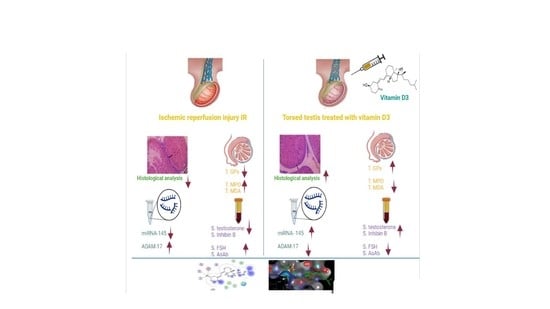
Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting miRNA-145 and ADAM17: In Silico and In Vivo Study
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Grouping
- Control Naïve group.
- Sham operated group (SHAM); Rats subjected to all surgical steps as the other two groups except for torsion/detorsion.
- Testicular Torsion/Detorsion group (T/D): T/D group: Rats were subjected to 720° torsion for 2 h then detorsion with subcutaneously injection of sesame oil (as a vehicle to vitamin D3) for 30 days.
- TT Testicular T/D; vitamin D3 treated group (T/D; D3); Rats subjected to 720° torsion for 2 h then detorsion with subcutaneous injection of vitamin D3 in a dose of 500 IU/Kg/day (20), starting half an hour before detorsion, then given daily, 5 days/week, for 30 days.
2.2. Chemicals and Reagents
2.3. Testicular Torsion/Detorsion Animal Model
2.4. Biochemical Measurements
- Assessment of testicular endocrinal function: Assessment of testicular endocrinal function was performed by measuring the serum level of total testosterone using Steroid EIA (enzyme immunoassay)-Testosterone, ALKPR-BIO, France. Inhibin B was measured by rat specific inhibin B ELISA (enzyme linked immunosorbent assay) kit, My Bio Source, San Diego, CA, USA.
- Determination of testicular oxidative stress markers: Assessment of testicular oxidative stress markers was calorimetrically performed using MDA OxiSelect “TBARS; thiobarbituric acid reactive substances” assay kit, CELL BIOLABS, USA, and GPx assay kit, Cayman Chemical, Ann-Arbor, MI, USA.
- Assessment of testicular inflammatory response: Assessment of testicular inflammatory response was evaluated by measuring MPO using rat specific CLIA (chemiluminescent immunoassay) kit, Life Span Bio Sciences, Seattle, WA, USA.
- Estimation of immunological reaction: Assessment of immunological reaction was estimated by evaluation of serum AsAb using rat specific ELISA kit, Cube Biosystems, College Park, MD, USA.
2.5. Assessment of Apoptotic Process
- Testicular ADAM17 Expression
- ii.
- Assessment of miRNA-145 by Real time PCR
2.6. Histological Studies
- (a)
- Light microscopic study
- (b)
- Transmission electron microscopic (TEM) study
- (c)
- Semen analysis study
2.7. Morphometric Study
- Mean diameter of seminiferous tubules (X20)
- Mean thickness of germinal epithelium (X20)
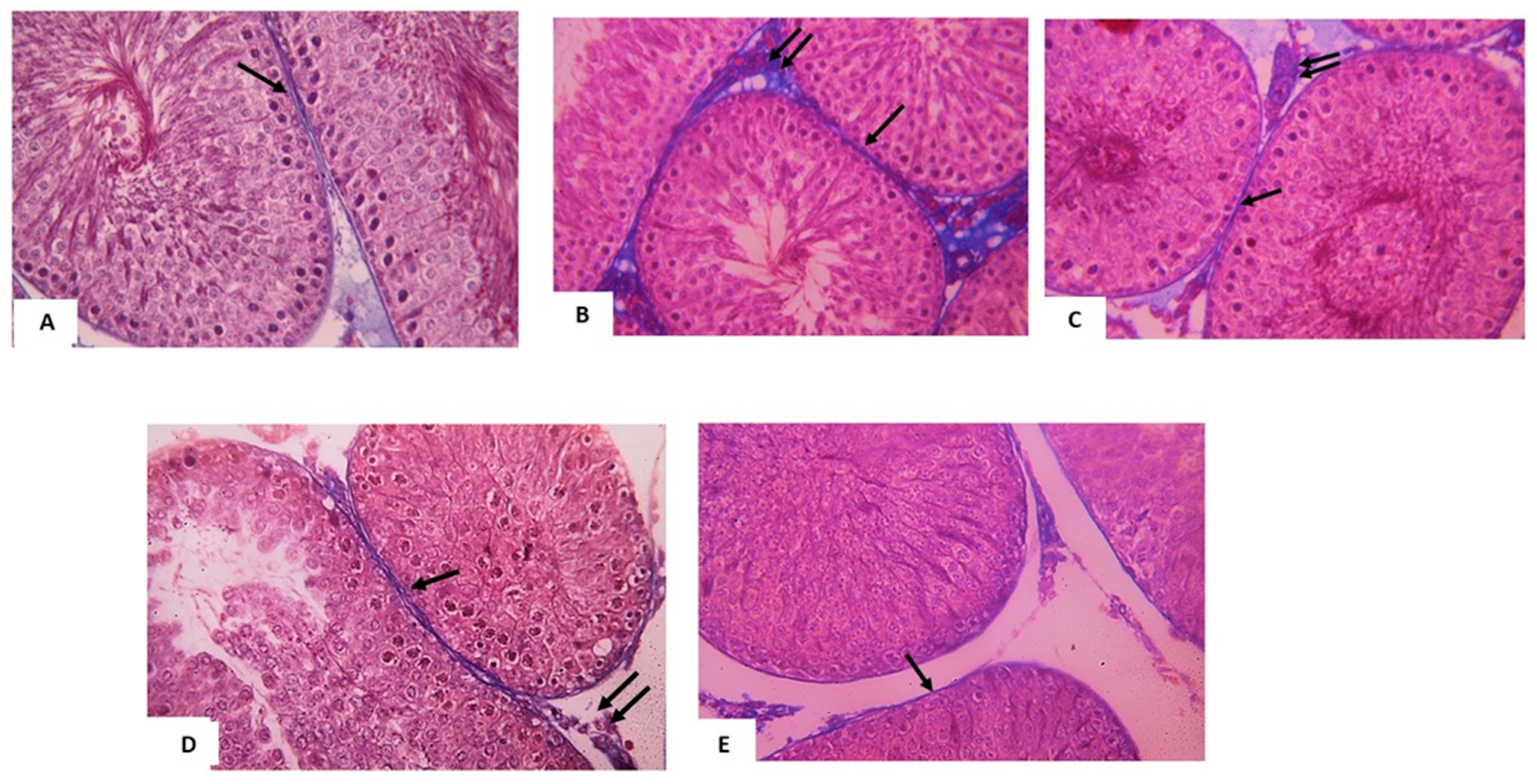
- Mean area percentage of collagen fibers in Mallory’s trichrome stained sections (X20)
2.8. In Silico Molecular Modelling Study
2.9. Statistical Study
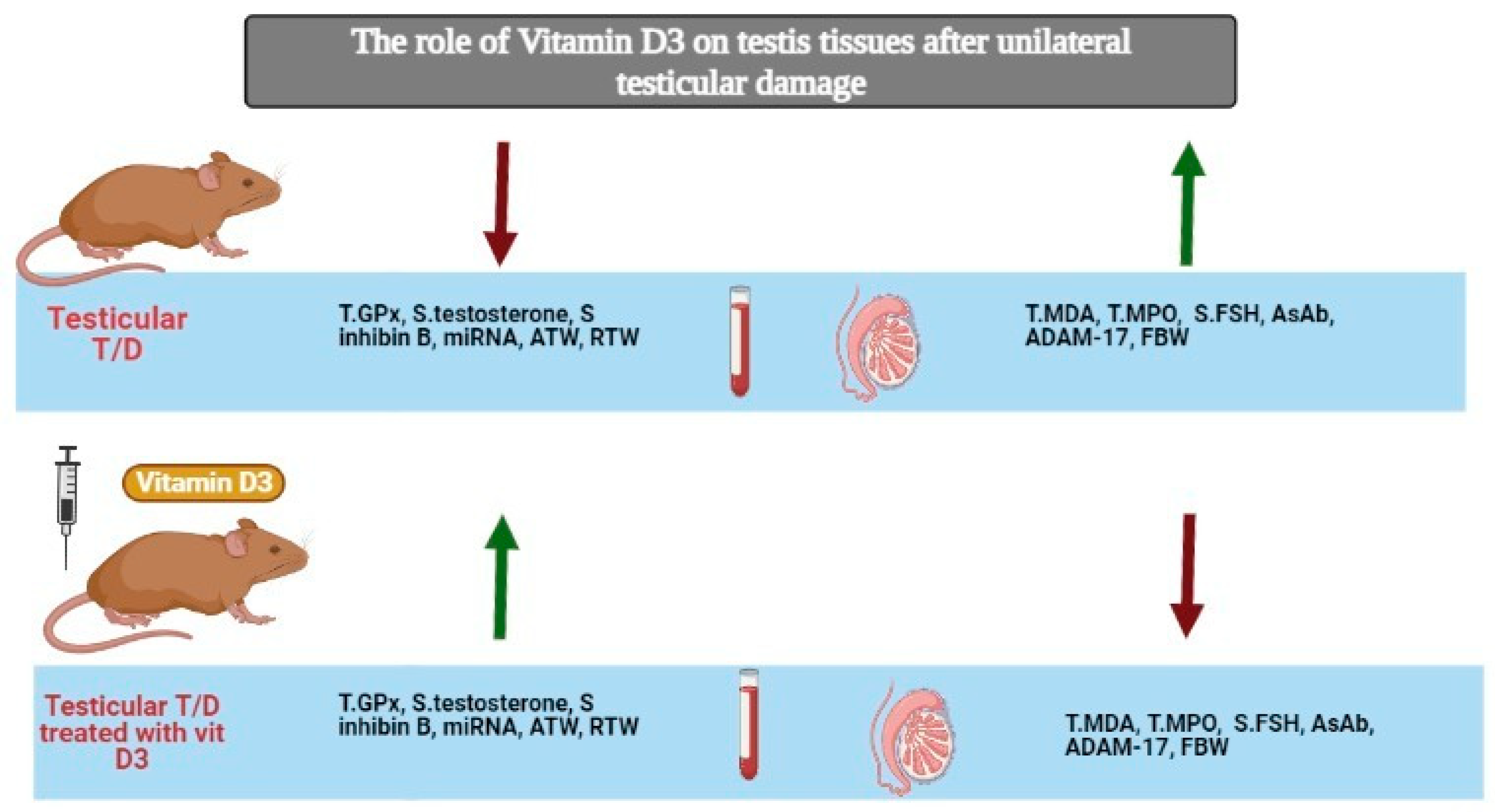
3. Results
3.1. Biochemical Analysis
3.1.1. Effect of Testicular T/D and Vitamin D3 Treatment on Oxidative Stress Markers in Ipsilateral and Contralateral Testis
3.1.2. Effect of Vitamin D3 Treatment on Serum Testosterone, FSH, Inhibin B, Anti-Sperm Antibody in Testicular T/D Rat Model
3.1.3. Effect of Testicular T/D and Vitamin D3 Treatment on Testicular miRNA-145 and ADAM17
3.1.4. Effect of Testicular T/D and Vitamin D3 Treatment on Relative Testicular Weight in Ipsilateral and Contralateral Testis
3.2. Histological Analysis
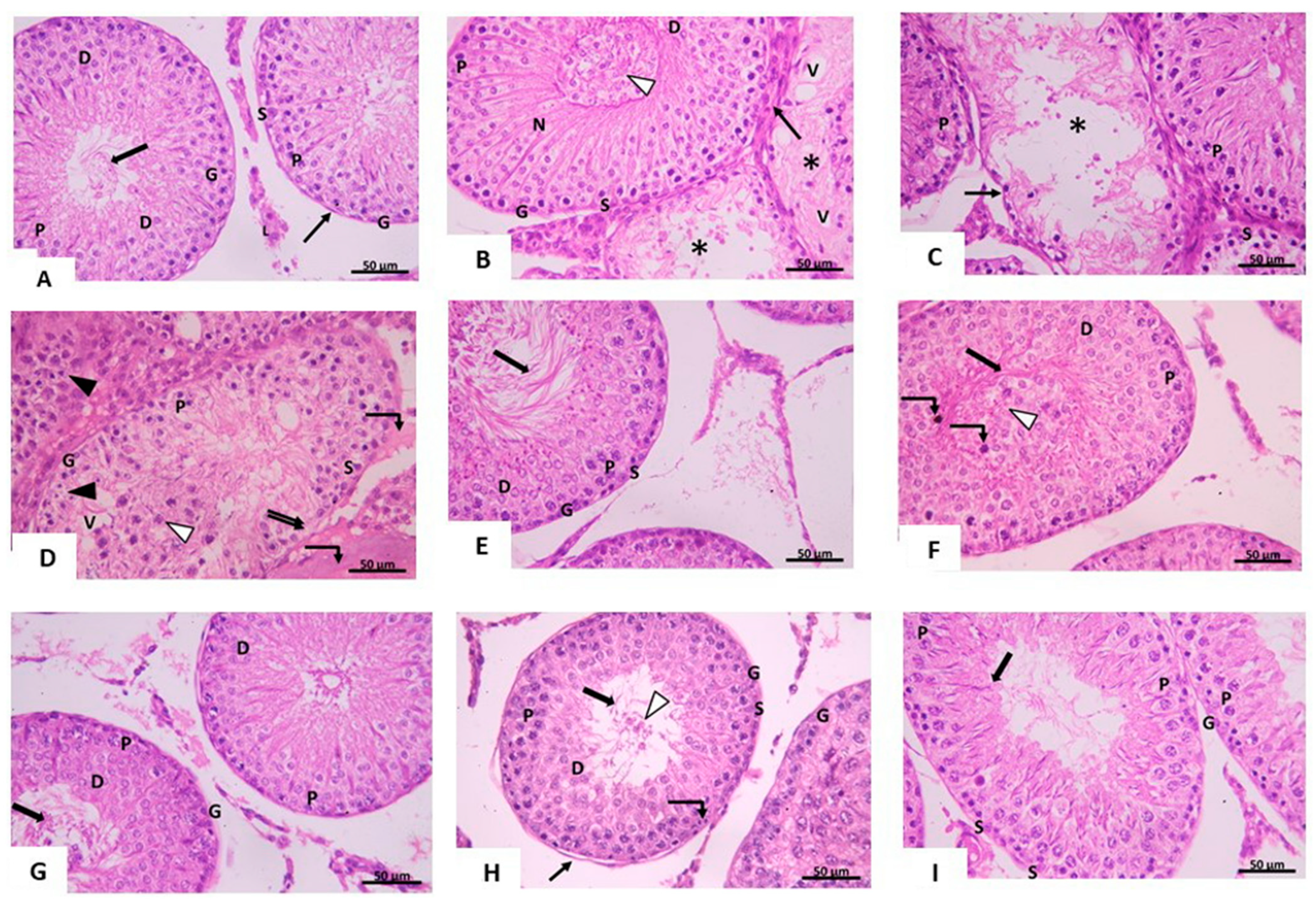
3.2.1. Light Microscopic Analysis
3.2.2. Semen Analysis
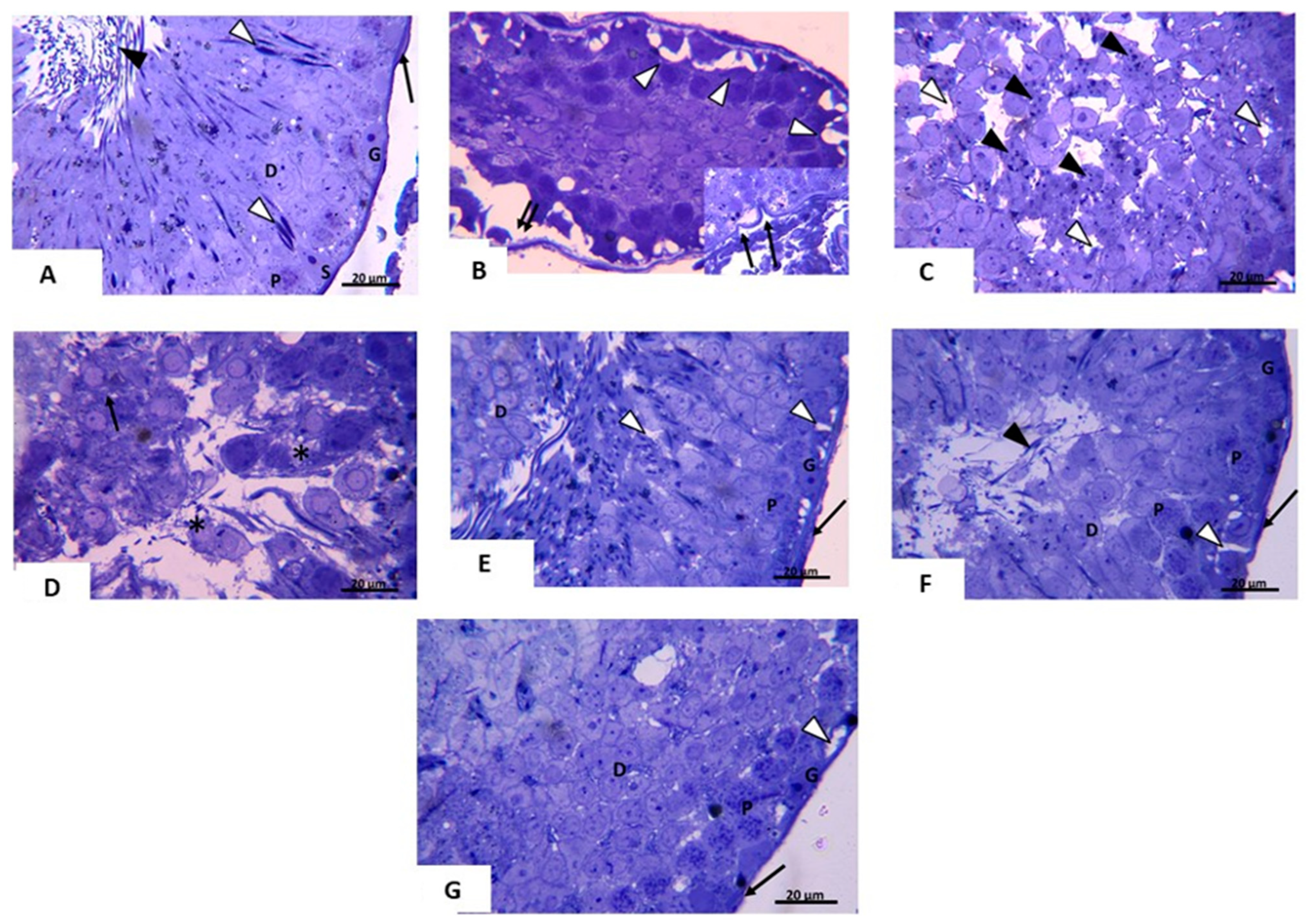
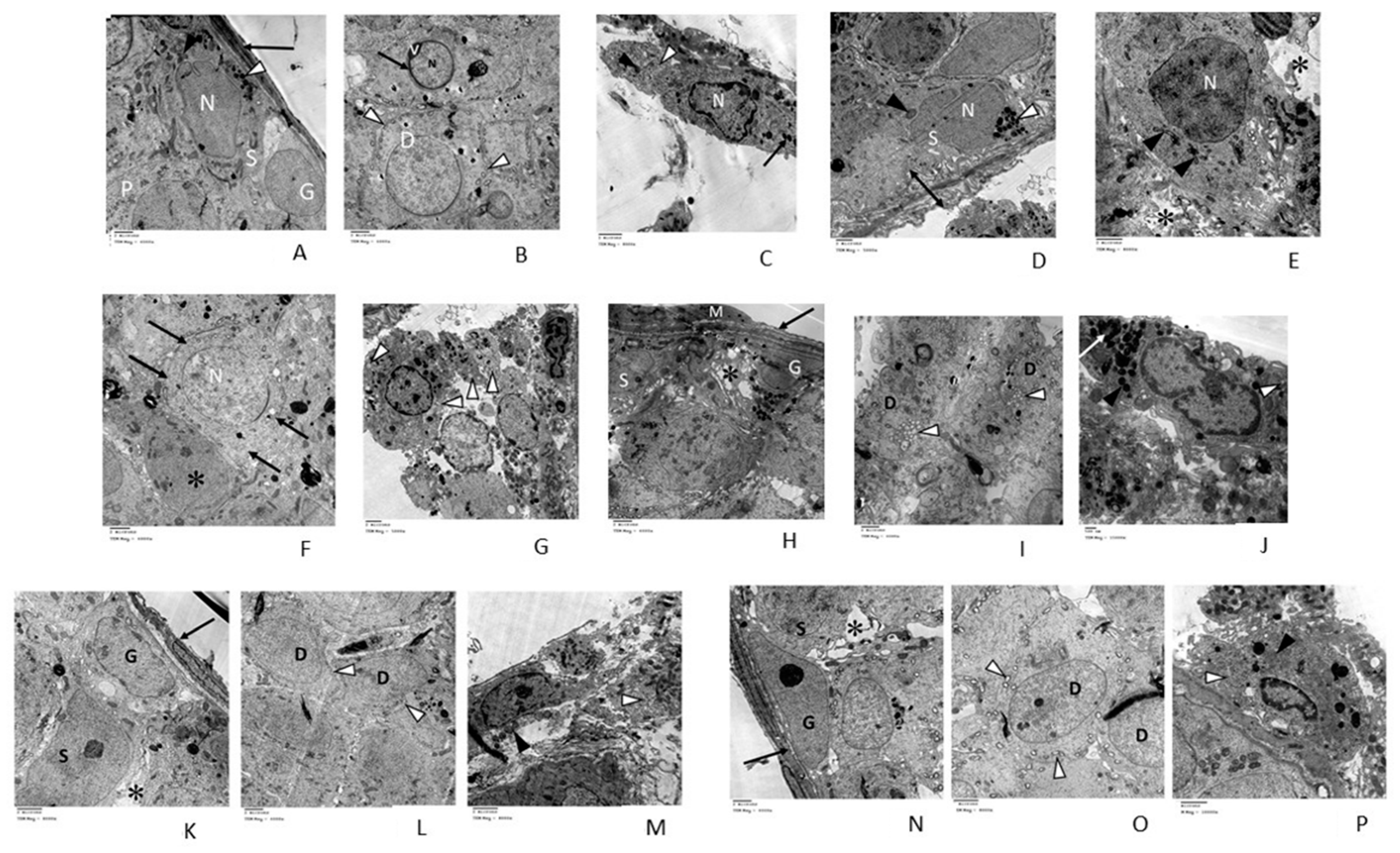
3.2.3. Transmission Electron Microscopic (TEM) Analysis
3.3. Histomorphometry Analysis
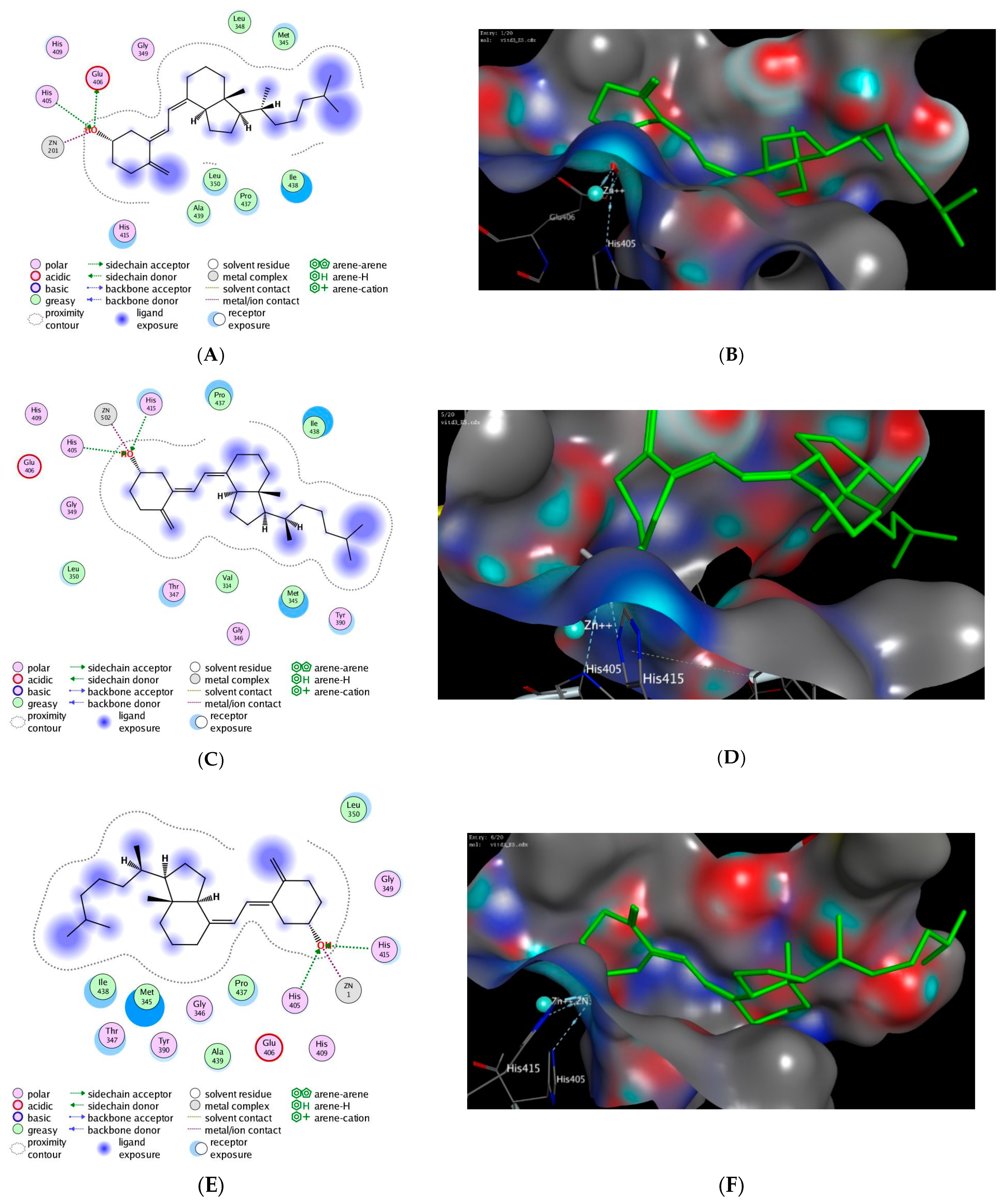
3.4. In Silico Molecular Modelling Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barada, J.H.; Weingarten, J.L.; Cromie, W.J. Testicular Salvage and Age-Related Delay in the Presentation of Testicular Torsion. J. Urol. 1989, 142, 746–748. [Google Scholar] [CrossRef]
