Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study
Abstract
1. Introduction
2. Results
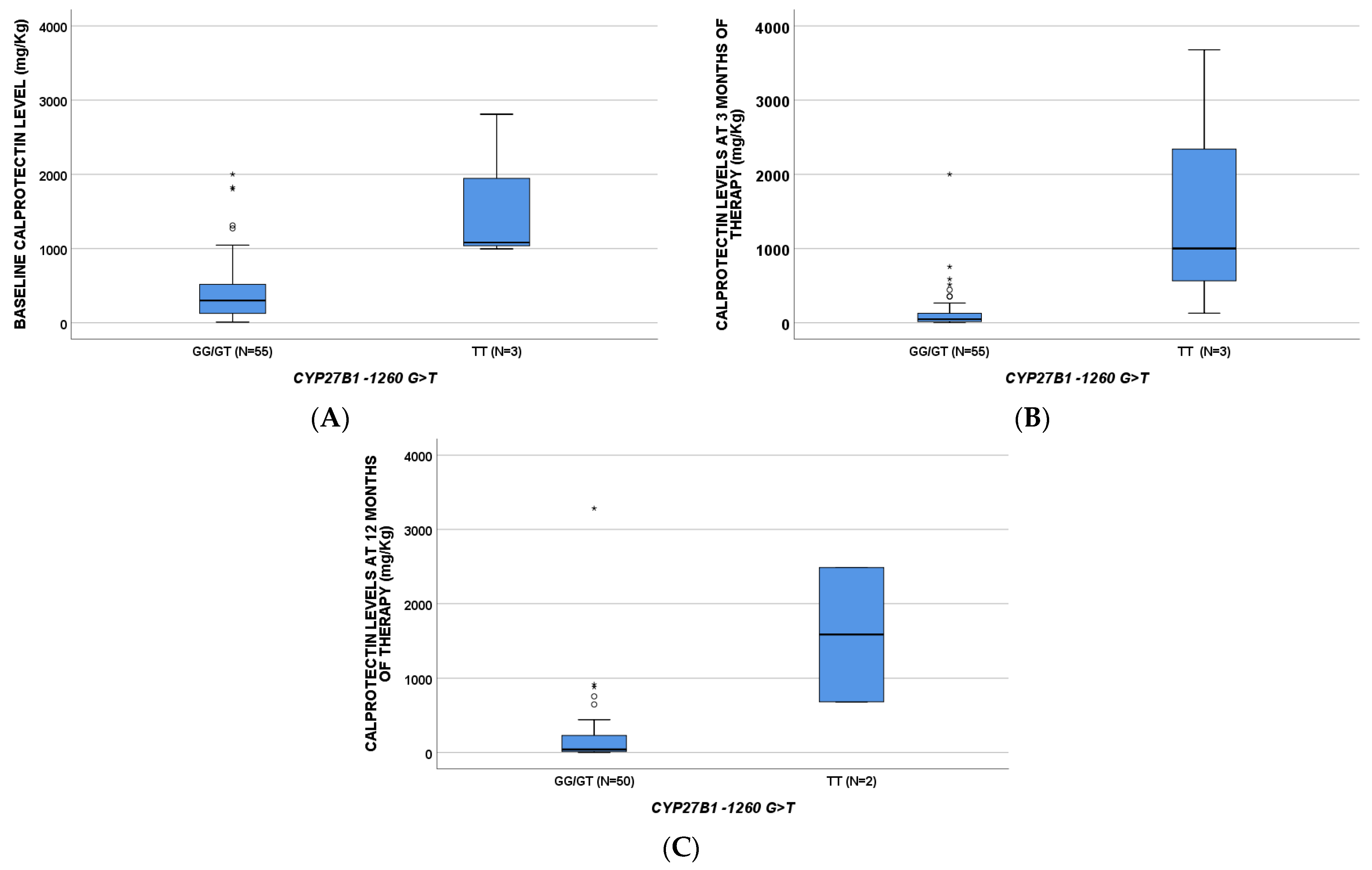
2.1. Calprotectin
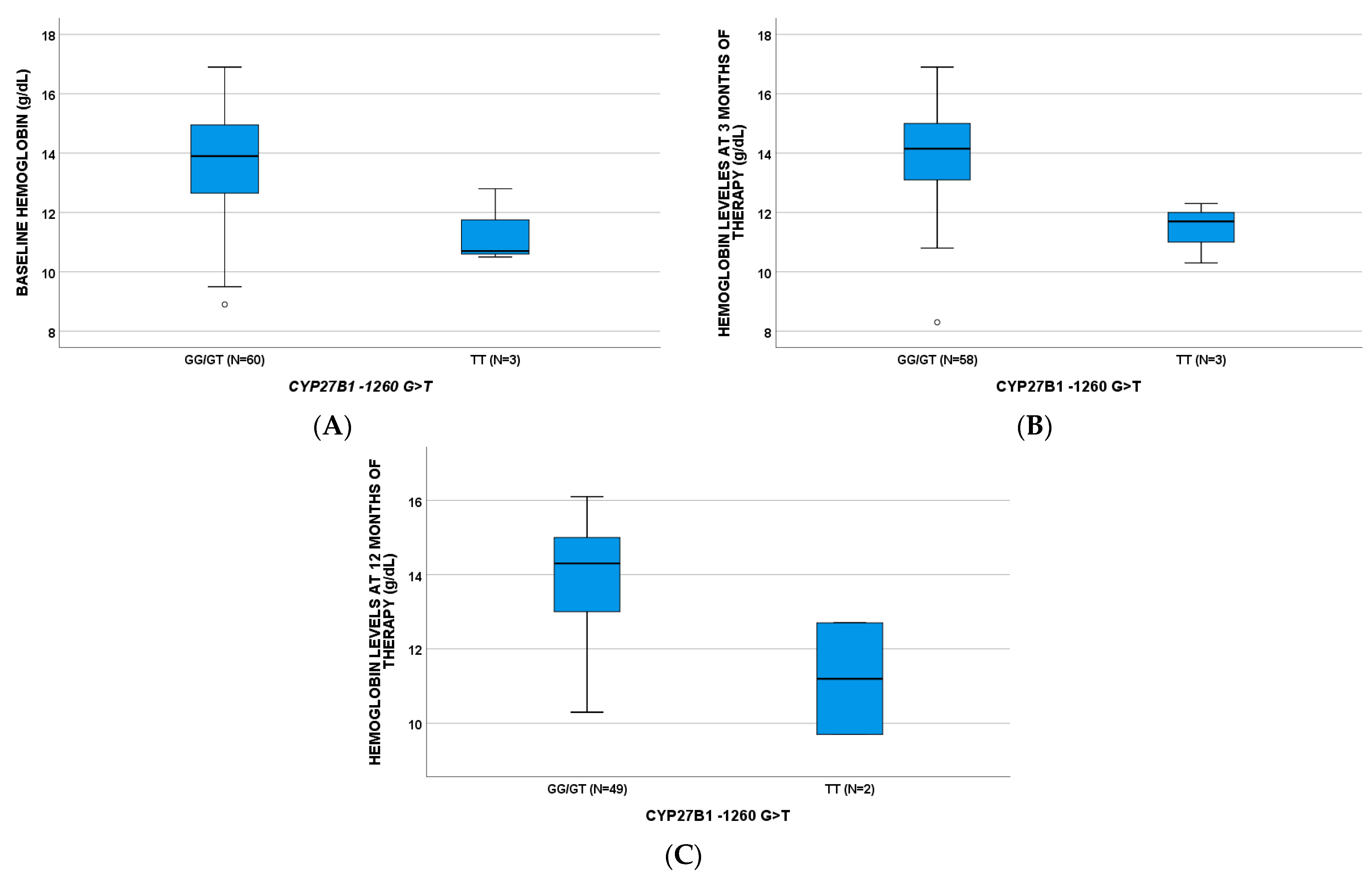
2.2. Hemoglobin
2.3. C-Reactive Protein > 1 mg/dL
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Vitamin D-Related Single Nucleotide Polymorphisms Analyses
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Actis, G.C.; Pellicano, R. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2021, 14, 4–22. [Google Scholar] [CrossRef]
- Denson, L.A. Challenges in IBD Research: Precision Medicine. Inflamm. Bowel Dis. 2019, 25, S31–S39. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Rosso, C. On-Treatment Decrease of Serum Interleukin-6 as a Predictor of Clinical Response to Biologic Therapy in Patients with Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 800. [Google Scholar] [CrossRef]
- Bertani, L.; Blandizzi, C. Fecal Calprotectin Predicts Mucosal Healing in Patients With Ulcerative Colitis Treated With Biological Therapies: A Prospective Study. Clin. Transl. Gastroenterol. 2020, 11, 00174. [Google Scholar] [CrossRef]
- Bertani, L.; Fornai, M. Serum oncostatin M at baseline predicts mucosal healing in Crohn’s disease patients treated with infliximab. Aliment. Pharmacol. Ther. 2020, 52, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bertani, L.; Rossari, F. Novel Prognostic Biomarkers of Mucosal Healing in Ulcerative Colitis Patients Treated With Anti-TNF: Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio. Inflamm. Bowel Dis. 2020, 26, 1579–1587. [Google Scholar] [CrossRef]
- Bertani, L.; Trico, D. Serum triiodothyronine-to-thyroxine (T3/T4) ratio predicts therapeutic outcome to biological therapies in elderly IBD patients. Aliment. Pharmacol. Ther. 2020, 53, 273–280. [Google Scholar] [PubMed]
- Bertani, L.; Caviglia, G.P. Serum Interleukin-6 and -8 as Predictors of Response to Vedolizumab in Inflammatory Bowel Diseases. J. Clin. Med. 2020, 9, 1323. [Google Scholar] [CrossRef]
- Dragoni, G.; Innocenti, T. Biomarkers of inflammation in Inflammatory Bowel Disease: How long before abandoning single-marker approaches? Dig. Dis. 2020, 39, 190–203. [Google Scholar] [CrossRef]
