Monitoring of Cardiorespiratory Parameters in Rats—Validation Based on Pharmacological Stimulation
Abstract
1. Introduction
2. Results
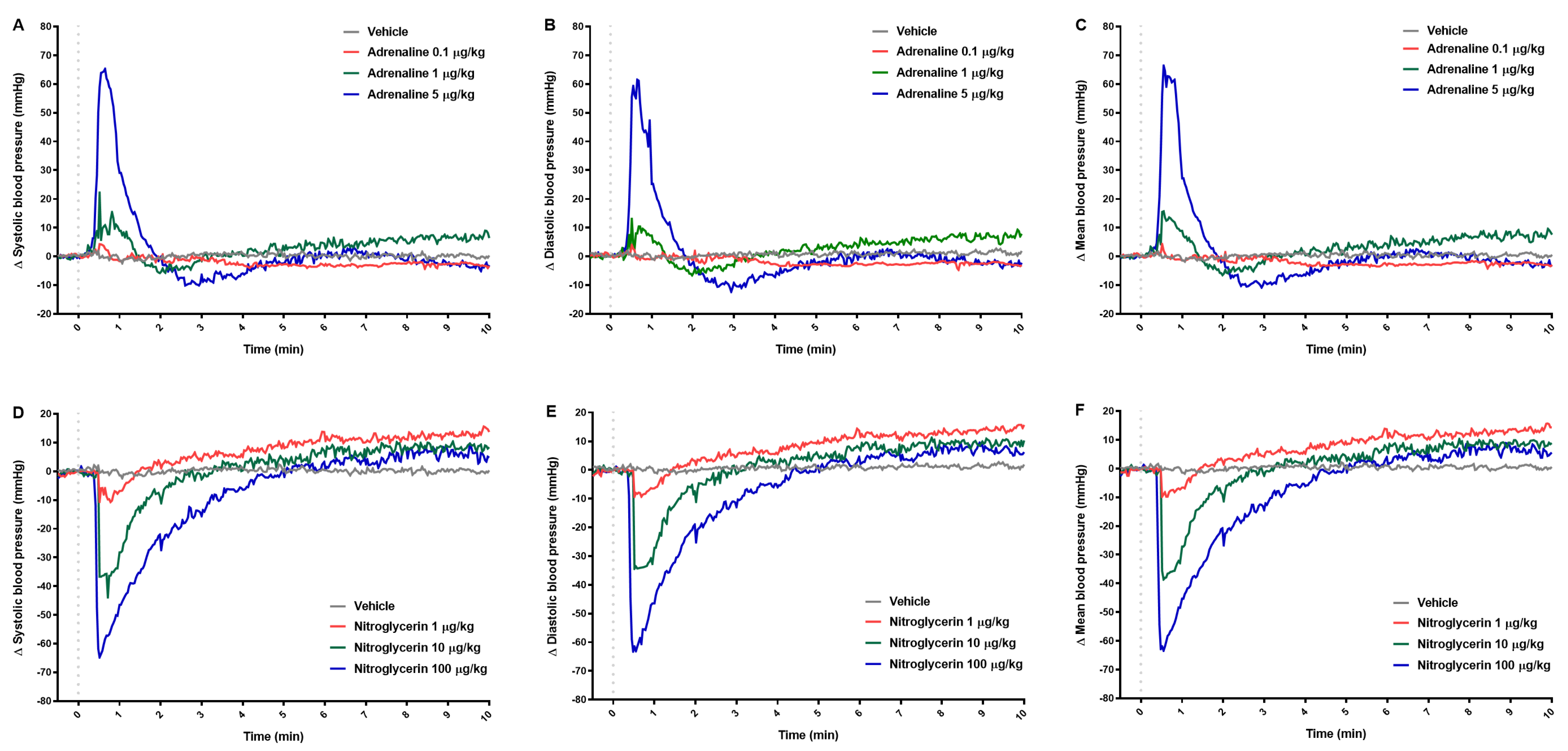
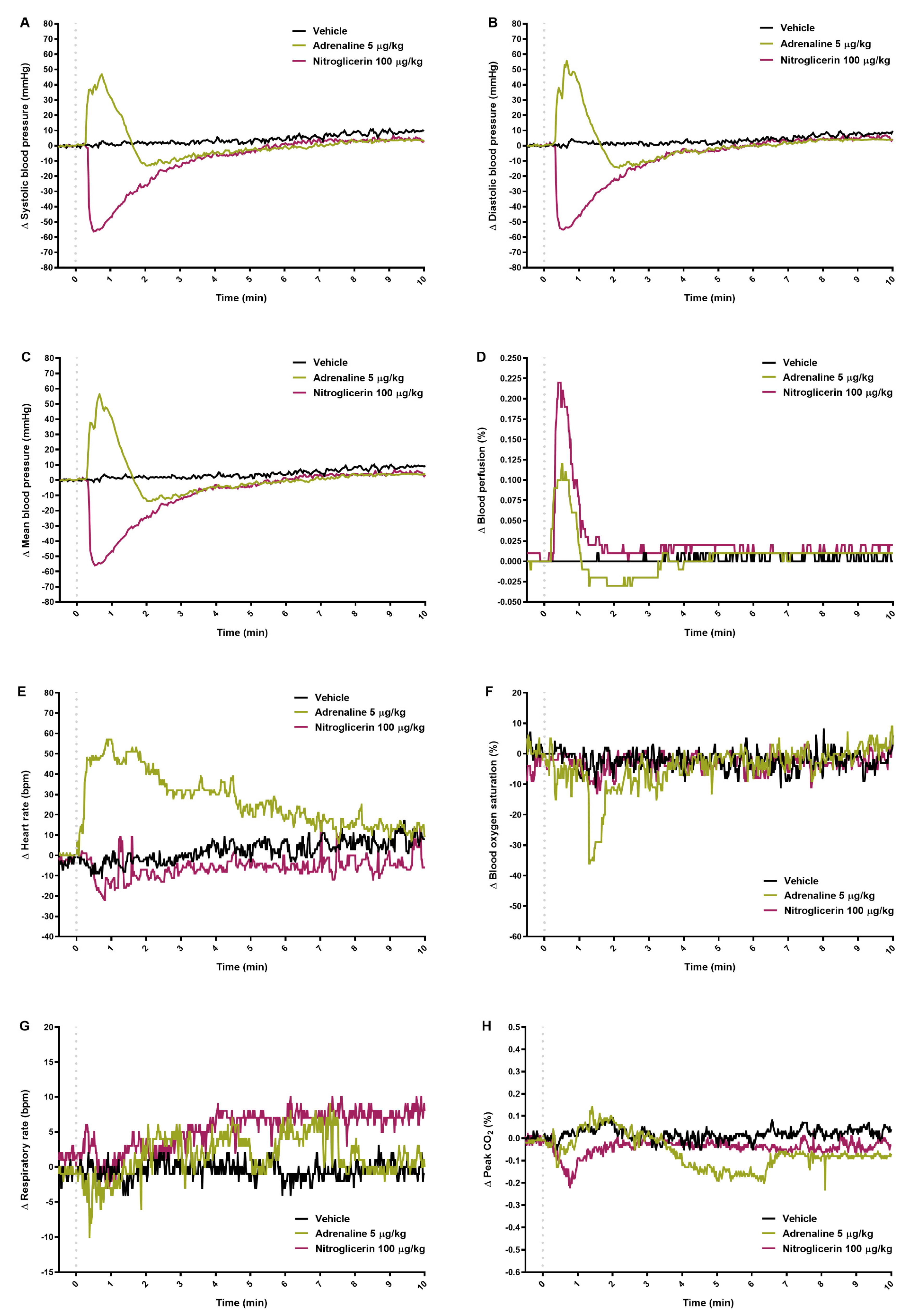
2.1. Blood Pressure
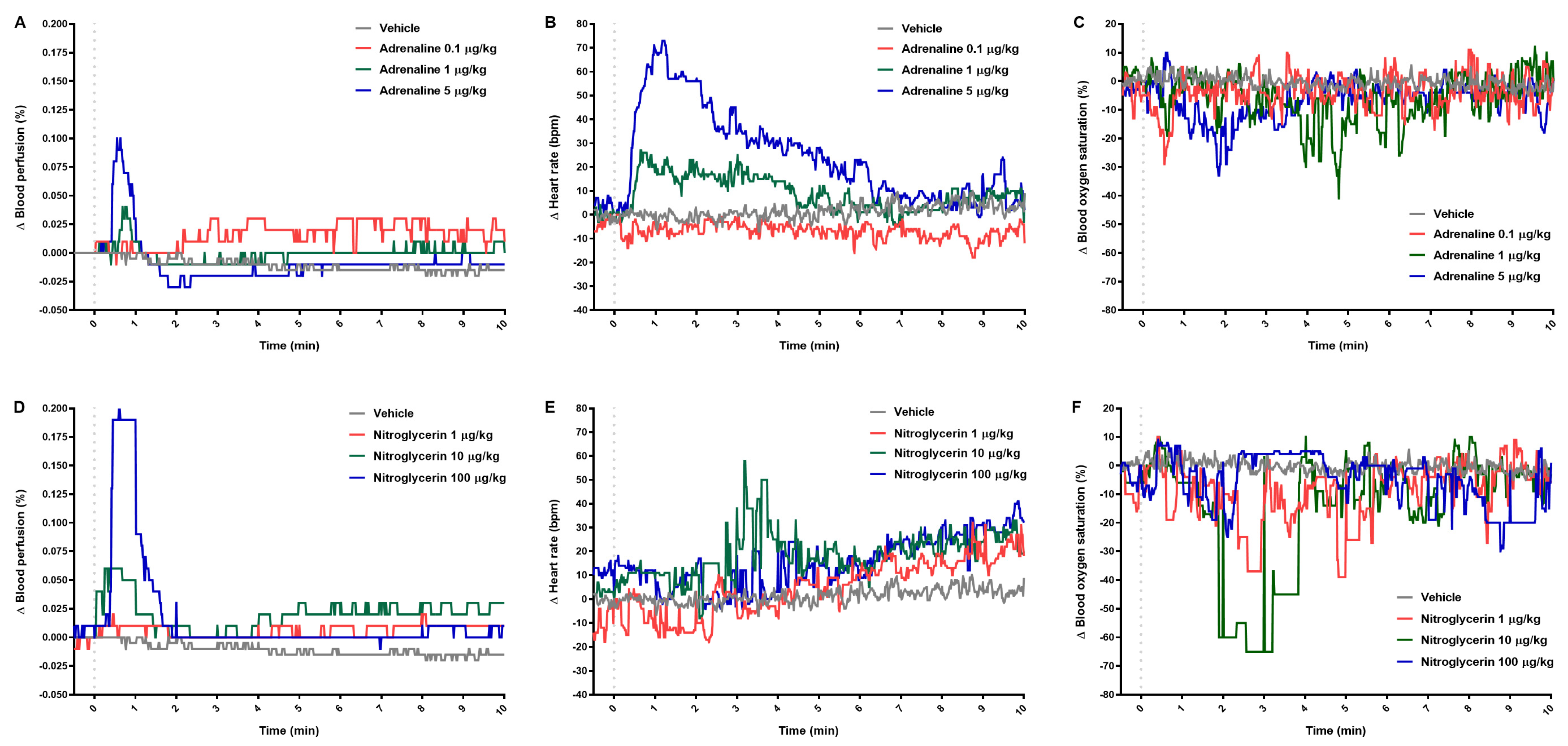
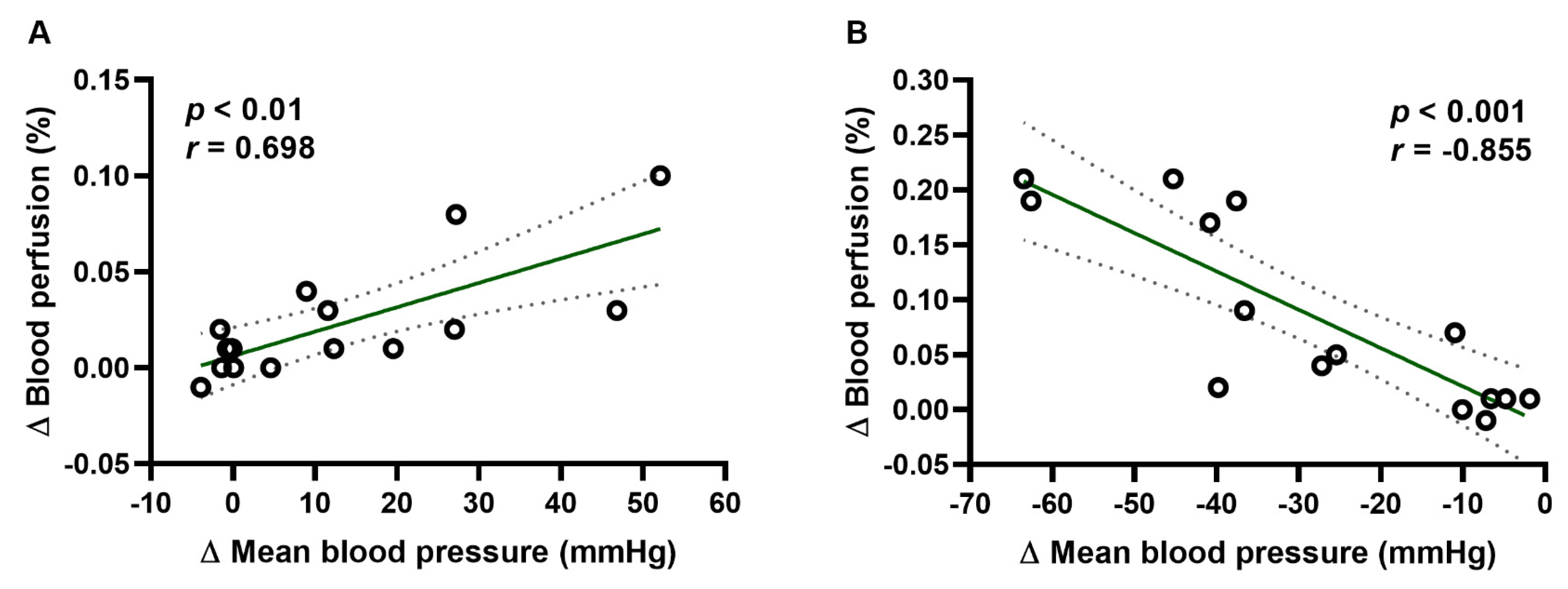
2.2. Blood Perfusion of Paw Vessels, Heart Rate, and Blood Oxygen Saturation
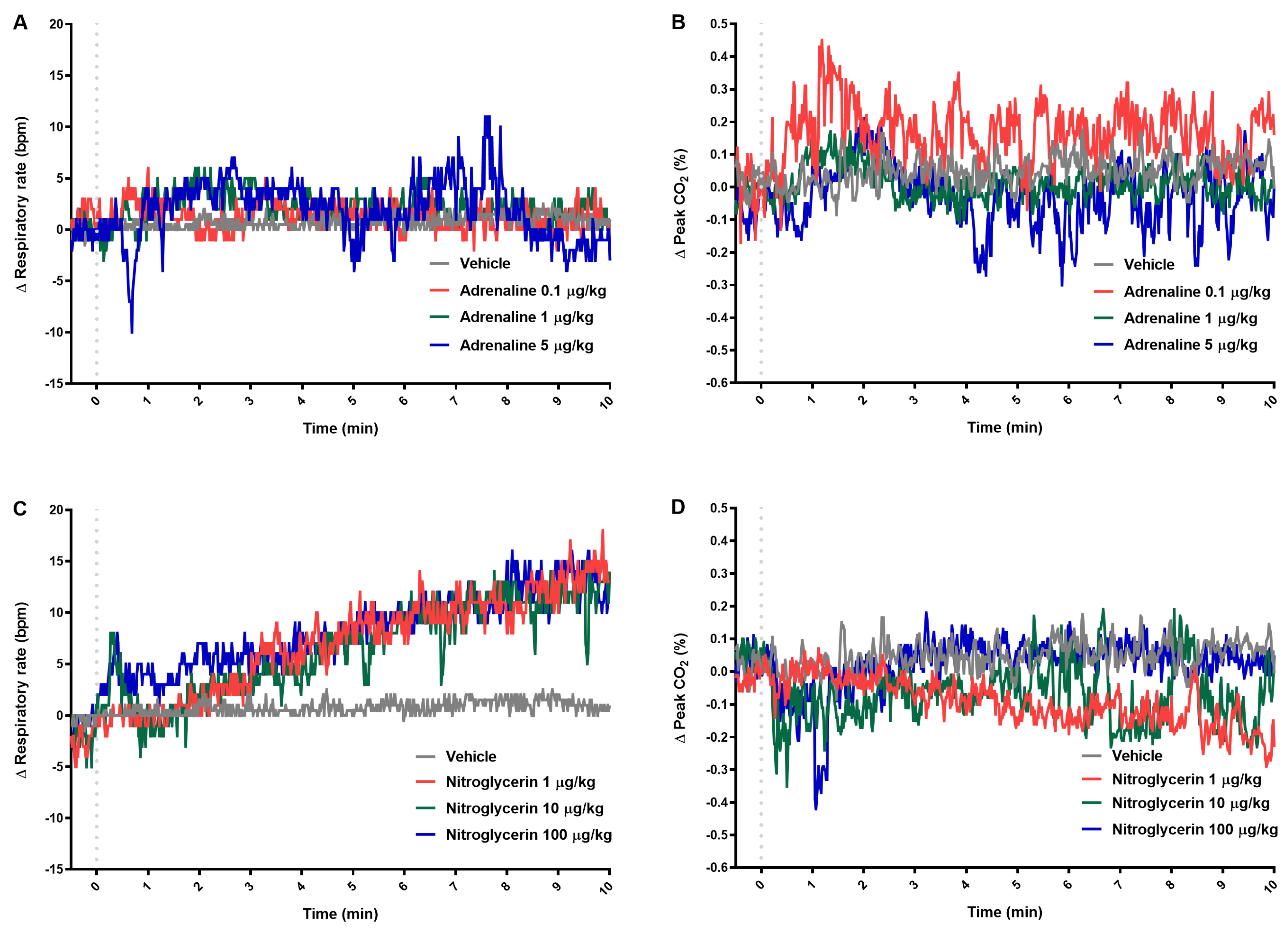
2.3. Respiratory Parameters
2.4. Results Obtained by Separated Methods
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Drugs
4.3. Animals
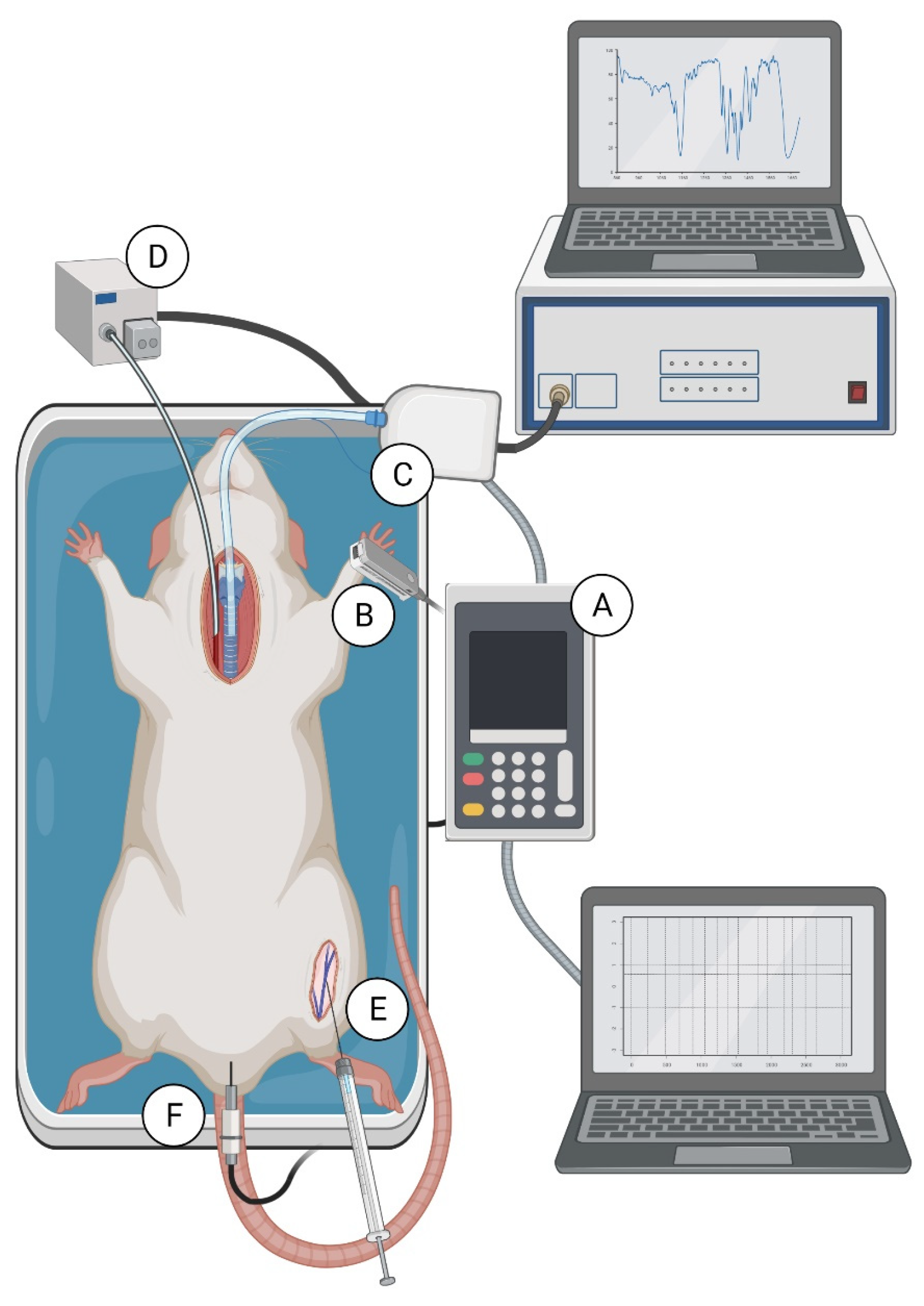
4.4. Experimental Procedure Performed with Both Methods Simultaneously
4.5. Experimental Procedure Performed with Both Methods Separately
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, C.; Nagayama, T.; Hashiba, M.; Fujiyoshi, M.; Hizue, M.; Kodaira, H.; Nishida, M.; Suzuki, K.; Takashima, Y.; Ogino, Y.; et al. Potentials and limitations of nonclinical safety assessment for predicting clinical adverse drug reactions: Correlation analysis of 142 approved drugs in Japan. J. Toxicol. Sci. 2013, 38, 581–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clark, M.; Steger-Hartmann, T. A big data approach to the concordance of the toxicity of pharmaceuticals in animals and humans. Regul. Toxicol. Pharmacol. 2018, 96, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Guth, B.D. Preclinical cardiovascular risk assessment in modern drug development. Toxicol. Sci. 2007, 97, 4–20. [Google Scholar] [CrossRef]
- Papadimitriou, D.; Xanthos, T.; Dontas, I.; Lelovas, P.; Perrea, D. The use of mice and rats as animal models for cardiopulmonary resuscitation research. Lab. Anim. 2008, 42, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.J. The rat: A model used in biomedical research. Methods Mol. Biol. 2010, 597, 1–11. [Google Scholar]