- Visser, A.J.; Heyns, C.F. Testicular function after torsion of the spermatic cord. BJU Int. 2003, 92, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Elshaari, F.A.; Elfagih, R.I.; Sheriff, D.S.; Barassi, I.F. Testicular torsion-detorsion-histological and biochemical changes in rat testis. J. Cytol. Histol. 2012, 3, 136. [Google Scholar] [CrossRef]
- Werns, S.W.; Lucchesi, B.R. Free radicals and ischemic tissue injury. Trends Pharmacol. Sci. 1990, 11, 161–166. [Google Scholar] [CrossRef]
- Wei, S.-M.; Huang, Y.-M.; Zhou, J. Probucol Reduces Testicular Torsion/Detorsion-Induced Ischemia/Reperfusion Injury in Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sharar, M.; Saied, E.M.; Rodriguez, M.C.; Arenz, C.; Montes-Bayón, M.; Linscheid, M.W. Elemental labelling and mass spectrometry for the specific detection of sulfenic acid groups in model peptides: A proof of concept. Anal. Bioanal. Chem. 2017, 409, 2015–2027. [Google Scholar] [CrossRef]
- Sertkaya, Z.; Koca, O.; Ozturk, M.; Akyuz, M.; Gumrukcu, G.; Kutluhan, M.A.; Karaman, M.I. Protective Effect of Udenafil Against Ischemia Reperfusion Injury Due to Testicular Torsion/Detorsion in Rat Model. Eurasian J. Med. 2020, 52, 115–119. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Szczurek, M.; Hassan, C.; Masrur, M.; Gangemi, A.; Phillips, S.A. Vitamin D Improves Nitric Oxide-Dependent Vasodilation in Adipose Tissue Arterioles from Bariatric Surgery Patients. Nutrients 2019, 11, 2521. [Google Scholar] [CrossRef]
- Celik, E.; Oğuztürk, H.; Şahin, N.; Turtay, M.G.; Oguz, F.; Çiftçi, O. Protective effects of hesperidin in experimental testicular ischemia/reperfusion injury in rats. Arch. Med. Sci. 2016, 12, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Parlaktas, B.S.; Atilgan, D.; Ozyurt, H.; Gencten, Y.; Akbaş, A.; Erdemir, F.; Uluocak, N. The biochemical effects of ischemia-reperfusion injury in the ipsilateral and contralateral testes of rats and the protective role of melatonin. Asian J. Androl. 2014, 16, 314–318. [Google Scholar] [CrossRef]
- Jeong, S.J.; Choi, W.S.; Chung, J.-S.; Baek, M.; Hong, S.K.; Choi, H. Preventive Effects of Cyclosporine A Combined With Prednisolone and Melatonin on Contralateral Testicular Damage After Ipsilateral Torsion-Detorsion in Pubertal and Adult Rats. J. Urol. 2010, 184, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.J.; Vooijs, M.A. The Role of Adams in Notch Signaling. Adv. Exp. Med. Biol. 2012, 727, 15–36. [Google Scholar] [CrossRef]
- Chemaly, M.; McGilligan, V.; Gibson, M.; Clauss, M.; Watterson, S.; Alexander, H.D.; Bjourson, A.J.; Peace, A. Role of tumour necrosis factor alpha converting enzyme (TACE/ADAM17) and associated proteins in coronary artery disease and cardiac events. Arch. Cardiovasc. Dis. 2017, 110, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.D.; Urriola-Muñoz, P.; Lagos-Cabré, R. The emerging role of matrix metalloproteases of the ADAM family in male germ cell apoptosis. Spermatogenesis 2011, 1, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Chauhan, A.K.; Canault, M.; Walsh, M.T.; Bergmeier, W.; Wagner, D.D. Oxidative stress activates ADAM17/TACE and induces its target receptor shedding in platelets in a p38-dependent fashion. Cardiovasc. Res. 2009, 84, 137–144. [Google Scholar] [CrossRef]
- Urriola-Muñoz, P.; Lizama, C.; Lagos-Cabré, R.; Reyes, J.G.; Moreno, R.D. Differential expression and localization of ADAM10 and ADAM17 during rat spermatogenesis suggest a role in germ cell differentiation. Biol. Res. 2014, 47, 31. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C.; Wang, Y.; Wen, S.; Wang, J.; Chen, Z.; He, Q.; Feng, D. MicroRNA-145 inhibits cell proliferation by directly targeting ADAM17 in hepatocellular carcinoma. Oncol. Rep. 2014, 32, 1923–1930. [Google Scholar] [CrossRef]