- Bangma, A.; Voskuil, M.D. Predicted efficacy of a pharmacogenetic passport for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2020, 51, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. External quality assessment in clinical neurochemistry: Survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin. Chem. 1995, 41, 256–263. [Google Scholar] [CrossRef]
- National Center for Health Statistics. National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes/about_nhanes.html (accessed on 24 September 2021).
- Maggio, D.; Cherubini, A. 25(OH)D Serum levels decline with age earlier in women than in men and less efficiently prevent compensatory hyperparathyroidism in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, V.; Modoni, S. Longitudinal evaluation of vitamin D status in healthy subjects from southern Italy: Seasonal and gender differences. Osteoporos. Int. 2001, 12, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- D’Avolio, A.; Avataneo, V. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients 2020, 12, 1359. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Annweiler, C.; Dursun, E. Vitamin D and cognition in older adults: International consensus guidelines. Gériatr. Psychol. Neuropsychiatr. Vieil. 2016, 14, 265–273. [Google Scholar] [CrossRef]
- Giusti, A.; Barone, A. High prevalence of secondary hyperparathyroidism due to hypovitaminosis D in hospitalized elderly with and without hip fracture. J. Endocrinol. Investig. 2006, 29, 809–813. [Google Scholar] [CrossRef]
- Balion, C.; Griffith, L.E. Vitamin D, cognition, and dementia: A systematic review and meta-analysis. Neurology 2012, 79, 1397–1405. [Google Scholar] [CrossRef]
- Avihingsanon, A.; Jitmitraparp, S. Advanced liver fibrosis by transient elastography, fibrosis 4, and alanine aminotransferase/platelet ratio index among Asian hepatitis C with and without human immunodeficiency virus infection: Role of vitamin D levels. J. Gastroenterol. Hepatol. 2014, 29, 1706–1714. [Google Scholar] [CrossRef]
