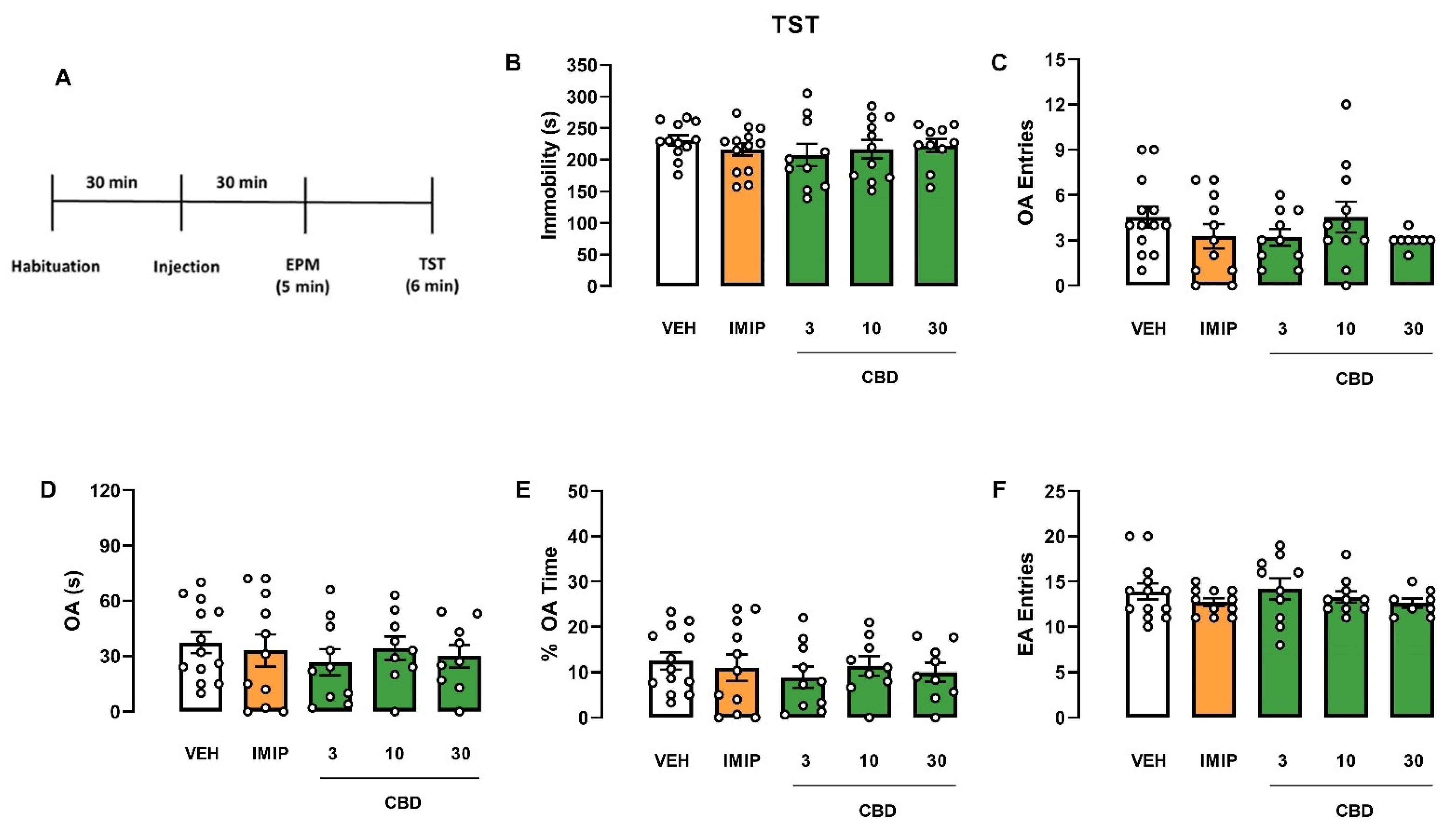
2.1. Swiss and C57BL/6 Mice
CBD Effects in Male and Female Swiss and C57BL/6 Mice Submitted to the Elevated Plus Maze (EPM) and Tail Suspension Test (TST)
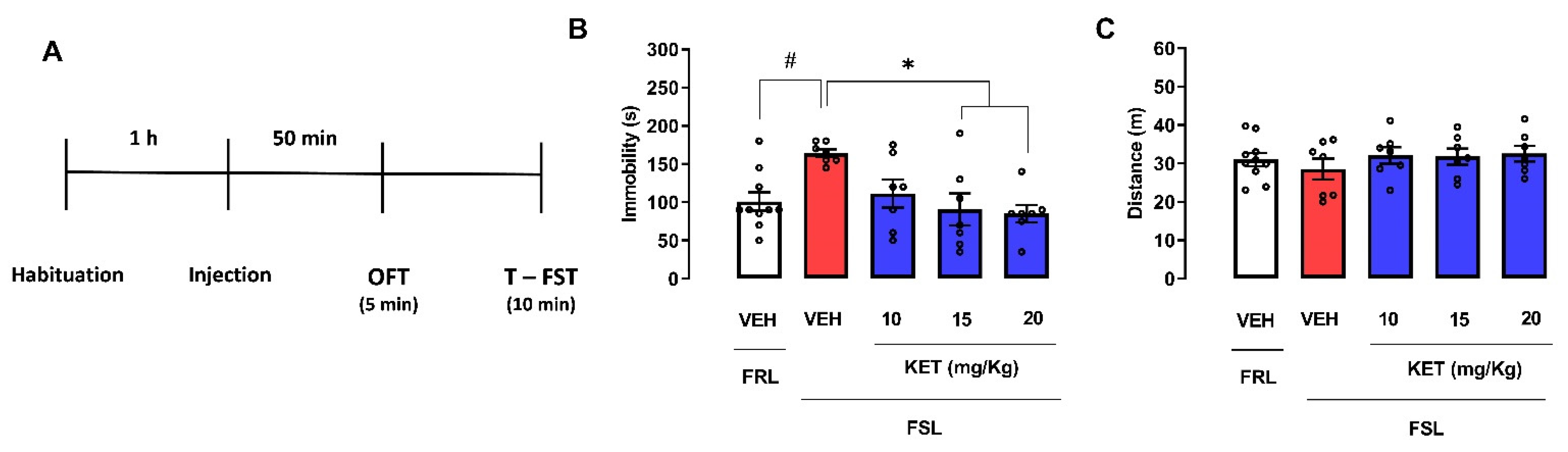
2.2. FSL Rats
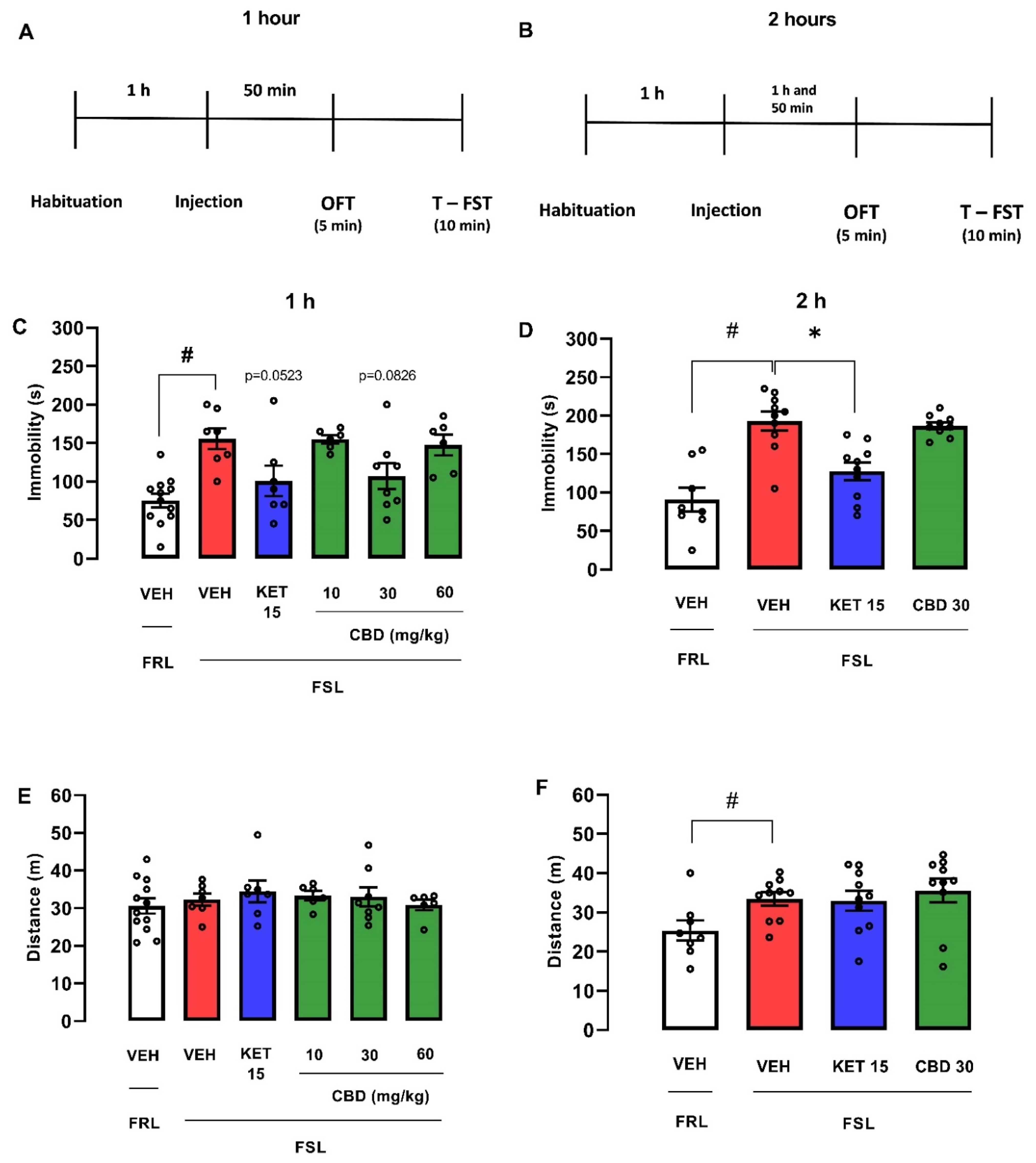
2.2.1. Dose-Response Curve of Ketamine in Female FSL Rats Exposed to the OFT/FST
2.2.2. Effect Produced by CBD Administered 1 or 2 h before the OFT/FST in Male and Female FSL Rats
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Methods and Experimental Design
4.3.1. Mice
Experiments 1 and 2—CBD Effects in Male and Female Mice Exposed to EPM and TST
4.3.2. Flinders Sensitive Line (FSL) Rats
Experiment 3—CBD Effect 1 and 2 h before OFT/FST in Male FSL Rats
Experiment 4—Dose-Response Curves with Ketamine in Female FSL Rats Exposed to OFT/FST
Experiment 5—CBD Effect 1 and 2 h before OFT/FST in Female FSL Rats
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- APA. Diagnostic and Statictical Manual of Mental Disorders; American Psychiatric Association: New York, NY, USA, 2013; ISBN 9780890425541. [Google Scholar]
- WHO. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Investing in Treatment for Depression and Anxiety Leads to Fourfold Return; WHO: Geneva, Switzerland, 2017; pp. 1–3. [Google Scholar]
- Hasin, D.S.; Sarvet, A.L.; Meyers, J.L.; Saha, T.D.; Ruan, W.J.; Stohl, M.; Grant, B.F. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018, 75, 336–346. [Google Scholar] [CrossRef]
- Kessler, R.; Bromet, E. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef]
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.U.; Jönsson, B. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012, 19, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Herzog, D.P.; Wegener, G.; Lieb, K.; Müller, M.B.; Treccani, G. Decoding the mechanism of action of rapid-acting antidepressant treatment strategies: Does gender matter? Int. J. Mol. Sci. 2019, 20, 949. [Google Scholar] [CrossRef]
- Will, T.R.; Proaño, S.B.; Thomas, A.M.; Kunz, L.M.; Thompson, K.C.; Ginnari, L.A.; Jones, C.H.; Lucas, S.C.; Reavis, E.M.; Dorris, D.M.; et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Zakiniaeiz, Y.; Cosgrove, K.P.; Potenza, M.N.; Mazure, C.M. Balance of the sexes: Addressing sex differences in preclinical research. Yale J. Biol. Med. 2016, 89, 255–259. [Google Scholar]