- Doberstein, K.; Steinmeyer, N.; Hartmetz, A.-K.; Eberhardt, W.; Mittelbronn, M.; Harter, P.N.; Juengel, E.; Blaheta, R.; Pfeilschifter, J.; Gutwein, P. MicroRNA-145 Targets the Metalloprotease ADAM17 and Is Suppressed in Renal Cell Carcinoma Patients. Neoplasia 2013, 15, 218–230, IN30–IN31. [Google Scholar] [CrossRef]
- Wu, J.; Yin, L.; Jiang, N.; Guo, W.-J.; Gu, J.-J.; Chen, M.; Xia, Y.-Y.; Wu, J.-Z.; Chen, D.; Wang, D.-J.; et al. MiR-145, a microRNA targeting ADAM17, inhibits the invasion and migration of nasopharyngeal carcinoma cells. Exp. Cell Res. 2015, 338, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Ayesh, B.M.; Hart, M.; Alles, J.; Fischer, U.; Hammadeh, M.; Keller, A.; Huleihel, M.; Meese, E. Differential expression of miR-23a/b-3p and its target genes in male patients with subfertility. Fertil. Steril. 2019, 112, 323–335.e2. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Obermayer-Pietsch, B. Vitamin D and fertility: A systematic review. Eur. J. Endocrinol. 2012, 166, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 14103. [Google Scholar] [CrossRef]
- Kanikarla, P.; Jain, S.K. 1,25(OH) 2 D 3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J. Steroid Biochem. Mol. Biol. 2016, 159, 94–101. [Google Scholar] [CrossRef]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of Vitamin D Supplementation on Testosterone Levels in Men. Horm. Metab. Res. 2010, 43, 223–225. [Google Scholar] [CrossRef]
- Asghari, S.; Hamedi-Shahraki, S.; Amirkhizi, F. Vitamin D status and systemic redox biomarkers in adults with obesity. Clin. Nutr. ESPEN 2021, 45, 292–298. [Google Scholar] [CrossRef]
- Ünsal, A.; Eroglu, M.; Avci, A.; Cimentepe, E.; Guven, C.; Balbay, M.D.; Durak, I. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand. J. Urol. Nephrol. 2006, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R. (Eds.) Andrological Evaluation of Male Infertility: A Laboratory Guide; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-26795-1. [Google Scholar]
- Saleh, S.R.; Attia, R.; Ghareeb, D.A. The Ameliorating Effect of Berberine-Rich Fraction against Gossypol-Induced Testicular Inflammation and Oxidative Stress. Oxidative Med. Cell. Longev. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. (Eds.) Theory and Practice of Histological Techniques, 7th ed.; Elsevier Churchill Livingston: Edinburgh, UK, 2013; ISBN 978-0-7020-4226-3. [Google Scholar]
- Raafat, M.H.; Hamam, G.G. The possible protective role of royal jelly against cisplatin-induced testicular lesions in adult albino rats: A Histological and Immunohistochemical Study. Egypt. J. Histol. 2012, 35, 353–365. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.N.; Orth, P.; Strickland, C.L.; Le, H.V.; Madison, V.; Beyer, B.M. Stabilization of the autoproteolysis of TNF-alpha converting enzyme (TACE) results in a novel crystal form suitable for structure-based drug design studies. Protein Eng. Des. Sel. 2006, 19, 155–161. [Google Scholar] [CrossRef]
- Maskos, K.; Fernandez-Catalan, C.; Huber, R.; Bourenkov, G.P.; Bartunik, H.; Ellestad, G.A.; Reddy, P.; Wolfson, M.F.; Rauch, C.T.; Castner, B.J.; et al. Crystal structure of the catalytic domain of human tumor necrosis factor- α-converting enzyme. Proc. Natl. Acad. Sci. USA 1998, 95, 3408–3412. [Google Scholar] [CrossRef]
- Bandarage, U.K.; Wang, T.; Come, J.H.; Perola, E.; Wei, Y.; Rao, B.G. Novel thiol-based TACE inhibitors. Part 2: Rational design, synthesis, and SAR of thiol-containing aryl sulfones. Bioorg. Med. Chem. Lett. 2008, 18, 44–48. [Google Scholar] [CrossRef]