- Lindh, J.D.; Bjorkhem-Bergman, L. Vitamin D and drug-metabolising enzymes. Photochem. Photobiol. Sci. 2012, 11, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Drocourt, L.; Ourlin, J.C. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J. Biol. Chem. 2002, 277, 25125–25132. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.D.; Andersson, M.L. Seasonal variation in blood drug concentrations and a potential relationship to vitamin D. Drug Metab. Dispos. 2011, 39, 933–937. [Google Scholar] [CrossRef]
- Thummel, K.E.; Brimer, C. Transcriptional control of intestinal cytochrome P-4503A by 1alpha,25-dihydroxy vitamin D3. Mol. Pharmacol 2001, 60, 1399–1406. [Google Scholar] [CrossRef]
- Fan, J.; Liu, S. Up-regulation of transporters and enzymes by the vitamin D receptor ligands, 1alpha,25-dihydroxyvitamin D3 and vitamin D analogs, in the Caco-2 cell monolayer. J. Pharmacol. Exp. Ther. 2009, 330, 389–402. [Google Scholar] [CrossRef]
- Robien, K.; Oppeneer, S.J. Drug-vitamin D interactions: A systematic review of the literature. Nutr. Clin. Pract. 2013, 28, 194–208. [Google Scholar] [CrossRef]
- Luo, G.; Guenthner, T. CYP3A4 induction by xenobiotics: Biochemistry, experimental methods and impact on drug discovery and development. Curr. Drug Metab. 2004, 5, 483–505. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Vidal, M. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS 2003, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Grober, U.; Kisters, K. Influence of drugs on vitamin D and calcium metabolism. Dermato-Endocrinology 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Bafutto, M.; Oliveira, E.C. Use of Vitamin D With Anti-Tumor Necrosis Factor Therapy for Crohn’s Disease. Gastroenterol. Res. 2020, 13, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.W.; Collins, E. Higher 25-hydroxyvitamin D levels are associated with greater odds of remission with anti-tumour necrosis factor-alpha medications among patients with inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2017, 45, 653–659. [Google Scholar] [CrossRef]
- Santos-Antunes, J.; Nunes, A.C. The Relevance of Vitamin D and Antinuclear Antibodies in Patients with Inflammatory Bowel Disease Under Anti-TNF Treatment: A Prospective Study. Inflamm. Bowel Dis. 2016, 22, 1101–1106. [Google Scholar] [CrossRef][Green Version]
