Humanization, Radiolabeling and Biodistribution Studies of an IgG1-Type Antibody Targeting Uncomplexed PSA for Theranostic Applications
Abstract
1. Introduction
2. Result
2.1. Humanization and Characterizations
2.2. Binding Kinetics and Affinity of Immunoconjugates
2.3. Radiolabeling and Stability Results
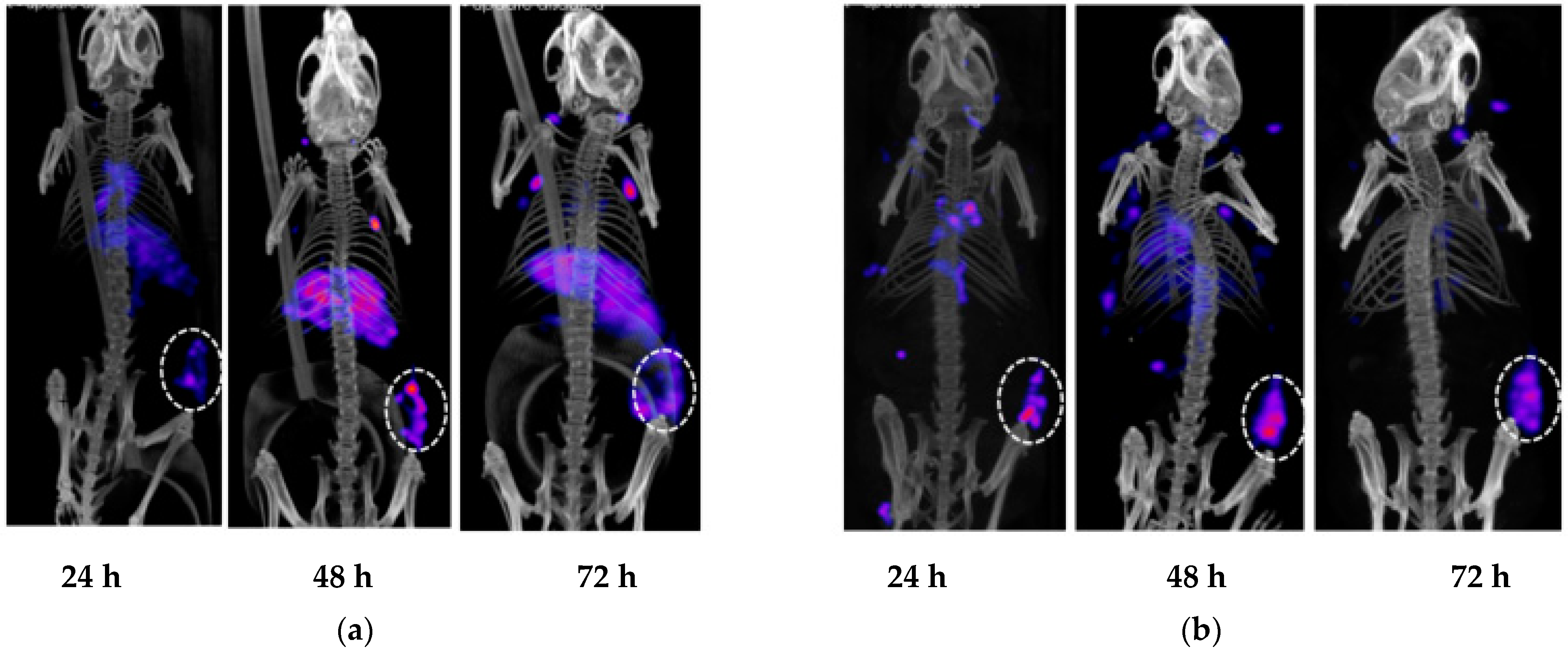
2.4. Evaluation of h5A10 Versus m5A10 for Diagnostic SPECT Imaging
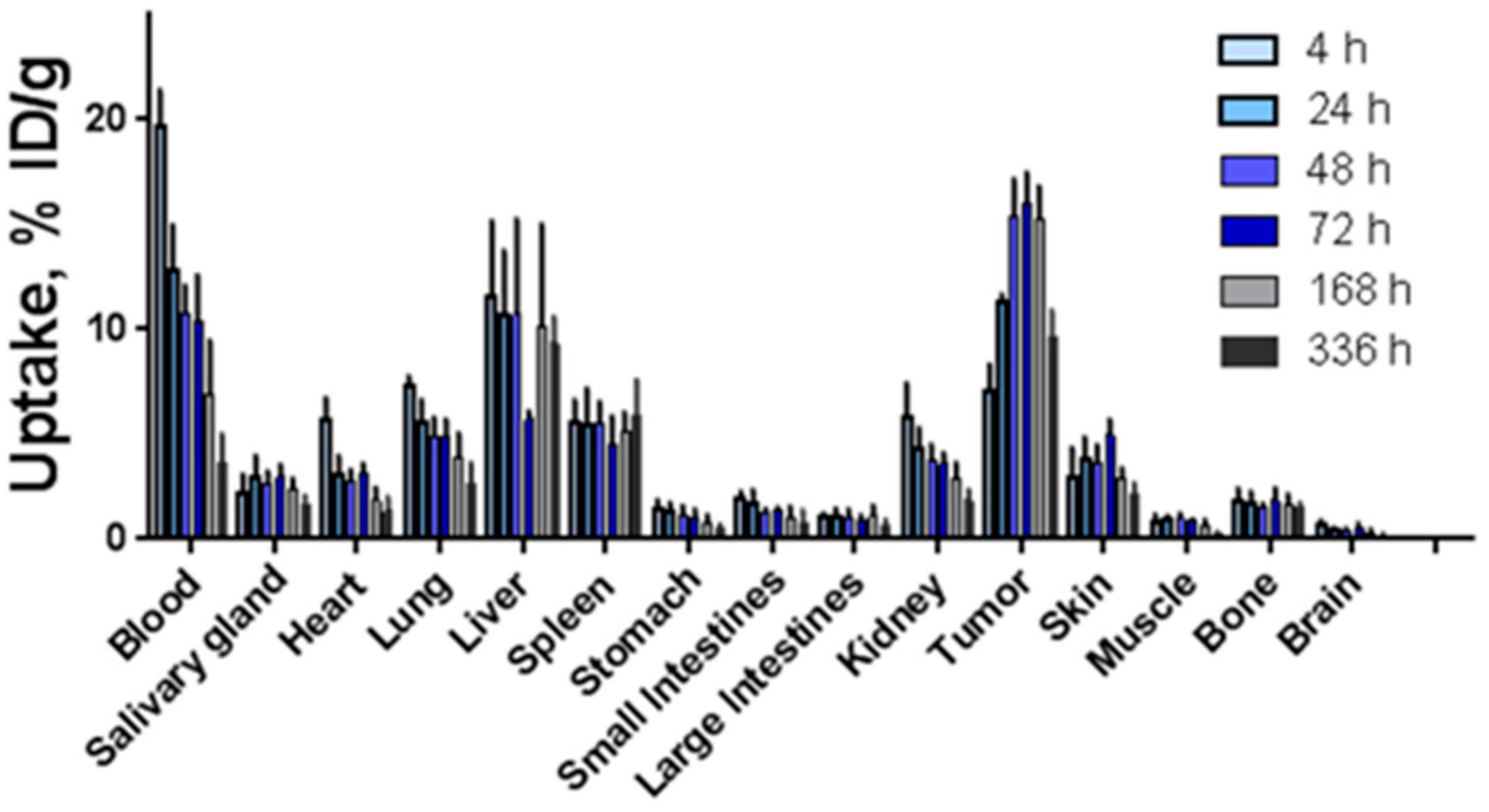
2.5. Biodistribution Studies of 177Lu-h5A10 and Specificity
3. Discussion
4. Materials and Methods
4.1. Humanization of m5A10
4.2. Expression, Purification, SEC and ELISA Analysis of the Humanized 5A10 IgG1
4.3. Conjugation and Binding Affinities by Surface Plasmon Resonance
4.4. Labeling Chemistry
4.5. Animal Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lilja, H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J. Clin. Investig. 1985, 76, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Christensson, A.; Laurell, C.-B.; Lilja, H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. JBIC J. Biol. Inorg. Chem. 1990, 194, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Haberkorn, U.; Eder, M.; Eisenhut, M.; Zechmann, C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1085–1086. [Google Scholar] [CrossRef] [PubMed]
- Behr, S.C.; Aggarwal, R.; Van Brocklin, H.F.; Flavell, R.R.; Gao, K.; Small, E.J.; Blecha, J.; Jivan, S.; Hope, T.A.; Simko, J.P.; et al. Phase I Study of CTT1057, an 18F-Labeled Imaging Agent with Phosphoramidate Core Targeting Prostate-Specific Membrane Antigen in Prostate Cancer. J. Nucl. Med. 2019, 60, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Proteinatlas The Human Protein Atlas. Available online: https://www.proteinatlas.org (accessed on 17 June 2020).