- Karp, N.A.; Reavey, N. Sex bias in preclinical research and an exploration of how to change the status quo. Br. J. Pharmacol. 2019, 176, 4107–4118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018, 51, 167–173. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Perez-Reyes, M.; Timmons, M.C.; Davis, K.H.; Wall, E.M. A comparison of the pharmacological activity in man of intravenously administered Δ9- Tetrahydrocannabinol, cannabinol, and cannabidiol. Experientia 1973, 29, 1368–1369. [Google Scholar] [CrossRef] [PubMed]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S. Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J. Chem. Neuroanat. 2019, 98, 104–116. [Google Scholar] [CrossRef]
- Vitale, R.M.; Iannotti, F.A.; Amodeo, P. The (Poly)pharmacology of cannabidiol in neurological and neuropsychiatric disorders: Molecular mechanisms and targets. Int. J. Mol. Sci. 2021, 22, 4876. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Bonaccorso, S.; Ricciardi, A.; Zangani, C.; Chiappini, S.; Schifano, F. Cannabidiol (CBD) use in psychiatric disorders: A systematic review. Neurotoxicology 2019, 74, 282–298. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef]
- El-Alfy, A.T.; Ivey, K.; Robinson, K.; Ahmed, S.; Radwan, M.; Slade, D.; Khan, I.; ElSohly, M.; Ross, S. Antidepressant-like effect of delta9-tetrahydrocannabinol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Biochem. Behav. 2010, 95, 434–442. [Google Scholar] [CrossRef]
- Réus, G.Z.; Stringari, R.B.; Ribeiro, K.F.; Luft, T.; Abelaira, H.M.; Fries, G.R.; Aguiar, B.W.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; et al. Administration of cannabidiol and imipramine induces antidepressant-like effects in the forced swimming test and increases brain-derived neurotrophic factor levels in the rat amygdala. Acta Neuropsychiatr. 2011, 23, 241–248. [Google Scholar] [CrossRef]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2018, 56, 1070–1081. [Google Scholar] [CrossRef]
- Sales, A.J.; Crestani, C.C.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zanelati, T.V.; Biojone, C.; Moreira, F.A.; Guimaraes, F.S.; Joca, S.R.L. Antidepressant-like effects of cannabidiol in mice: Possible involvement of 5-HT 1A receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef]
- Xu, C.; Chang, T.; Du, Y.; Yu, C.; Tan, X.; Li, X. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like e ff ects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 2019, 70, 103202. [Google Scholar] [CrossRef]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of chronic cannabidiol treatment in the rat chronic unpredictable mild stress model of depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef]
- Shoval, G.; Shbiro, L.; Hershkovitz, L.; Hazut, N.; Zalsman, G.; Mechoulam, R.; Weller, A. Prohedonic effect of cannabidiol in a rat model of depression. Neuropsychobiology 2016, 73, 123–129. [Google Scholar] [CrossRef]