- Mechie, N.C.; Mavropoulou, E. Distinct Association of Serum Vitamin D Concentration with Disease Activity and Trough Levels of Infliximab and Adalimumab during Inflammatory Bowel Disease Treatment. Digestion 2019, 101, 761–770. [Google Scholar] [CrossRef]
- Prosser, D.E.; Jones, G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 2004, 29, 664–673. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Heaney, R.P. Estimates of optimal vitamin D status. Osteoporos. Int. 2005, 16, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B.; Harris, S.S. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am. J. Clin. Nutr. 1997, 65, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Krajcovicova, A. Vitamin D therapy in inflammatory bowel diseases: Who, in what form, and how much? J. Crohns Colitis 2015, 9, 198–209. [Google Scholar] [CrossRef]
- Al Khoury, A.; Singh, K. The Burden of Anemia Remains Significant over Time in Patients with Inflammatory Bowel Diseases at a Tertiary Referral Center. J. Gastrointest. Liver Dis. 2020, 29, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Long, Z. Dietary factors and polymorphisms in vitamin D metabolism genes: The risk and prognosis of colorectal cancer in northeast China. Sci. Rep. 2017, 7, 8827. [Google Scholar] [CrossRef]
- Allegra, S.; Cusato, J. The effect of vitamin D pathway genes and deferasirox pharmacogenetics on liver iron in thalassaemia major patients. Pharm. J. 2019, 19, 417–427. [Google Scholar] [CrossRef]
- Sawada, N.; Sakaki, T. Structure-function analysis of CYP27B1 and CYP27A1. Studies on mutants from patients with vitamin D-dependent rickets type I (VDDR-I) and cerebrotendinous xanthomatosis (CTX). Eur. J. Biochem. 2001, 268, 6607–6615. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.M.; Bojunga, J. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 2010, 54, 887–893. [Google Scholar] [CrossRef]
- Kitanaka, S.; Isojima, T. Association of vitamin D-related gene polymorphisms with manifestation of vitamin D deficiency in children. Endocr. J. 2012, 59, 1007–1014. [Google Scholar] [CrossRef]
- Kato, S.; Yanagisawa, J. The importance of 25-hydroxyvitamin D3 1 alpha-hydroxylase gene in vitamin D-dependent rickets. Curr. Opin. Nephrol. Hypertens. 1998, 7, 377–383. [Google Scholar] [CrossRef]