- Condon, J.S.; Joseph-McCarthy, D.; Levin, J.I.; Lombart, H.-G.; Lovering, F.E.; Sun, L.; Wang, W.; Xu, W.; Zhang, Y. Identification of potent and selective TACE inhibitors via the S1 pocket. Bioorg. Med. Chem. Lett. 2007, 17, 34–39. [Google Scholar] [CrossRef]
- Dai, C.; Li, D.; Popovici-Muller, J.; Zhao, L.; Girijavallabhan, V.M.; Rosner, K.E.; Lavey, B.J.; Rizvi, R.; Shankar, B.B.; Wong, M.K.; et al. 2-(2-Aminothiazol-4-yl)pyrrolidine-based tartrate diamides as potent, selective and orally bioavailable TACE inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3172–3176. [Google Scholar] [CrossRef]
- Düsterhöft, S.; Jung, S.; Hung, C.-W.; Tholey, A.; Sönnichsen, F.D.; Grötzinger, J.; Lorenzen, I. Membrane-Proximal Domain of a Disintegrin and Metalloprotease-17 Represents the Putative Molecular Switch of Its Shedding Activity Operated by Protein-disulfide Isomerase. J. Am. Chem. Soc. 2013, 135, 5776–5781. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.G.; Bandarage, U.K.; Wang, T.; Come, J.H.; Perola, E.; Wei, Y.; Tian, S.-K.; Saunders, J.O. Novel thiol-based TACE inhibitors: Rational design, synthesis, and SAR of thiol-containing aryl sulfonamides. Bioorg. Med. Chem. Lett. 2007, 17, 2250–2253. [Google Scholar] [CrossRef]
- Guo, Z.; Orth, P.; Wong, S.-C.; Lavey, B.J.; Shih, N.-Y.; Niu, X.; Lundell, D.J.; Madison, V.; Kozlowski, J.A. Discovery of novel spirocyclopropyl hydroxamate and carboxylate compounds as TACE inhibitors. Bioorg. Med. Chem. Lett. 2008, 19, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.I.; Chen, J.M.; Laakso, L.M.; Du, M.; Du, X.; Venkatesan, A.M.; Sandanayaka, V.; Zask, A.; Xu, J.; Xu, W.; et al. Acetylenic TACE inhibitors. Part 2: SAR of six-membered cyclic sulfonamide hydroxamates. Bioorg. Med. Chem. Lett. 2005, 15, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.I.; Chen, J.M.; Laakso, L.M.; Du, M.; Schmid, J.; Xu, W.; Cummons, T.; Xu, J.; Jin, G.; Barone, D.; et al. Acetylenic TACE inhibitors. Part 3: Thiomorpholine sulfonamide hydroxamates. Bioorg. Med. Chem. Lett. 2006, 16, 1605–1609. [Google Scholar] [CrossRef]
- Li, D.; Popovici-Muller, J.; Belanger, D.B.; Caldwell, J.; Dai, C.; David, M.; Girijavallabhan, V.M.; Lavey, B.J.; Lee, J.F.; Liu, Z.; et al. Structure and activity relationships of tartrate-based TACE inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 4812–4815. [Google Scholar] [CrossRef]
- Mazzola, R.D.; Zhu, Z.; Sinning, L.; McKittrick, B.; Lavey, B.; Spitler, J.; Kozlowski, J.; Neng-Yang, S.; Zhou, G.; Guo, Z.; et al. Discovery of novel hydroxamates as highly potent tumor necrosis factor-α converting enzyme inhibitors. Part II: Optimization of the S3′ pocket. Bioorg. Med. Chem. Lett. 2008, 18, 5809–5814. [Google Scholar] [CrossRef]
- Niu, X.; Umland, S.; Ingram, R.; Beyer, B.M.; Liu, Y.-H.; Sun, J.; Lundell, D.; Orth, P. IK682, a tight binding inhibitor of TACE. Arch. Biochem. Biophys. 2006, 451, 43–50. [Google Scholar] [CrossRef]
- Park, K.; Gopalsamy, A.; Aplasca, A.; Ellingboe, J.W.; Xu, W.; Zhang, Y.; Levin, J.I. Synthesis and activity of tryptophan sulfonamide derivatives as novel non-hydroxamate TNF-α converting enzyme (TACE) inhibitors. Bioorg. Med. Chem. 2009, 17, 3857–3865. [Google Scholar] [CrossRef]
- Rosner, K.E.; Guo, Z.; Orth, P.; Shipps, G.W.; Belanger, D.B.; Chan, T.Y.; Curran, P.J.; Dai, C.; Deng, Y.; Girijavallabhan, V.M.; et al. The discovery of novel tartrate-based TNF-α converting enzyme (TACE) inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Goettig, P.; Maskos, K.; Belouski, E.; Winters, D.; Hecht, R.; Black, R.; Bode, W. Structural Determinants of the ADAM Inhibition by TIMP-3: Crystal Structure of the TACE-N-TIMP-3 Complex. J. Mol. Biol. 2008, 381, 1307–1319. [Google Scholar] [CrossRef]