- Shbiro, L.; Hen-shoval, D.; Hazut, N.; Rapps, K.; Dar, S.; Zalsman, G.; Mechoulam, R.; Weller, A.; Shoval, G. Effects of cannabidiol in males and females in two different rat models of depression. Physiol. Behav. 2019, 201, 59–63. [Google Scholar] [CrossRef]
- Bambico, F.R.; Katz, N.; Debonnel, G.; Gobbi, G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 2007, 27, 11700–11711. [Google Scholar] [CrossRef]
- Kirkedal, C.; Wegener, G.; Moreira, F.; Joca, S.R.L.; Liebenberg, N. A dual inhibitor of FAAH and TRPV1 channels shows dose-dependent effect on depression-like behaviour in rats. Acta Neuropsychiatr. 2017, 29, 324–329. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zou, J.; Wu, R.; Yang, Y.; Tai, F. Strain and Sex Differences in Anxiety-Like and Social Behaviors in C57BL/6J and BALB/cJ Mice. Exp. Anim. 2011, 60, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Li, X.; Clay, M.; Lindstrom, T.; Skolnick, P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol. Biochem. Behav. 2001, 70, 187–192. [Google Scholar] [CrossRef]
- David, D.J.P.; Renard, C.E.; Jolliet, P.; Hascoët, M.; Bourin, M. Antidepressant-like effects in various mice strains in the forced swimming test. Psychopharmacology 2003, 166, 373–382. [Google Scholar] [CrossRef]
- Liu, X.; Gershenfeld, H.K. Genetic differences in the tail-suspension test and its relationship to imipramine response among 11 inbred strains of mice. Biol. Psychiatry 2001, 49, 575–581. [Google Scholar] [CrossRef]
- Marchette, R.C.N.; Bicca, M.A.; Santos, E.C.D.S.; de Lima, T.C.M. Distinctive stress sensitivity and anxiety-like behavior in female mice: Strain differences matter. Neurobiol. Stress 2018, 9, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, N.; Joseph, D.; David, P.; Dailly, E.; Hascoët, M.; Bourin, M. Antidepressant-like effects in various mice strains in the tail suspension test. Behav. Brain Res. 2003, 143, 193–200. [Google Scholar] [CrossRef]
- Võikar, V.; Kõks, S.; Vasar, E.; Rauvala, H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 2001, 72, 271–281. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Jalfre, M. “Behavioural despair” in rats and mice: Strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978, 51, 291–294. [Google Scholar] [CrossRef]
- Liu, X.; Gershenfeld, H.K. An exploratory factor analysis of the Tail Suspension Test in 12 inbred strains of mice and an F2 intercross. Brain Res. Bull. 2003, 60, 223–231. [Google Scholar] [CrossRef]
- Sales, A.J.; Guimarães, F.S.; Joca, S.R.L. CBD modulates DNA methylation in the prefrontal cortex and hippocampus of mice exposed to forced swim. Behav. Brain Res. 2020, 388, 112627. [Google Scholar] [CrossRef]