- Strisciuglio, C.; Cenni, S. The Role of Inflammation on Vitamin D Levels in a Cohort of Pediatric Patients With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 501–506. [Google Scholar] [CrossRef]
- Allegra, S.; Cusato, J. Role of CYP24A1, VDR and GC gene polymorphisms on deferasirox pharmacokinetics and clinical outcomes. Pharm. J. 2017, 18, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Royce, S.G. The intestinal vitamin D receptor in inflammatory bowel disease: Inverse correlation with inflammation but no relationship with circulating vitamin D status. Therap. Adv. Gastroenterol. 2019, 12, 1756284818822566. [Google Scholar] [CrossRef]
- Cauci, S.; Maione, V. BsmI (rs1544410) and FokI (rs2228570) vitamin D receptor polymorphisms, smoking, and body mass index as risk factors of cutaneous malignant melanoma in northeast Italy. Cancer Biol. Med. 2017, 14, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Chaparro, M. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- D’Haens, G.; Ferrante, M. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Sandborn, W. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am. J. Gastroenterol. 2015, 110, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Values |
|---|---|
| Number of patients | 63 |
| Age (year), median [IQR] | 42 [32;55] |
| Male sex, n (%) | 36 (57.1) |
| Perianal Disease, n (%) | 10 (15.9) |
| Smoke (0 = no, 1 = smoker, 2 = ex), n (%) | 31 (49.2) |
| 11 (17.5) | |
| 21 (33) | |
| Alcohol, n (%) | 2 (3.2) |
| Intestinal localization, n (%) | L1 24 (38.1) |
| L2 9 (14.3) | |
| L3 29 (46) | |
| L4 1 (1.6) | |
| Surgery, n (%) | 39 (61.9) |
| Comorbidities, n (%) | 23 (36.5) |
| VD Supplementation, n (%) | 31 (49.2) |
| Weight, median [IQR] | 61 [57.5–70.5] |
| Height, median [IQR] | 170 [164–174.5] |
| Years of disease, median [IQR] | 13 [6.5–18.5] |
| Hb t0, median [IQR] | 13.9 [12.58–14.56] |
| Calprotectin t0, median [IQR] | 322.5 [130.5–657.5] |
| CRP > 1 t0, n (%) | 34 (54.1) |
| Clinical response 3 months, n (%) | 46 (73) |
| Clinical response 12 months, n (%) | 40 (63.5) |