- Yu, W.; Tong, L.; Kim, S.H.; Wong, M.K.; Chen, L.; Yang, D.-Y.; Shankar, B.B.; Lavey, B.J.; Zhou, G.; Kosinski, A.; et al. Biaryl substituted hydantoin compounds as TACE inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 5286–5289. [Google Scholar] [CrossRef] [PubMed]
- El Azab, I.H.; Saied, E.M.; A Osman, A.; E Mehana, A.; A Saad, H.; Elkanzi, N.A. Novel N-bridged pyrazole-1-carbothioamides with potential antiproliferative activity: Design, synthesis, in vitro and in silico studies. Futur. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.; Belal, A.; El-Deen, I.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.; Alsanie, W.; Saied, E.M. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef] [PubMed]
- Sliwoski, G.; Kothiwale, S.; Meiler, J.; Lowe, E.W., Jr. Computational Methods in Drug Discovery. Pharmacol. Rev. 2013, 66, 334–395. [Google Scholar] [CrossRef]
- Yecies, T.; Bandari, J.; Schneck, F.; Cannon, G. Direction of Rotation in Testicular Torsion and Identification of Predictors of Testicular Salvage. Urology 2018, 114, 163–166. [Google Scholar] [CrossRef]
- Abou-Bakr, D.A. Vitamin D3 Administration before and after Detorsion could Salvage the Testicular Endocrine Function in an Experimental Model of Testicular Torsion/Detortion. Ain Shams Med. J. 2020, 71, 1–14. [Google Scholar] [CrossRef]
- Asadi, N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Osemlak, P.; Miszczuk, K.; Jędrzejewski, G.; Nachulewicz, P.; Beń-Skowronek, I.; Brzozowska, A. Testicular torsion: Its effect on autoimmunisation, pituitary–testis axis and correlation with primary gonadal dysfunction in boys. Pediatr. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Ozturk, H.; Ozturk, H.; Terzi, E.H.; Bugdayci, G.; Duran, A. Interleukin 10 Reduces Testicular Damage in Experimental Testicular Ischemia/Reperfusion Injury. Urology 2014, 83, 508.e1–508.e6. [Google Scholar] [CrossRef]
- Jacobsen, F.M.; Rudlang, T.M.; Fode, M.; Østergren, P.B.; Sønksen, J.; Ohl, D.A.; Jensen, C.F.S.; On behalf of the CopMich Collaborative. The Impact of Testicular Torsion on Testicular Function. World J. Men’s Health 2020, 38, 298–307. [Google Scholar] [CrossRef]
- Moritoki, Y.; Kojima, Y.; Mizuno, K.; Kamisawa, H.; Kohri, K.; Hayashi, Y. Intratesticular pressure after testicular torsion as a predictor of subsequent spermatogenesis: A rat model. BJU Int. 2011, 109, 466–470. [Google Scholar] [CrossRef]
- Ikebuaso, A.D.; Yama, O.E.; Duru, F.; Oyebadejo, S. Experimental Testicular Torsion in a Rat Model: Effects of Treatment with Pausinystalia macroceras on Testis Functions. J. Reprod. Infertil. 2012, 13, 218–224. [Google Scholar]
- Romeo, C.; Impellizzeri, P.; Arrigo, T.; Antonuccio, P.; Valenzise, M.; Mirabelli, S.; Borruto, F.A.; Scalfari, G.; Arena, F.; De Luca, F. Late hormonal function after testicular torsion. J. Pediatr. Surg. 2010, 45, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef]
- Aktaş, A.; Tuncer, M.C.; Yildirim, A.; Nergiz, Y.; Akkus, M. Protective Effects of Melatonin on Testicular Torsion and Detorsion Damage in Sprague-Dawley Rats. Int. J. Morphol. 2011, 29, 7–15. [Google Scholar] [CrossRef]
- Wang, X.; Pan, L.; Zou, Z.; Wang, D.; Lu, Y.; Dong, Z.; Zhu, L. Hypoxia reduces testosterone synthesis in mouse Leydig cells by inhibiting NRF1-activated StAR expression. Oncotarget 2017, 8, 16401–16413. [Google Scholar] [CrossRef] [PubMed]
- Romeo, C.; Arrigo, T.; Impellizzeri, P.; Manganaro, A.; Antonuccio, P.; Di Pasquale, G.; Messina, M.F.; Marseglia, L.; Formica, I.; Zuccarello, B. Altered serum inhibin b levels in adolescents with varicocele. J. Pediatr. Surg. 2007, 42, 390–394. [Google Scholar] [CrossRef]