- Campos, A.C.; Ortega, Z.; Palazuelos, J.; Fogaca, M.V.; Aguiar, D.C.; Diaz-Alonso, J.; Ortega-Gutierrez, S.; Vazquez-Villa, H.; Moreira, F.A.; Guzmán, M.; et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int. J. Neuropsychopharmacol. 2013, 16, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Fogaça, M.V.; Campos, A.C.; Coelho, L.D.; Duman, R.S.; Guimarães, F.S. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018, 135, 22–33. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Shoji, H.; Takao, K.; Hattori, S.; Miyakawa, T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol. Brain 2016, 9, 11. [Google Scholar] [CrossRef]
- Mason, S.S.; Baker, K.B.; Davis, K.W.; Pogorelov, V.M.; Malbari, M.M.; Ritter, R.; Wray, S.P.; Gerhardt, B.; Lanthorn, T.H.; Savelieva, K.V. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur. J. Pharmacol. 2009, 602, 306–315. [Google Scholar] [CrossRef]
- Mitchell, N.C.; Koek, W.; Daws, L.C.; Antonio, S.; Antonio, S.; Antonio, S. Antidepressant-like effects and basal immobility depend on age and serotonin transporter genotype. Genes Brain Behav. 2015, 14, 543–549. [Google Scholar] [CrossRef]
- Wang, S.; Huang, G.; Yan, J.; Li, C.; Feng, J.; Chen, Q.; Zheng, X.; Li, H.; Li, J.; Wang, L.; et al. Influence of aging on chronic unpredictable mild stress-induced depression-like behavior in male C57BL/6J mice. Behav. Brain Res. 2021, 414, 113486. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ 9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behav. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Kasten, C.R.; Zhang, Y.; Boehm, S.L. Acute cannabinoids produce robust anxiety-like and locomotor effects in mice, but long-term consequences are age- And sex-dependent. Front. Behav. Neurosci. 2019, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Long, L.E.; Chesworth, R.; Huang, X.F.; McGregor, I.S.; Arnold, J.C.; Karl, T. A behavioural comparison of acute and chronic 9- tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 2010, 13, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Lucki, I.; Dalvi, A.; Mayorga, A.J. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology 2001, 155, 315–322. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, X.; Ran, Y.; Li, X.; Xiong, J.; Zheng, Y. Mouse strain differences in SSRI sensitivity correlate with serotonin transporter binding and function. Sci. Rep. 2017, 7, 8631. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Lavanco, G.; Maurel, O.M.; Gulisano, W.; Laudani, S.; Geraci, F.; Grasso, M.; Barbagallo, C.; Caraci, F.; Bucolo, C.; et al. A novel arousal-based individual screening reveals susceptibility and resilience to PTSD-like phenotypes in mice. Neurobiol. Stress 2021, 14, 100286. [Google Scholar] [CrossRef]