| Remission 3 months, n (%) | 37 (58.7) |
| Remission 12 months, n (%) | 36 (57.1) |
| CYP27B1-1260 GG/GT | CYP27B1-1260 TT | CYP27B1 + 2838 CC | CYP27B1 + 2838 CT/TT | VDR ApaI CC | VDR ApaI CA/AA | VDR Cdx2 AA | VDR Cdx2 AG/GG | GC 1296 AA/AC | GC 1296 CC | CYP24A1 8620 AA/AG | CYP24A1 8620 GG | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CALPROTECTIN ug/g (BL) | 301 (117–534) | 1082 (996–/) | 1064 (784–3608) | 503 (300–1300) | 798 (321–1829) | 298 (92–511) | 115 (74–196) | 330 (163–830) | 405 (213–997) | 170 (53–500) | ||
| CALPROTECTIN ug/g (T3) | 49 (17–138) | 1001 (129–/) | 359 (70–1670) | 49 (15–133) | 224 (55–898) | 42 (15–131) | ||||||
| CALPROTECTIN (T12) | 41 (15–237) | 1585 (683–/) | ||||||||||
| HEMOGLOBIN g/dL (BL) | 13.90 (12.63–14.98) | 10.70 (10.5–/) | 13.50 (12.48–14.43) | 14.9 (13.50–15.80) | ||||||||
| HEMOGLOBIN g/dL (T3) | 14.15 (13.08–15.03) | 11.70 (10.30–/) | 13.65 (12.33–14.88) | 14.9 (13.65–15.90) | ||||||||
| HEMOGLOBIN g/dL (T12) | 14.30 (13.00–15.05) | 11.20 (9.70–/) | 13.70 (12.65–14.70) | 14.50 (13.73–15.60) |
| Remission after 3 Months of Therapy | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| p-Value | OR (95% IC) | p-Value | OR (95% IC) | |
| Age [≥50] | 0.621 | 0.768 (0.269–2.190) | ||
| Sex | 0.941 | 0.963 (0.349–2.653) | ||
| Weight [≥70 Kg] | 0.889 | 1.08 (0.367–3.182) | ||
| Smoke | 0.023 | 0.506 (0.281–0.909) | 0.035 | 0.480 (0.243–0.948) |
| Alcohol | NSC | |||
| Years of disease >10 | 0.367 | 0.605 (0.203–1.804) | ||
| Perianal Disease | 0.543 | 0.656 (0.169–2.547) | ||
| Surgery | 0.634 | 0.776 (0.274–2.199) | ||
| Systemic steroids | 0.002 | 0.103 (0.025–0.422) | 0.001 | 0.081 (0.018–0.366) |
| Mesar | 0.808 | 0.871 (0.285–2.661) | ||
| Topical steroids | 0.386 | 0.438 (0.068–2.828) | ||
| Immunosuppression | 0.213 | 2.464 (0.597–10.178) | ||
| Ab | 0.434 | 1.789 (0.416–7.684) | ||
| VD Supplementation | 0.685 | 1.231 (0.415–3.364) | ||
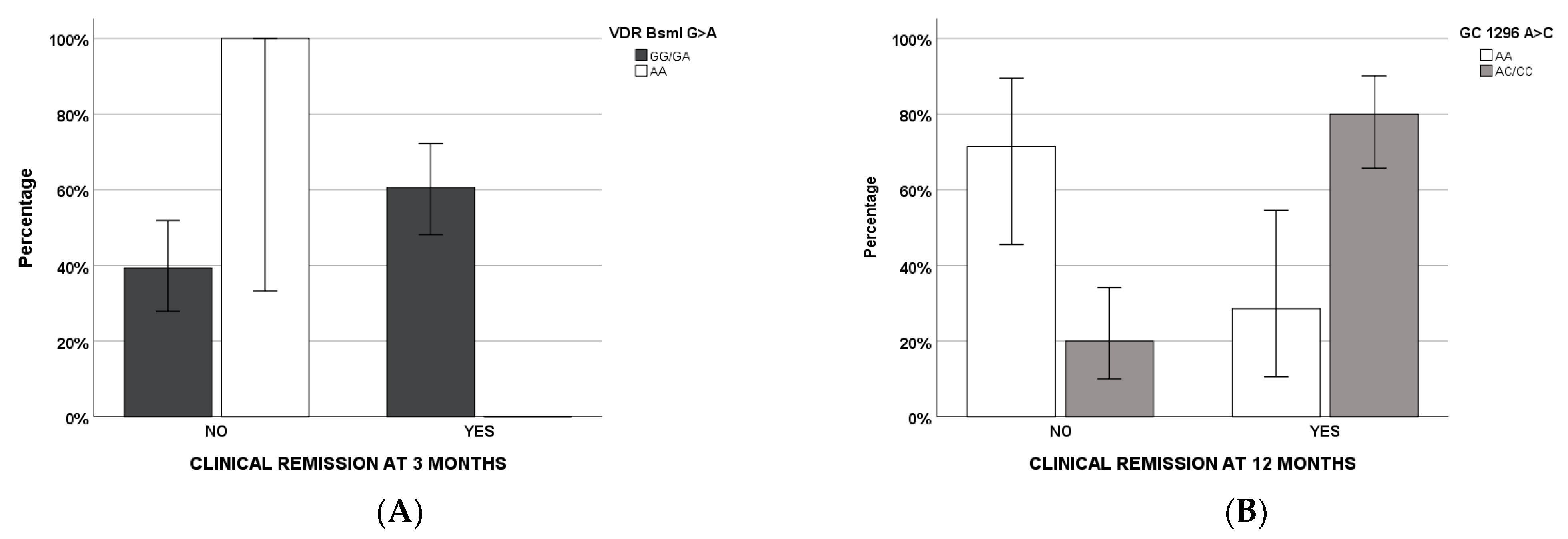
| VDR BsmI AA | NSC | NSC | ||
| GC 1296 CA/AA | 0.049 | 3.229 (0.994–10.491) | ||
| Remission after 12 Months of Therapy | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| p-Value | OR (95% IC) | p-Value | OR (95% IC) | |