- Salazar, G.M.; Revilla Monsalve, M.C.; Palomino, G.M.A.; Arteaga, M.M.; Díaz-Cintra, S.; De La Rosa-Santander, P.; Roque-Ramírez, B.; Concepción, D.G. Chronological and morphological study of heart development in the rat. Anat. Rec. 2012, 295, 1267–1290. [Google Scholar]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Janssen, P.M. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol. Ther. 2014, 141, 235–249. [Google Scholar] [CrossRef]
- Malkoff, J. Non-Invasive Blood Pressure for Mice and Rats. In Animal Lab News; Kent Scientific Corporation: Torrington, CT, USA, 2005; pp. 1–12. [Google Scholar]
- Ossenberg, F.W.; Peignoux, M.; Bourdiau, D.; Benhamou, J.P. Pentobarbital pharmacokinetics in the normal and in the hepatectomized rat. J. Pharmacol. Exp. Ther. 1975, 194, 111–116. [Google Scholar]
- Stokes, E.L.; Flecknell, P.A.; Richardson, C.A. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 2009, 43, 149–154. [Google Scholar] [CrossRef]
- Gross, D.R. Animal Models in Cardiovascular Research; Springer: New York, NY, USA, 2009. [Google Scholar]
- Benessiano, J.; Levy, B.I.; Michel, J.B. Instantaneous aortic blood flow measurement with range-gated Doppler flowmeter in anesthetized rat. J. Pharmacol. Methods 1985, 14, 99–110. [Google Scholar] [CrossRef]
- Tuma, R.F.; Irion, G.L.; Vasthare, U.S.; Heinel, L.A. Age-related changes in regional blood flow in the rat. Am. J. Physiol. 1985, 249, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, L.I.; Manriquez, M.C.; Nath, K.A.; Romero, J.C.; Juncos, L.A. Low-dose angiotensin II enhances pressor responses without causing sustained hypertension. Hypertension 2003, 42, 798–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kurtz, T.W.; Griffin, K.A.; Bidani, A.K.; Davisson, R.L.; Hall, J.E. Recommendations for blood pressure measurement in humans and experimental animals: Part 2: Blood pressure measurement in experimental animals: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e22–e23. [Google Scholar] [PubMed]
- Sano, Y.; Ito, S.; Yoneda, M.; Nagasawa, K.; Matsuura, N.; Yamada, Y.; Uchinaka, A.; Bando, Y.K.; Murohara, T.; Nagata, K. Effects of various types of anesthesia on hemodynamics, cardiac function, and glucose and lipid metabolism in rats. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1360–H1366. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 2009, 15, 1291. [Google Scholar] [CrossRef] [PubMed]