- Pascual Cuadrado, D.; Todorov, H.; Lerner, R.; Islami, L.; Bindila, L.; Gerber, S.; Lutz, B. Long-term molecular differences between resilient and susceptible mice after a single traumatic exposure. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Fernández-Guasti, A.; Olivares-Nazario, M.; Reyes, R.; Martínez-Mota, L. Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim test. Pharmacol. Biochem. Behav. 2017, 152, 81–89. [Google Scholar] [CrossRef]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-nai¨ve and ‘“depressed”’ mice exposed to chronic mild stress. Neuroscience 2015, 290, 49–60. [Google Scholar] [CrossRef]
- Gómez, M.L.; Martínez-Mota, L.; Estrada-Camarena, E.; Fernández-Guasti, A. Influence of the brain sexual differentiation process on despair and antidepressant-like effect of fluoxetine in the rat forced swim test. Neuroscience 2014, 261, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.; Ryan, C.; Curley, A.; Mulcaire, J.; Kelly, J.P. Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 37, 227–236. [Google Scholar] [CrossRef]
- Wright, K.N.; Kabbaj, M. Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr. Opin. Behav. Sci. 2018, 23, 36–41. [Google Scholar] [CrossRef]
- Melo, A.; Kokras, N.; Dalla, C.; Ferreira, C.; Ventura-Silva, A.P.; Sousa, N.; Pego, J.M. The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl. Psychiatry 2015, 5, e573. [Google Scholar] [CrossRef][Green Version]
- Carrier, N.; Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Green, M.R.; Martin, B.R. Pharmacological Characterization of Cannabinoids in the Elevated Plus Maze. J. Pharmacol. Exp. Ther. 1990, 253, 1002–1009. [Google Scholar] [PubMed]
- Liu, J.; Scott, B.W.; Burnham, W.M. Effects of cannabidiol and Δ9-tetrahydrocannabinol in the elevated plus maze in mice. Behav Pharmacol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Charney, D. Comorbidity of mood and anxiety disorders. Depress. Anxiety 2000, 12, 69–76. [Google Scholar] [CrossRef]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef]
- de Morais, H.; Chaves, Y.C.; Waltrick, A.P.F.; Jesus, C.H.A.; Genaro, K.; Crippa, J.A.; da Cunha, J.M.; Zanoveli, J.M. Sub-chronic treatment with cannabidiol but not with URB597 induced a mild antidepressant-like effect in diabetic rats. Neurosci. Lett. 2018, 682, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Shvo, Y. The structure of cannabidiol. Tetrahedrom 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef]
- Harvey, D.J. Absorption, Distribution, and Biotransformation of the Cannabinoids. Marihuana Med. 1999, 91–103. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- McGilveray, I.J. Pharmacokinetics of cannabinoids. Pain Res. Manag. 2005, 10, 15A–22A. [Google Scholar] [CrossRef]