- Lilja, H.; Christensson, A.; Dahlén, U.; Matikainen, M.T.; Nilsson, O.; Pettersson, K.; Lövgren, T. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin. Chem. 1991, 37, 1618–1625. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmsson-Timmermand, O.; Santos, E.; Thorek, D.L.; Evans-Axelsson, S.; Bjartell, A.; Lilja, H.; Larson, S.M.; Strand, S.-E.; Tran, T.A.; Ulmert, D. Radiolabeled antibodies in prostate cancer: A case study showing the effect of host immunity on antibody bio-distribution. Nucl. Med. Biol. 2015, 42, 375–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ulmert, D.; Evans, M.J.; Holland, J.; Rice, S.; Wongvipat, J.; Pettersson, K.; Abrahamsson, P.-A.; Scardino, P.T.; Larson, S.M.; Lilja, H.; et al. Imaging Androgen Receptor Signaling with a Radiotracer Targeting Free Prostate-Specific Antigen. Cancer Discov. 2012, 2, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Veach, D.R.; Storey, C.M.; Lueckerath, K.; Braun, K.; von Bodman, C.; Lamminmäki, U.; Kalidindi, T.M.; Strand, S.-E.; Strand, J.; Altai, M.; et al. PSA-Targeted Alpha-, Beta-, and Positron-Emitting Immunotheranostics in Murine Prostate Cancer Models and Nonhuman Primates. Clin. Cancer Res. 2021, 27, 2050–2060. [Google Scholar] [CrossRef] [PubMed]
- Kabat, E.A.; Wu, T.T.; Perry, H.M.; Gottesman, K.S.; Foeller, C. Sequences of Proteins of Immunological Interest; DIANE Publishing: Collingdale, PA, USA, 1991. [Google Scholar]
- Timmermand, O.V.; Örbom, A.; Altai, M.; Zedan, W.; Holmqvist, B.; Safi, M.; Tran, T.; Strand, S.-E.; Strand, J. A Conjugation Strategy to Modulate Antigen Binding and FcRn Interaction Leads to Improved Tumor Targeting and Radioimmunotherapy Efficacy with an Antibody Targeting Prostate-Specific Antigen. Cancers 2021, 13, 3469. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar] [PubMed]
- Tagawa, S.T.; Akhtar, N.H.; Nikolopoulou, A.; Kaur, G.; Robinson, B.; Kahn, R.; Vallabhajosula, S.; Goldsmith, S.J.; Nanus, D.M.; Bander, N.H. Bone Marrow Recovery and Subsequent Chemotherapy Following Radiolabeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 in Men with Metastatic Castration-Resistant Prostate Cancer. Front. Oncol. 2013, 3, 214. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Jhanwar, Y.S.; Kaur, G.; Tagawa, S.T.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Radioimmunotherapy of Metastatic Prostate Cancer with 177Lu-DOTAhuJ591 Anti Prostate Specific Membrane Antigen Specific Monoclonal Antibody. Curr. Radiopharm. 2015, 9, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.J.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177–Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-H.; Gao, D.-W.; Feng, J.; He, J.; Seo, Y.; Tedesco, J.; Wolodzko, J.G.; Hasegawa, B.H.; Franc, B.L. Biodistributions of 177Lu- and 111In-Labeled 7E11 Antibodies to Prostate-Specific Membrane Antigen in Xenograft Model of Prostate Cancer and Potential Use of 111In-7E11 as a Pre-therapeutic Agent for 177Lu-7E11 Radioimmunotherapy. Mol. Imaging Biol. 2009, 11, 159–166. [Google Scholar] [CrossRef]
- Jones, M.D.; Brand, N.J.; Fersht, A.R. Single-stranded M13 DNA: Use as a cloning vector. Nucleic Acids Res. 1986, 14, 10116. [Google Scholar] [CrossRef][Green Version]