- Brockway, B.P.; Mills, P.A.; Azar, S.H. A new method for continuous chronic measurement and recording of blood pressure, heart rate and activity in the rat via radio-telemetry. Clin. Exp. Hypertens. 1991, 13, 885–895. [Google Scholar] [CrossRef]
- Mattson, D.L. Long-term measurement of arterial blood pressure in conscious mice. Am. J. Physiol. 1998, 274, R564–R5670. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.; Kinter, L.B. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol. Genom. 2003, 13, 197–205. [Google Scholar] [CrossRef]
- Accardi, M.V.; Troncy, E.; Abtout, S.; Ascah, A.; Maghezzi, S.; Authier, S. Rat cardiovascular telemetry: Marginal distribution applied to positive control drugs. J. Pharmacol. Toxicol Methods 2016, 81, 120–127. [Google Scholar] [CrossRef]
- Sarazan, R.D.; Schweitz, K.T. Standing on the shoulders of giants: Dean Franklin and his remarkable contributions to physiological measurements in animals. Adv. Physiol. Educ. 2009, 33, 144–156. [Google Scholar] [CrossRef]
- Huetteman, D.A.; Bogie, H. Direct Blood Pressure Monitoring in Laboratory Rodents via Implantable Radio Telemetry. Methods Mol. Biol. 2009, 573, 57–73. [Google Scholar]
- Niemeyer, J.E. Telemetry for small animal physiology. Lab. Anim. 2016, 45, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Gross, V.; Luft, C.F. Exercising Restraint in Measuring Blood Pressure in Conscious Mice. Hypertension 2003, 41, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Irvine, R.J.; White, I.J.; Chan, R. The influence of restraint on blood pressure in the rat. J. Pharmacol. Toxicol. Methods 1997, 38, 157–162. [Google Scholar] [CrossRef]
- Delaunois, A.; Dedoncker, P.; Hanon, E.; Guyaux, M. Repeated assessment of cardiovascular and respiratory functions using combined telemetry and whole-body plethysmography in the rat. J. Pharmacol. Toxicol. Methods 2009, 60, 117–129. [Google Scholar] [CrossRef]