- Harvey, D.J.; Samara, E.; Mechoulam, R. Comparative metabolism of cannabidiol in dog, rat and man. Pharmacol. Biochem. Behav. 1991, 40, 523–532. [Google Scholar] [CrossRef]
- Harvey, D.J.; Brown, N.K. Comparative in vitro metabolism of the cannabinoids. Pharmacol. Biochem. Behav. 1991, 40, 533–540. [Google Scholar] [CrossRef]
- Samara, E.; Bialer, M.; Harvey, D.J. Metabolism of cannabidiol by the rat. Eur. J. Drug Metab. Pharmacokinet. 1991, 16, 305–313. [Google Scholar] [CrossRef]
- Narimatsu, S.; Watanabe, K.; Yamamoto, I.; Yoshimura, H. Sex difference in the oxidative metabolism of Delta9-tetrahydrocannabinol in the rat. Biochem. Pharmacol. 1991, 41, 1187–1194. [Google Scholar] [CrossRef]
- Wiley, J.L.; Burston, J.J. Sex differences in δ9-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci. Lett. 2014, 576, 51–55. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Rimmerman, N.; Krey, J.F.; Walker, J.M. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, 349–358. [Google Scholar] [CrossRef]
- Carlsson, M.; Carlsson, A. A regional study of sex differences in rat brain serotonin. Prog. Neuropsychopharmacol. Biol. Psychiatry 1988, 12, 53–61. [Google Scholar] [CrossRef]
- de Fonseca, F.R.; Cebeira, M.; Ramos, J.A.; Martín, M.; Fernández-Ruiz, J.J. Cannabinoid receptors in rat brain areas: Sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci. 1994, 54, 159–170. [Google Scholar] [CrossRef]
- Riebe, C.J.N.; Hill, M.N.; Lee, T.T.Y.; Hillard, C.J.; Gorzalka, B.B. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 2010, 35, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Fischette, C.T.; Biegon, A.; McEwen, B.S. Sex differences in serotonin 1 receptor binding in rat brain. Science 1983, 222, 333–335. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, W.; Barker, J.L.; Rubinow, D.R. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: A possible role of testosterone. Neuroscience 1999, 94, 251–259. [Google Scholar] [CrossRef]
- Carrier, N.; Wang, X.; Sun, L.; Lu, X.Y. Sex-specific and estrous cycle-dependent antidepressant-like effects and hippocampal Akt signaling of leptin. Endocrinology 2015, 156, 3695–3705. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.; Peng, H.Y.; Lin, T.B.; Lai, C.Y.; Hsieh, M.C.; Wen, Y.C.; Lee, A.S.; Wang, H.H.; Yang, P.S.; Chen, G.D.; et al. (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 2018, 139, 1–12. [Google Scholar] [CrossRef]
- Rincón-Cortés, M.; Grace, A.A. Sex-dependent effects of stress on immobility behavior and VTA dopamine neuron activity: Modulation by ketamine. Int. J. Neuropsychopharmacol. 2017, 20, 823–832. [Google Scholar] [CrossRef]
- Sarkar, A.; Kabbaj, M. Sex differences in effects of ketamine on behavior, spine density and synaptic proteins in socially isolated rats. Biol. Psychiatry 2017, 80, 448–456. [Google Scholar] [CrossRef]