- Fu, G.-B.; Qian, L.-X.; Cui, Y.-G.; Xu, Z.-Y.; Xuan, H.-B.; Zhu, J.-G.; Zhang, W. Antisperm-antibodies induced by testicular torsion and its influence on testicular function. Zhonghua Nan Ke Xue 2006, 12, 988–991. [Google Scholar]
- Semercioz, A.; Baltaci, A.K.; Mogulkoc, R.; Avunduk, M.C. Effect of Zinc and Melatonin on Oxidative Stress and Serum Inhibin-B Levels in a Rat Testicular Torsion–Detorsion Model. Biochem. Genet. 2017, 55, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Arap, M.A.; Vicentini, F.C.; Cocuzza, M.; Hallak, J.; Athayde, K.; Lucon, A.M.; Arap, S.; Srougi, M. Late Hormonal Levels, Semen Parameters, and Presence of Antisperm Antibodies in Patients Treated for Testicular Torsion. J. Androl. 2007, 28, 528–532. [Google Scholar] [CrossRef]
- Shimizu, S.; Saito, M.; Kinoshita, Y.; Ohmasa, F.; Dimitriadis, F.; Shomori, K.; Hayashi, A.; Satoh, K. Nicorandil ameliorates ischaemia-reperfusion injury in the rat kidney. Br. J. Pharmacol. 2011, 163, 272–282. [Google Scholar] [CrossRef]
- Oroszi, M.; Szabó, A.; Fehér, M.; Deak, G.; Bajory, Z. Microcirculatory effects of sildenafil in experimental testicular torsion in rats. World J. Urol. 2018, 36, 2081–2087. [Google Scholar] [CrossRef]
- Arena, S.; Iacona, R.; Antonuccio, P.; Russo, T.; Salvo, V.; Gitto, E.; Impellizzeri, P.; Romeo, C. Medical perspective in testicular ischemia-reperfusion injury. Exp. Ther. Med. 2017, 13, 2115–2122. [Google Scholar] [CrossRef]
- Turner, T.T.; Lysiak, J.J. Oxidative Stress: A Common Factor in Testicular Dysfunction. J. Androl. 2008, 29, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.G.; Farias, J.G.; Henríquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The Hypoxic Testicle: Physiology and Pathophysiology. Oxidative Med. Cell. Longev. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, J.; Elenberg, Y.; Lurie, M.; Shiloni, E.; Coran, A.; Sukhotnik, I. Effect of dexamethasone on germ cell apoptosis in the contralateral testis after testicular ischemia−reperfusion injury in the rat. Fertil. Steril. 2006, 85, 1111–1117. [Google Scholar] [CrossRef]
- Bejarano, I.; Rodríguez, A.B.; Pariente, J.A. Apoptosis Is a Demanding Selective Tool During the Development of Fetal Male Germ Cells. Front. Cell Dev. Biol. 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, J.; Drmić-Hofman, I.; Pogorelić, Z.; Vilović, K.; Šupe-Domić, D.; Šešelja-Perišin, A.; Čapkun, V. Beneficial Effect of Nifedipine on Testicular Torsion-detorsion Injury in Rats. Urology 2014, 84, 1194–1198. [Google Scholar] [CrossRef]
- Lizama, C.; Rojas-Benítez, D.; Antonelli, M.; Ludwig, A.; Bustamante-Marín, X.; Brouwer-Visser, J.; Moreno, R.D. TACE/ADAM17 is involved in germ cell apoptosis during rat spermatogenesis. Reproduction 2010, 140, 305–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Izzotti, A.; Calin, G.; Arrigo, P.; Steele, V.E.; Croce, C.M.; De Flora, S. Downregulation of microRNA expression in the lungs of rats exposed to cigarette smoke. FASEB J. 2008, 23, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Shen, Z.; Zhou, S. Function of microRNA-145 and mechanisms underlying its role in malignant tumor diagnosis and treatment. Cancer Manag. Res. 2019, 11, 969–979. [Google Scholar] [CrossRef]
- Grabiec, M.; Walentowicz, P.; Sadłecki, P.; Walentowicz-Sadłecka, M. The role of vitamin D in the carcinogenesis of breast and ovarian cancer. Ginekol. Pol. 2013, 84, 305–308. [Google Scholar] [CrossRef]
- Tokgoz, V.; Sipahi, M.; Keskin, O.; Guvendi, G.F.; Takir, S. Protective effects of vitamin D on ischemia-reperfusion injury of the ovary in a rat model. Iran J. Basic Med. Sci. 2018, 21, 593–599. [Google Scholar] [CrossRef]