- Nirogi, R.; Shanmuganathan, D.; Jayarajan, P.; Abraham, R.; Kancharla, B. Comparison of whole body and head out plethysmography using respiratory stimulant and depressant in conscious rats. J. Pharmacol. Toxicol. Methods 2012, 65, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, Health and Human Services. International Conference on Harmonisation; guidance on S7A safety pharmacology studies for human pharmaceuticals; availability. Notice. Fed. Regist. 2001, 66, 36791–36792. [Google Scholar]
- Jing, W.; Mu-Huo, J.; Zhong-Yun, W.; Wei, Z.; Jian-Jun, Y.; Yong, G.P. Blood pressure reduction induced by low dose of epinephrine via different routes in rats. J. Cardiovasc. Pharmacol. 2013, 62, 325–328. [Google Scholar]
- Westfall, T.C.; Westfall, D.P. Adrenergic agonists and antagonists. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics; Brunton, L.L., Chabner, B.A., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Yamaguchi, S.; Chiba, M.; Yamane, A.; Ohshima, S.; Komatsu, K. Dose-response effects of adrenergic drugs on axial movements of the rat mandibular incisor and on arterial blood pressure. Arch. Oral. Biol. 1997, 42, 801–809. [Google Scholar] [CrossRef]
- Friström, S.; Marvola, M.; Klinge, E.; Halmekoski, J. Effect of Acetyl Derivatives of some Sympathomimetic Amines on the Blood Pressure of the Rat. Acta Pharmacol. Toxicol. 1977, 40, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hafen, B.B.; Sharma, S. Oxygen Saturation; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Heinzow, B.; Ziegler, A. Comparison of the Effects of Nitroglycerin Administered to Rats by Different Routes. J. Cardiovasc. Pharmacol. 1981, 3, 573–580. [Google Scholar] [CrossRef]
- McDonald, L.J.; Murad, F. Nitric Oxide and Cyclic GMP Signaling. Proc. Soc. Exp. Biol. Med. 1996, 211, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, T.M.; Mendes-Júnior, L.G.; Guimarães, D.D.; França-Silva, M.S.; Nalivaiko, E.; Braga, V.A. Cardiorespiratory effects induced by 2-nitrate-1,3-dibuthoxypropan are reduced by nitric oxide scavenger in rats. Auton. Neurosci. 2014, 181, 31–36. [Google Scholar] [CrossRef]