- Tizabi, Y.; Bhatti, B.H.; Manaye, K.F.; Das, J.R.; Akinfiresoye, L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience 2012, 213, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Elfving, B.; Rafati, A.H.; Mansouri, M.; Zarate, C.A.; Mathe, A.A.; Wegener, G. Rapid effects of S-ketamine on the morphology of hippocampal astrocytes and BDNF serum levels in a sex-dependent manner. Eur. Neuropsychopharmacol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Consoli, D.; Fedotova, J.; Micale, V.; Sapronov, N.S.; Drago, F. Stressors affect the response of male and female rats to clomipramine in a model of behavioral despair (forced swim test). Eur. J. Pharmacol. 2005, 520, 100–107. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, D.; Sadananda, M. Estrous cycle phase-dependent changes in anxiety-and depression-like profiles in the late adolescent Wistar-Kyoto rat. Ann. Neurosci. 2017, 24, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Jaric, I.; Rocks, D.; Cham, H.; Herchek, A.; Kundakovic, M. Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model. Front. Mol. Neurosci. 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lébron-Milad, K.; Tsareva, A.; Ahmed, N.; Milad, M.R. Sex differences and estrous cycle in female rats interact with the effects of fluoxetine treatment on fear extinction. Behav. Brain Res. 2013, 253, 217–222. [Google Scholar] [CrossRef]
- Eskelund, A.; Budac, D.P.; Sanchez, C.; Elfving, B.; Wegener, G. Female flinders sensitive line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 2016, 329, 337–348. [Google Scholar] [CrossRef]
- Stukalin, Y.; Lan, A.; Einat, H. Revisiting the validity of the mouse tail suspension test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neurosci. Biobehav. Rev. 2020, 112, 39–47. [Google Scholar] [CrossRef]
- Griebel, G.; Belzung, C.; Perrault, G.; Sanger, D.J. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology 2000, 148, 164–170. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, e1088. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychohparmacology 1987, 92, 180–185. [Google Scholar] [CrossRef]
- Liebenberg, N.; Joca, S.; Wegener, G. Nitric oxide involvement in the antidepressant-like effect of ketamine in the Flinders sensitive line rat model of depression. Acta Neuropsychiatr. 2014, 27, 90–96. [Google Scholar] [CrossRef]
- Monleon, S.; D’Aquila, P.S.; Parra, A.; Simon, V.M.; Brain, P.F.; Willner, P. Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology 1995, 117, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bespalov, A.; Michel, M.C.; Steckler, T. Good Research Practice in Pharmacology and Biomedicine; Springer: Cham, Switzerland, 2020; ISBN 9783030336554. [Google Scholar]