- Yao, X.; El-Samahy, M.; Yang, H.; Feng, X.; Li, F.; Meng, F.; Nie, H.; Wang, F. Age-associated expression of vitamin D receptor and vitamin D-metabolizing enzymes in the male reproductive tract and sperm of Hu sheep. Anim. Reprod. Sci. 2018, 190, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Tirabassi, G.; Cutini, M.; Muscogiuri, G.; Muti, N.D.; Corona, G.; Galdiero, M.; Pivonello, R.; Colao, A.; Balercia, G. Association between vitamin D and sperm parameters: Clinical evidence. Endocrine 2016, 58, 194–198. [Google Scholar] [CrossRef]
- Alzoubi, A.; Mahdi, H.; Al Bashir, S.; Halalsheh, O.; Al Ebbini, M.; Alzarir, M.; Al-Ahmar, K.; Alfaqih, M.; Al-Hadidi, A. Normalization of Serum Vitamin D Improves Semen Motility Parameters in Patients with Idiopathic Male Infertility. Acta Endocrinol. 2017, 13, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.B.; Lawaetz, J.G.; Petersen, J.H.; Juul, A.; Jørgensen, N. Effects of Vitamin D Supplementation on Semen Quality, Reproductive Hormones, and Live Birth Rate: A Randomized Clinical Trial. J. Clin. Endocrinol. Metab. 2017, 103, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Cito, G.; Cocci, A.; Micelli, E.; Gabutti, A.; Russo, G.I.; Coccia, M.E.; Franco, G.; Serni, S.; Carini, M.; Natali, A. Vitamin D and Male Fertility: An Updated Review. World J. Men’s Health 2020, 38, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Huang, W.; Liu, S.; Cai, S.; Hong, L.; Xiao, W.; Thiele, K.; Zeng, Y.; Song, M.; Diao, L. Impacts of Immunometabolism on Male Reproduction. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Basalamah, M.; Abdelghany, A.H.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018, 8, 4853. [Google Scholar] [CrossRef]
- Wang, J.; Knapp, S.; Pyne, N.; Pyne, S.; Elkins, J.M. Crystal Structure of Sphingosine Kinase 1 with PF-543. ACS Med. Chem. Lett. 2014, 5, 1329–1333. [Google Scholar] [CrossRef]

| Parameter | Control Group | Sham Group | T/D Group | T/D (Vitamin D3) Group |
|---|---|---|---|---|
| T.GPx.I | 1.812 0.06534 | 1.793 0.07967 | 0.1715 0.08693 ab | 1.525 0.1369 abc |
| T.GPx.C | 1.823 0.2111 | 1.702 0.1143 | 0.2933 0.08824 ab | 1.142 0.0332 abc |
| T.MDA.I | 255.7 125.3 | 268.8 127.9 | 2635 238.2 ab | 1231 221.3 abc |
| T.MDA.C | 240.5 50.23 | 238.3 44.35 | 436.2 25.16 ab | 271.2 39.28 bc |
| T.MPO.I | 30.32 2.345 | 36.72 3.706 | 95.90 3.484 ab | 74.95 5.430 abc |
| T.MPO.C | 29.55 1.456 | 33.23 1.564 | 55.75 3.653 ab | 36.48 2.373 abc |
| Parameter | Control Group | Sham Group | T/D Group | T/D (Vitamin D3) Group |
|---|---|---|---|---|
| S. testosterone | 138.5 6.345 | 134.3 5.982 | 64.40 5.168 ab | 119.4 3.838 abc |
| S. FSH | 0.7700 0.1674 | 0.6500 0.1378 | 2.212 0.1429 ab | 1.717 0.1472 abc |
| S. inhibin B | 40.34 3.453 | 36.85 2.359 | 22.93 1.749 ab | 35.68 2.982 abc |
| Anti-sperm antibody | 0.8012 0.07623 | 0.7483 0.06853 | 1.750 0.1378 ab | 0.8083 0.1209 abc |
| Parameter | Control Group | Sham Group | T/D Group | T/D (Vitamin D3) Group |
|---|---|---|---|---|
| T.micRNA145.I | 40.23 1.675 | 35.70 1.953 | 13.30 4.763 ab | 20.98 9.640 abc |
| T.micRNA145.C | 43.56 2.342 | 32.40 2.605 | 19.73 2.115 ab | 27.97 3.881 abc |
| T.ADAM17.I | 1.950 0.1912 | 2.250 0.1871 | 8.100 0.6356 ab | 3.252 0.4450 abc |
| T.ADAM17.C | 2.119 0.1366 | 2.133 0.1366 | 3.517 0.3061 ab | 2.465 0.3252 abc |
| Parameter | Control Group | Sham Group | T/D Group | T/D (Vitamin D3) Group |
|---|---|---|---|---|
| FBW | 150.09 ± 5.23 | 149.10 ± 4.23 | 158.65 ± 2.83 | 165.23 ± 6.45 a,b |
| ATW-I | 0.93 ± 0.12 | 0.86 ± 0.05 | 0.58 ± 0.03 a,b | 0.72 ± 0.08 a,b,c |
| RTW-I | 0.66 0.091 | 0.53 0.082 | 0.29 0.045 a,b | 0.40 ± 0.094 a,b,c |
| ATW-C | 0.100 ± 0.03 | 0.91 ± 0.01 | 0.63 ± 0.04 a,b | 0.86 ± 0.02 a,b,c |