| Adrenaline Dose | Nitroglycerin Dose | |||||
|---|---|---|---|---|---|---|
| T (min) | Low | Medium | High | Low | Medium | High |
| Δ Systolic blood pressure (mmHg) | ||||||
| 0.5 | 4.3 (0.9–8.3) | 22.2 (10.9–26.4) b | 63.9 (39.9–67.0) c | −10.6 (−17.6–−5.7) | −36.7 (−44.0–−31.4) b | −62.5 (−74.0–−56.8) c |
| 1 | −1.7 (−3.7–1.8) | 8.9 (4.4–13.5) | 29.1 (21.2–57.8) b | −6.8 (−10.0–−2.3) | −28.2 (−40.5–10.3) a | −46.5 (−64.0–−37.6) c |
| 2 | −0.9 (−2.0–2.2) | −5.3 (−6.1–−0.2) | 0.0 (−8.4–6.8) | 1.1 (−0.1–4.4) | −11.1 (−23.2– −3.4) | −27.4 (−49.8–−9.4) a |
| 3 | −1.3 (−3.9–0.9) | −1.6 (−6.2–0.3) | −6.4 (−13.4–−5.0) c | 3.7 (1.4–7.4) | −2.8 (−7.0–1.5) | −15.6 (−44.2–1.4) |
| 10 | −3.3 (−5.2–3.1) | 6.4 (−0.2–10.3) | −3.0 (−9.2–4.5) | 13.7 (8.8–17.0) a | 8.4 (2.9–13.9) | 4.8 (−2.6–15.1) |
| 30 | −10.9 (−21.4–3.6) | 10.3 (−5.9–24.4) | 0.6 (−7.3–13.5) | 10.4 ± 2.5 | 4.3 ± 6.8 | 4.9 ± 11.7 |
| 60 | −21.5 (−24.9–9.5) | 10.1 (−16.9–24.0) | −2.9 (−10.9–13.5) | 6.8 ± 4.6 | −2.6 ± 6.1 | 2.9 ± 11.5 |
| Δ Diastolic blood pressure (mmHg) | ||||||
| 0.5 | 4.0 (0.9–7.6) | 13.1 (7.6–23.4) a | 55.5 (33.2–65.9) c | −9.3 (−15.5–−6.1) | −34.4 (−41.9–−30.7) b | −61.5 (−73.0–−53.8) c |
| 1 | −0.8 (−4.0–0.8) | 7.3 (0.2–10.9) | 25.2 (17.9–50.6) b | −6.4 (−9.3–−1.9) | −27.1 (−40.1–−10.9) a | −46.5 (−66.0–−42.7) c |
| 2 | −0.6 ± 2.0 | −4.8 ± 3.8 | −2.8 ± 7.4 | 1.8 (1.1–4.6) | −11.1 (−19.3–−2.4) | −25.2 (−48.6–−11.1) a |
| 3 | −0.2 (−4.0–1.5) | −3.5 (−7.1–−0.2) | −9.2 (−14.0–−6.2) c | 4.6 (2.3–7.8) | −1.4 (−6.5–2.8) | −12.9 (−42.2–2.2) |
| 10 | −3.0 (−5.5–4.7) | 7.9 (0.5–9.9) | −2.2 (−8.2–6.0) | 15.7 (9.7–19.1) a | 10.3 (3.4–15.0) | 6.2 (−7.6–17.0) |
| 30 | −10.7 (−21.0–4.8) | 9.9 (−5.4–23.3) | 1.3 (−6.2–13.5) | 12.7 ± 2.3 | 5.1 ± 8.0 | 5.7 ± 12.9 |
| 60 | −17.8 (−24.6–9.5) | 10.7 (−15.1–22.8) | −3.2 (−12.0–10.6) | 9.2 ± 3.0 | −0.5 ± 7.1 | 3.9 ± 11.4 |
| Δ Mean blood pressure (mmHg) | ||||||