- Carobrez, A.P.; Bertoglio, L.J. Ethological and temporal analyses of anxiety-like behavior: The elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev. 2005, 29, 1193–1205. [Google Scholar] [CrossRef]
- Sales, A.J.; Biojone, C.; Terceti, M.S.; Guimarães, F.S.; Gomes, M.V.M.; Joca, S.R.L. Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors. Br. J. Pharmacol. 2011, 164, 1711–1721. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2011, e3769. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Müller, H.K.; Elfving, B.; Eskelund, A.; Joca, S.R.L.; Wegener, G. Antidepressant-like effect induced by P2×7 receptor blockade in FSL rats is associated with BDNF signalling activation. J. Psychopharmacol. 2019, 33, 1436–1446. [Google Scholar] [CrossRef]
- Pereira, V.S.; Joca, S.R.L.; Harvey, B.H.; Elfving, B.; Wegener, G. Esketamine and rapastinel, but not imipramine, have antidepressant-like effect in a treatment-resistant animal model of depression. Acta Neuropsychiatr. 2019, 31, 258–265. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex Differ. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A felixble statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, S.; Happ, D.F.; Mikkelsen, P.F.; Geisel, J.; Wegener, G.; Obeid, R. Behavioral and metabolic effects of S-adenosylmethionine and imipramine in the Flinders Sensitive Line rat model of depression. Behav. Brain Res. 2019, 364, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Silote, G.P.; Gatto, M.C.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Strain, sex, and time dependent antidepressant-like effects of cannabidiol. Preprints 2021, 2021100347. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silote, G.P.; Gatto, M.C.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S.R.L. Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol. Pharmaceuticals 2021, 14, 1269. https://doi.org/10.3390/ph14121269
Silote GP, Gatto MC, Eskelund A, Guimarães FS, Wegener G, Joca SRL. Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol. Pharmaceuticals. 2021; 14(12):1269. https://doi.org/10.3390/ph14121269
Chicago/Turabian StyleSilote, Gabriela P., Michelle C. Gatto, Amanda Eskelund, Francisco S. Guimarães, Gregers Wegener, and Sâmia R. L. Joca. 2021. "Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol" Pharmaceuticals 14, no. 12: 1269. https://doi.org/10.3390/ph14121269
APA StyleSilote, G. P., Gatto, M. C., Eskelund, A., Guimarães, F. S., Wegener, G., & Joca, S. R. L. (2021). Strain-, Sex-, and Time-Dependent Antidepressant-like Effects of Cannabidiol. Pharmaceuticals, 14(12), 1269. https://doi.org/10.3390/ph14121269