- Williams, D.G.; Matthews, D.J.; Jones, T. Humanizing Antibodies by CDR Grafting. In Antibody Engineering; Kontermann, R., Dubel, S., Eds.; Springer Protocols Handbooks; Springer: Berlin/Heidelberg, Germany, 2010; pp. 319–339. [Google Scholar]
- Meares, C.F.; McCall, M.J.; Reardan, D.T.; Goodwin, D.A.; Diamanti, C.I.; McTigue, M. Conjugation of antibodies with bifunctional chelating agents: Isothiocyanate and bromoacetamide reagents, methods of analysis, and subsequent addition of metal ions. Anal. Biochem. 1984, 142, 68–78. [Google Scholar] [CrossRef]




| Conjugate | Koff (10−6s−1) | Kon (106 M−1s−1) | KD (10−12 M) |
|---|---|---|---|
| m5A10 | 5.4 | 0.25 | 22 |
| h5A10 | 4.8 | 0.62 | 7.7 |
| m5A10-DTPA | 6.3 | 0.27 | 23 |
| h5A10-DTPA | 4.8 | 0.39 | 12 |
| Organs | 4 h | 24 h | 48 h | 72 h | 168 h | 336 h |
|---|---|---|---|---|---|---|
| Blood | 0.4 ± 0.1 | 0.2 ± 0.0 | 1.5 ± 0.2 | 1.5 ± 1.0 | 2.4 ± 0.8 | 3.0 ± 1.3 |
| Salivary gland | 3.6 ± 0.17 | 0.3 ± 0.0 | 5.3 ± 0.8 | 5.2 ± 1.0 | 6.9 ± 2.1 | 6.1 ± 2.0 |
| Heart | 1.3 ± 0.4 | 1.6 ± 0.5 | 5.4 ± 1.0 | 4.8 ± 0.5 | 8.8 ± 2.7 | 8.5 ± 4.4 |
| Lung | 1.0 ± 0.2 | 1.5 ± 0.2 | 2.9 ± 0.4 | 3.1 ± 0.5 | 4.1 ± 1.1 | 4.1 ± 1.8 |
| Liver | 0.7 ± 0.2 | 0.8 ± 0.0 | 1.9 ± 0.9 | 2.6 ± 0.3 | 1.8 ± 1.0 | 1.0 ± 0.2 |
| Spleen | 1.3 ± 0.4 | 0.6 ± 0.4 | 2.9 ± 0.9 | 3.4 ± 0.8 | 3.1 ± 0.9 | 1.8 ± 0.7 |
| Stomach | 5.3 ± 2.5 | 0.8 ± 0.1 | 13.0 ± 3.7 | 15.3 ± 4.4 | 21.5 ± 9.2 | 19.6 ± 7.5 |
| Small intestines | 3.9 ± 1.3 | 3.3 ± 0.4 | 11.7 ± 0.2 | 11.1 ± 2.6 | 18.8 ± 11.8 | 19.6 ± 14.1 |
| Large intestines | 6.9 ± 2.3 | 2.8 ± 0.6 | 13.5 ± 4.2 | 17.4 ± 2.6 | 16.9 ± 7.3 | 18.0 ± 8.1 |
| Kidneys | 1.3 ± 0.5 | 4.1 ± 1.1 | 3.8 ± 0.5 | 4.2 ± 0.6 | 5.4 ± 1.2 | 5.6 ± 1.9 |
| Skin | 2.9± 1.5 | 0.5 ± 0.2 | 3.9 ± 0.5 | 3.1 ± 0.8 | 5.2 ± 0.4 | 4.9 ± 1.7 |
| Muscle | 9.9 ± 5.6 | 1.1 ± 1.1 | 14.6 ± 1.5 | 16.9 ± 2.8 | 25.5 ± 10.0 | 35.2 ± 13.2 |
| Bone | 4.7 ± 1.7 | 4.7 ± 1.0 | 9.9 ± 1.7 | 8.8 ± 2.5 | 9.6 ± 2.9 | 6.4 ± 1.2 |
| Brain | 12.5 ± 7.8 | 2.8 ± 0.8 | 38.4 ± 8.4 | 33.9 ± 15.9 | 74.1 ± 66.6 | 67.1 ± 44.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strand, J.; Sjöström, K.; Lamminmaki, U.J.; Vilhelmsson Timmermand, O.; Strand, S.-E.; Tran, T.A. Humanization, Radiolabeling and Biodistribution Studies of an IgG1-Type Antibody Targeting Uncomplexed PSA for Theranostic Applications. Pharmaceuticals 2021, 14, 1251. https://doi.org/10.3390/ph14121251
Strand J, Sjöström K, Lamminmaki UJ, Vilhelmsson Timmermand O, Strand S-E, Tran TA. Humanization, Radiolabeling and Biodistribution Studies of an IgG1-Type Antibody Targeting Uncomplexed PSA for Theranostic Applications. Pharmaceuticals. 2021; 14(12):1251. https://doi.org/10.3390/ph14121251
Chicago/Turabian StyleStrand, Joanna, Kjell Sjöström, Urpo J. Lamminmaki, Oskar Vilhelmsson Timmermand, Sven-Erik Strand, and Thuy A. Tran. 2021. "Humanization, Radiolabeling and Biodistribution Studies of an IgG1-Type Antibody Targeting Uncomplexed PSA for Theranostic Applications" Pharmaceuticals 14, no. 12: 1251. https://doi.org/10.3390/ph14121251
APA StyleStrand, J., Sjöström, K., Lamminmaki, U. J., Vilhelmsson Timmermand, O., Strand, S.-E., & Tran, T. A. (2021). Humanization, Radiolabeling and Biodistribution Studies of an IgG1-Type Antibody Targeting Uncomplexed PSA for Theranostic Applications. Pharmaceuticals, 14(12), 1251. https://doi.org/10.3390/ph14121251