| 0.5 | 4.3 (1.1–8.0) | 15.5 (13.5–24.9) b | 56.4 (36.8–66.5) c | −9.8 (−16.6–−5.9) | −35.6 (−42.2–−31.4) b | −62.0 (−73.5–−55.4) c |
| 1 | −1.4 (−3.9–0.1) | 8.9 (−0.1–12.3) | 27.2 (19.6–52.1) b | −6.6 (−10.1–−1.9) | −27.2 (−39.8–−11.0) a | −45.3 (−63.5–−37.6) c |
| 2 | −0.8 ± 1.9 | −4.3 ± 3.4 | −2.6 ± 6.7 | 1.4 (0.7–3.9) | −11.4 (−21.3– −3.0) | −26.7 (−49.1–−10.2) a |
| 3 | −1.0 (−4.2–1.1) | −2.6 (−6.7–1.1) | −8.7 (−17.4–−5.8) c | 4.1 (1.8–7.2) | −2.5 (−6.5–2.0) | −14.5 (−43.1–1.8) |
| 10 | −2.9 (−5.6–3.8) | 7.7 (0.9–10.1) | −3.1 (−10.0–5.1) | 14.3 (9.4–17.5) a | 8.9 (2.6–14.3) | 5.6 (−4.2–15.7) |
| 30 | −10.8 (−21.0–4.0) | 10.1 (−5.7–23.9) | 1.0 (−6.6–13.6) | 11.2 ± 2.4 | 4.6 ± 7.4 | 5.3 ± 12.2 |
| 60 | −19.6 (−24.7–9.1) | 10.4 (−16.0–23.4) | −3.1 (−11.0–13.3) | 7.9 ± 3.8 | −1.7 ± 6.5 | 3.1 ± 11.4 |
| Adrenaline Dose | Nitroglycerin Dose | |||||
|---|---|---|---|---|---|---|
| T (min) | Low | Medium | High | Low | Medium | High |
| Δ Blood perfusion (%) | ||||||
| 0.5 | 0.0 (−0.01–0.02) | 0.01 (−0.01–0.03) | 0.08 (0.0–0.12) a | 0.01 (0.0–0.03) | 0.06 (0.02–0.09) a | 0.19 (0.0–0.29) b |
| 1 | 0.0 (−0.01–0.02) | 0.01 (0.0–0.04) a | 0.03 (0.01–0.1) b | 0.01 (0.0–0.01) | 0.05 (0.02–0.09) a | 0.19 (0.17–0.21) c |
| 2 | 0.0 (0.0–0.03) | −0.01 (−0.04–0.02) | −0.03 (−0.04–0.01) | 0.01 (−0.01–0.01) | 0.02 (0.0–0.03) a | 0.03 (0.01–0.04) b |
| 3 | 0.03 (0.01–0.03) | −0.01 (−0.03–0.02) | −0.02 (−0.04–0.01) | 0.0 (−0.01–0.01) | 0.01 (0.0–0.02) | 0.0 (0.0–0.02) |
| 10 | 0.01 (−0.01–0.05) a | 0.0 (−0.01–0.03) | −0.01 (−0.01–0.0) | 0.01 (−0.01–0.02) | 0.03 (0.0–0.04) b | 0.01 (−0.01–0.06) |
| 30 | −0.01 (−0.01–0.02) | 0.0 (−0.05–0.01) | 0.0 (−0.01–0.01) a | 0.0 (−0.01–0.01) | 0.02 (0.0–0.03) b | −0.01 (−0.03–0.08) |
| 60 | −0.02 (−0.03–0.0) | −0.01 (−0.06–−0.01) | −0.01 (−0.01–0.0) a | −0.01 (−0.03–0.0) | 0.0 (−0.02–0.01) a | −0.01 (−0.03–0.07) |
| Δ Heart rate (bpm) | ||||||
| 0.5 | −2.0 (−4.0–0.0) | 11.0 (3.0–21.0) | 43.0 (35.0–47.0) b | 2.0 (−33.0–5.0) | 11.0 (−4.0–16.0) | 12.0 (0.0–27.0) |
| 1 | −6.0 (−16.0–4.0) | 21.0 (16.0–31.0) | 69.0 (−61.0–102.0) b | −2.0 (−10.0–0.0) | 9.0 (6.0–33.0) a | 12.0 (3.0–30.0) a |