| Age [>50] | 0.03 | 0.267 (0.081–0.883) | ||
| Sex | 0.248 | 1.964 (0.624–6.179) | ||
| Weight [>70 Kg] | 0.836 | 0.880 (0.263–2.950) | ||
| Smoke | 0.279 | 0.702 (0.370–1.332) | ||
| Alcohol | NSC | |||
| >10 aa mal | 0.017 | 0.140 (0.028–0.698) | ||
| Mal xian | 0.072 | 0.236 (0.049–1.135) | ||
| Surgery | 0.691 | 0.786 (0.240–2.575) | ||
| Systemic steroids | 0.621 | 0.700 (0.170–2.882) | ||
| Mesar | 0.521 | 0.649 (0.174–2.426) | ||
| Topical steroids | 0.104 | 0.143 (0.014–1.487) | ||
| Immunosuppression | 0.331 | 2.286 (0.432–12.103) | ||
| Ab | 0.444 | 1.931 (0.358–10.422) | ||
| VD Supplementation | 0.004 | 0.143 (0.039–0.529) | 0.006 | 0.050 (0.006–0.428) |
| CYP24A1 22776 TT | NSC | |||
| VDR ApaI CA/AA | NSC | |||
| VDR BsmI AA | NSC | |||
| GC 1296 CA/AA | 0.001 | 10 (2.479–40.331) | 0.003 | 29.285 (3.160–271.367) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cusato, J.; Bertani, L.; Antonucci, M.; Tomasello, C.; Caviglia, G.P.; Dibitetto, S.; Massano, A.; Mangia, M.; Mula, J.; Ceccarelli, L.; et al. Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study. Pharmaceuticals 2021, 14, 1230. https://doi.org/10.3390/ph14121230
Cusato J, Bertani L, Antonucci M, Tomasello C, Caviglia GP, Dibitetto S, Massano A, Mangia M, Mula J, Ceccarelli L, et al. Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study. Pharmaceuticals. 2021; 14(12):1230. https://doi.org/10.3390/ph14121230
Chicago/Turabian StyleCusato, Jessica, Lorenzo Bertani, Miriam Antonucci, Cristina Tomasello, Gian Paolo Caviglia, Simone Dibitetto, Alessandro Massano, Michela Mangia, Jacopo Mula, Linda Ceccarelli, and et al. 2021. "Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study" Pharmaceuticals 14, no. 12: 1230. https://doi.org/10.3390/ph14121230
APA StyleCusato, J., Bertani, L., Antonucci, M., Tomasello, C., Caviglia, G. P., Dibitetto, S., Massano, A., Mangia, M., Mula, J., Ceccarelli, L., Costa, F., Zanzi, F., Astegiano, M., Ribaldone, D. G., & D’Avolio, A. (2021). Vitamin D-Related Genetics as Predictive Biomarker of Clinical Remission in Adalimumab-Treated Patients Affected by Crohn’s Disease: A Pilot Study. Pharmaceuticals, 14(12), 1230. https://doi.org/10.3390/ph14121230