| RTW-C | 0.75 ± 0.056 | 0.68 ± 0.064 | 0.39 0.043 a,b | 0.64 0.075 a,b,c |
| Mean Diameter of Seminiferous Tubules (µm) | Mean Thickness of Germinal Epithelium (µm) | Mean Area % of Collagen Fibers | |
|---|---|---|---|
| Control group | 250.83 ± 2.04 | 74.50 ± 2.58 | 6.50 ± 1.76 |
| Testicular T/D group (Ipsilateral testis) | 212.00 ± 2.75 * | 47.16 ± 2.93 * | 14.50 ± 1.87 * |
| Testicular T/D group (Contralateral testis) | 221.83 ± 4.70 * | 59.00 ± 2.36 * | 13.50 ± 2.42 * |
| Vit. D 3 treated group (Ipsilateral testis) | 238.16 ± 2.92 *▲ | 66.16 ± 2.92 *▲ | 9.66 ± 1.63 *▲ |
| Vit. D 3 treated group (Contralateral testis) | 246.80 ± 3.96 ▲ | 72.20 ± 2.28 ▲ | 8.28 ± 1.11 ▲ |
| PDB | Docking Score (kcal/mol) | Interactive Residues | |
|---|---|---|---|
| Hydrophilic Interactions | Hydrophobic Interactions | ||
| 2ddf | −13.63 | Zn+2, His415 | Pro437, Ile438, Ala439, Met345, Leu359 |
| 3l0v | −10.71 | Gly346, His415 | Ile438, Pro437, Ala439, Met345, Leu350, Ala351, Pro356, Leu348 |
| 3ewl | −17.65 | Zn+2, His415, Glu406 | Val440, Ile438, Pro437, Ala439, Met345, Leu350 |
| 3kmc | −11.25 | Zn+2, Glu406 | Met345, Ile438, Pro437, Leu350 |
| 3kme | −16.42 | Zn+2, His409, Glu406 | Pro437, Ile438, Ala439, Leu348, Met345, Leu350, |
| 3le9 | −15.23 | Zn+2, His415, Asp313 | Val314, Leu348, Pro437 |
| 3o64 | −13.84 | Zn+2, His415 | Ile394, Leu348, Pro437, Ile438, Val434, Val440, Leu 401, Ala439, Val402 |
| 2i47 | −15.76 | Zn+2, His415, Glu406 | Met345, Leu348, Leu350, Pro437, Ile438, Ala439 |
| 3e8r | −12.94 | Zn+2, Glu406 | Leu350, Pro437, Ile438, Leu348, Ala439, Met345 |
| 3edz | −16.72 | Zn+2, His415, Glu406 | Leu350, Met345, Leu348, Ileu438, Ala439, Pro437 |
| 3lgp | −10.37 | Zn+2, Glu406 | Ala439, Pro437, Ile438, Leu348 |
| 3l0t | −16.48 | Zn+2, His409, His415 | Leu350, Met345, Leu348, Ala439, Ile438, Pro437 |
| 1bkc | −17.21 | Zn+2, His415, Glu406 | Met345, Ile438, Leu348, Pro437, Leu350, Ala439 |
| 2oi0 | −11.96 | Zn+2, His415 | Leu350, Leu348, Met345, Ala439, Ile438, Pro437 |
| 3b92 | −16.39 | Zn+2, His415, His405 | Pro437, Ile438, Met345, Val314, Leu350 |
| 3lea | −14.53 | Zn+2, His415 | Trp312, Met345, Leu384, Val314, Pro437, Ala439, Leu350 |
| 2fv9 | −17.41 | Zn+2, His415, His405 | Leu350, Leu348, Ile438, Ala439, Met345, Pro437 |
| 2fv5 | −16.90 | Zn+2, His415, His405 | Ile438, Leu350, Ala439, Met345, Pro437, Leu348 |
| 3g42 | −15.79 | Zn+2, His415, His409 | Ala439, Met345, Ile438, Leu438, Pro437 |
| 1zxc | −16.47 | Zn+2, His405, Glu406 | Ile438, Pro437, Leu350, Ala439, Met345, Leu348 |
| 2a8h | −17.87 | Zn+2, Glu406, His415, His405 | Pro437, Met345, Ile438, Leu348, Ala439, Leu350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting miRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. https://doi.org/10.3390/ph14121222
Mohamed DI, Abou-Bakr DA, Ezzat SF, El-Kareem HFA, Nahas HHA, Saad HA, Mehana AE, Saied EM. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting miRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals. 2021; 14(12):1222. https://doi.org/10.3390/ph14121222
Chicago/Turabian StyleMohamed, Doaa I., Doaa A. Abou-Bakr, Samar F. Ezzat, Hanaa F. Abd El-Kareem, Hebatallah H. Abo Nahas, Hosam A. Saad, Amir E. Mehana, and Essa M. Saied. 2021. "Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting miRNA-145 and ADAM17: In Silico and In Vivo Study" Pharmaceuticals 14, no. 12: 1222. https://doi.org/10.3390/ph14121222
APA StyleMohamed, D. I., Abou-Bakr, D. A., Ezzat, S. F., El-Kareem, H. F. A., Nahas, H. H. A., Saad, H. A., Mehana, A. E., & Saied, E. M. (2021). Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting miRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals, 14(12), 1222. https://doi.org/10.3390/ph14121222