| 2 | −5.0 (−17.0–3.0) | 22.0 (17.0–34.0) | 57.0 (43.0–82.0) b | −5.0 (−8.0–3.0) | 15.0 (8.0–42.0) b | 13.0 (−2.0–30.0) |
| 3 | −6.0 (−16.0–0.0) | 25.0 (10.0–36.0) | 35.0 (18.0–61.0) a | −8.0 (−10.0–9.0) | 38.0 (26.0–50.0) a | 18.0 (1.0–30.0) |
| 10 | −12.0 (−23.0–−3.0) a | 9.0 (−13.0–16.0) | 2.0 (−9.0–33.0) | 9.0 (2.0–30.0) | 20.0 (16.0–29.0) | 32.0 (8.0–43.0) a |
| 30 | −25.0 (−34.0–−9.0) | 19.0 (0.0–36.0) | 8.0 (−42.0–50.0) | 15.0 (7.0–33.0) | 22.0 (18.0–33.0) b | 24.0 (−11.0–50.0) |
| 60 | −38.0 (−48.0–−3.0) | 15.0 (5.0–53.0) | 43.0 (−56.0–55.0) | 8.0 (−2.0–40.0) | 1.0 (1.0–13.0) | 26.0 (−22.0–35.0) |
| Δ Blood oxygen saturation (%) | ||||||
| 0.5 | −14.8 ± 19.4 | −9.4 ± 13.2 | 1.2 ± 11.1 | 0.0 (−34.0–10.0) | 8.0 (−6.0–15.0) | 7.0 (−21.0–12.0) |
| 1 | −0.6 ± 6.3 | −0.2 ± 6.1 | −1.0 ± 10.9 | −3.4 ± 6.5 | −4.8 ± 3.0 | −4.8 ± 7.4 |
| 2 | −4.0 (−9.0–5.0) | 0.0 (−16.0–2.0) | −13.0 (−44.0–0.0) a | −6.0 (−13.0–−2.0) a | 1.0 (−3.0–2.0) | 1.0 (−3.0–4.0) |
| 3 | −4.0 ± 4.5 | −6.0 ± 7.7 | −14.2 ± 18.4a | −7.0 (−11.0–−3.0) | 3.0 (0.0–3.0) | 4.0 (−9.0–5.0) |
| 10 | 1.0 (−2.0–4.0) | 0.0 (−6.0–18.0) | 1.0 (−13.0–3.0) | −6.0 (−8.0–3.0) | −3.0 (−5.0–6.0) | 1.0 (−8.0–7.0) |
| 30 | −6.0 (−11.0–−4.0) | −11.0 (−19.0–4.0) | −23.0 (−42.0–0.0) a | −5.0 ± 5.8 | −1.8 ± 3.3 | −3.6 ± 4.7 |
| 60 | −14.0 (−25.0–−11.0) a | −8.0 (−23.0–3.0) | −12.0 (−13.0–−8.0) | −4.2 ± 3.1 | −3.4 ± 2.2 | −6.2 ± 6.8 |
| Adrenaline Dose | Nitroglycerin Dose | |||||
|---|---|---|---|---|---|---|
| T (min) | Low | Medium | High | Low | Medium | High |
| Δ Respiratory rate (bpm) | ||||||
| 0.5 | 2.2 ± 2.0 | 2.2 ± 6.7 | −0.4 ± 7.9 | 0.8 ± 5.2 | 5.0 ± 4.5 | 3.0 ± 8.6 |
| 1 | 6 (2–23) b | 2 (1–4) | 4 (2–17) a | −1.0 (−2.0–0.0) | 0.0 (−2.0–5.0) | 5.0 (2.0–8.0) |
| 2 | 1.2 ± 1.8 | 3.2 ± 4.8 | 5.4 ± 4.5 | 0.0 (−1.0–2.0) | 1.0 (−2.0–7.0) | 7.0 (1.0–7.0) |
| 3 | 2.4 ± 2.2 | 3.2 ± 3.7 | 6.4 ± 6.2 | 2.0 (−3.0–5.0) | 4.0 (1.0–5.0) | 6.0 (3.0–10.0) b |
| 10 | 0.6 ± 2.7 | 1.2 ± 3.0 | 0.0 ± 6.8 | 13.0 (5.0–20.0) a | 14.0 (7.0–18.0) a | 13.0 (9.0–17.0) b |
| 30 | −1.8 ± 3.7 | 3.2 ± 2.8 | 0.4 ± 7.2 | 21.0 (14.0–34.0) b | 17.0 (8.0–19.0) | 17.0 (1.0–21.0) a |
| 60 | 1.0 ± 3.5 | 3.4 ± 3.5 | 4.6 ± 2.8 | 26.0 (10.0–31.0) b | 21.0 (10.0–24.0) | 19.0 (7.0–30.0) |
| Δ Peak CO2 (%) | ||||||
| 0.5 | 0.1 ± 0.6 | 0.0 ± 0.1 | −0.1 ± 0.2 | −0.1 ± 0.2 | −0.4 ± 0.4 | −0.1 ± 0.1 |
| 1 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 (−0.1–0.1) | −0.1 (−0.2–0.0) | −0.1 (−0.4–−0.1) a |
| 2 | 0.1 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.0 ± 0.1 | −0.1 ± 0.1 | −0.1 ± 0.2 |
| 3 | 0.1 ± 0.1 | 0.0 ± 0.1 | 0.1 ± 0.1 | −0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.2 |
| 10 | 0.2 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.1 | −0.2 (−0.3–−0.1) a | 0.0 (−0.3–0.1) | 0.1 (−0.1–0.1) |
| 30 | 0.2 (0.1–0.5) | −0.1 (−0.3–−0.1) a | −0.1 (−0.2–0.2) | −0.3 (−0.4–−0.2) c | −0.1 (−0.3–0.0) | −0.1 (−0.4–0.1) |
| 60 | 0.1 (−0.1–0.3) | −0.1 (−0.2–0.0) | −0.2 (−0.3–−0.1) | −0.4 (−0.4–−0.3 ) b | −0.2 (−0.2–−0.1) | −0.2 (−0.2–−0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miklosz, J.; Kalaska, B.; Zajaczkowski, S.; Pawlak, D.; Mogielnicki, A. Monitoring of Cardiorespiratory Parameters in Rats—Validation Based on Pharmacological Stimulation. Pharmaceuticals 2021, 14, 1223. https://doi.org/10.3390/ph14121223
Miklosz J, Kalaska B, Zajaczkowski S, Pawlak D, Mogielnicki A. Monitoring of Cardiorespiratory Parameters in Rats—Validation Based on Pharmacological Stimulation. Pharmaceuticals. 2021; 14(12):1223. https://doi.org/10.3390/ph14121223
Chicago/Turabian StyleMiklosz, Joanna, Bartlomiej Kalaska, Stanislaw Zajaczkowski, Dariusz Pawlak, and Andrzej Mogielnicki. 2021. "Monitoring of Cardiorespiratory Parameters in Rats—Validation Based on Pharmacological Stimulation" Pharmaceuticals 14, no. 12: 1223. https://doi.org/10.3390/ph14121223
APA StyleMiklosz, J., Kalaska, B., Zajaczkowski, S., Pawlak, D., & Mogielnicki, A. (2021). Monitoring of Cardiorespiratory Parameters in Rats—Validation Based on Pharmacological Stimulation. Pharmaceuticals, 14(12), 1223. https://doi.org/10.3390/ph14121223







