Assessment of the In Vitro Cytotoxicity Effects of the Leaf Methanol Extract of Crinum zeylanicum on Mouse Induced Pluripotent Stem Cells and Their Cardiomyocytes Derivatives
Abstract
:1. Introduction
2. Results
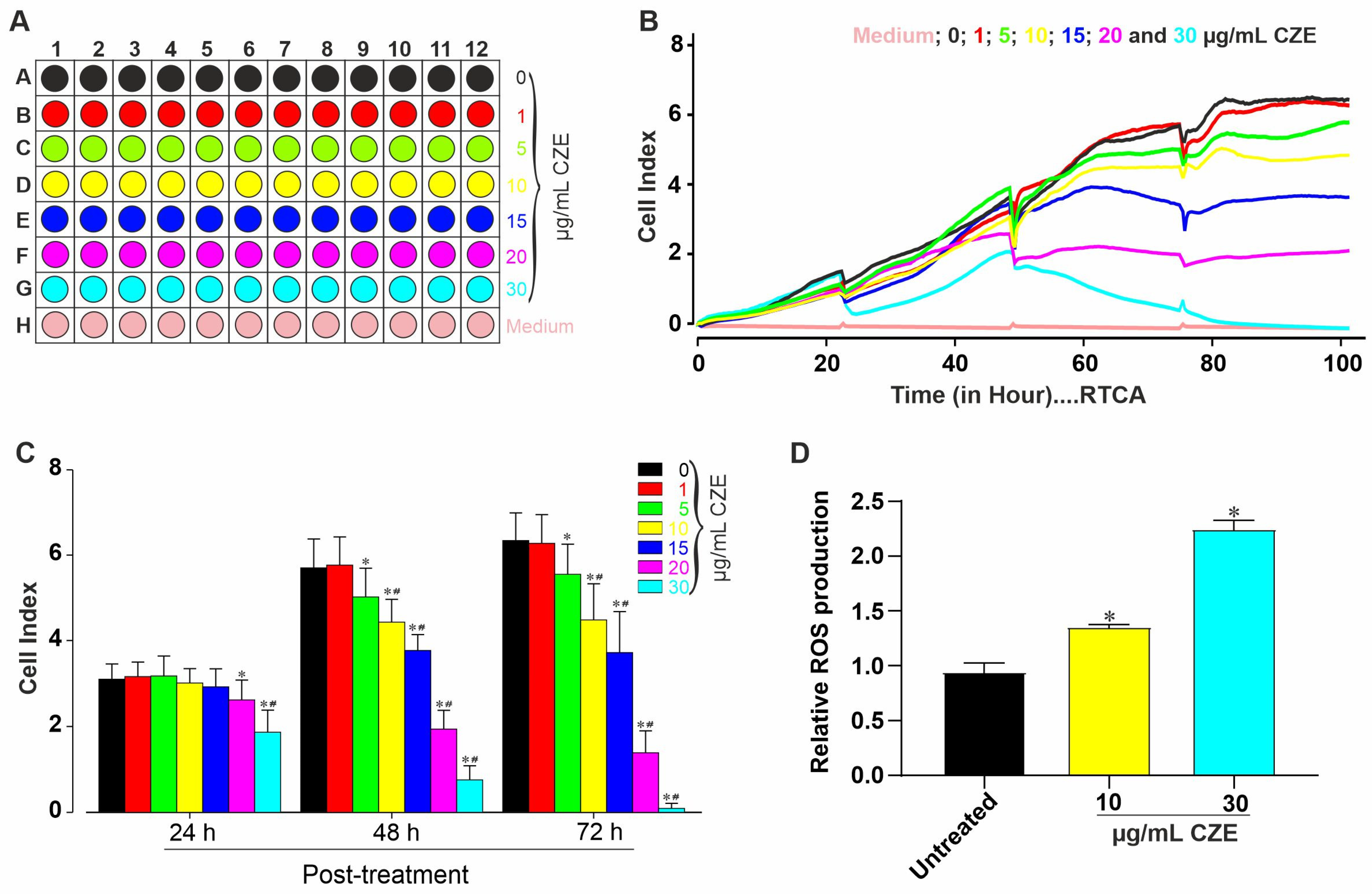
2.1. Cytotoxic Effects of CZE
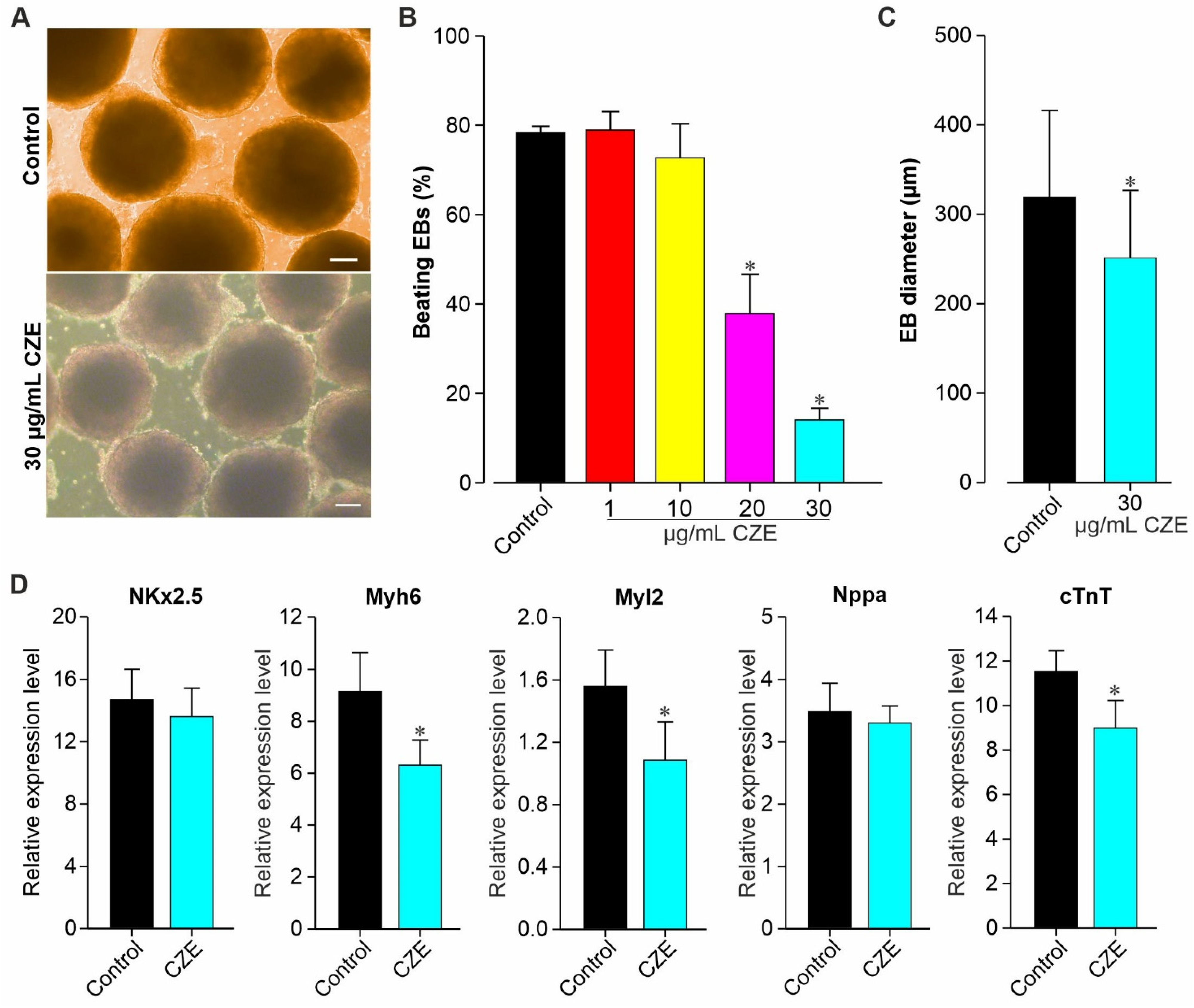
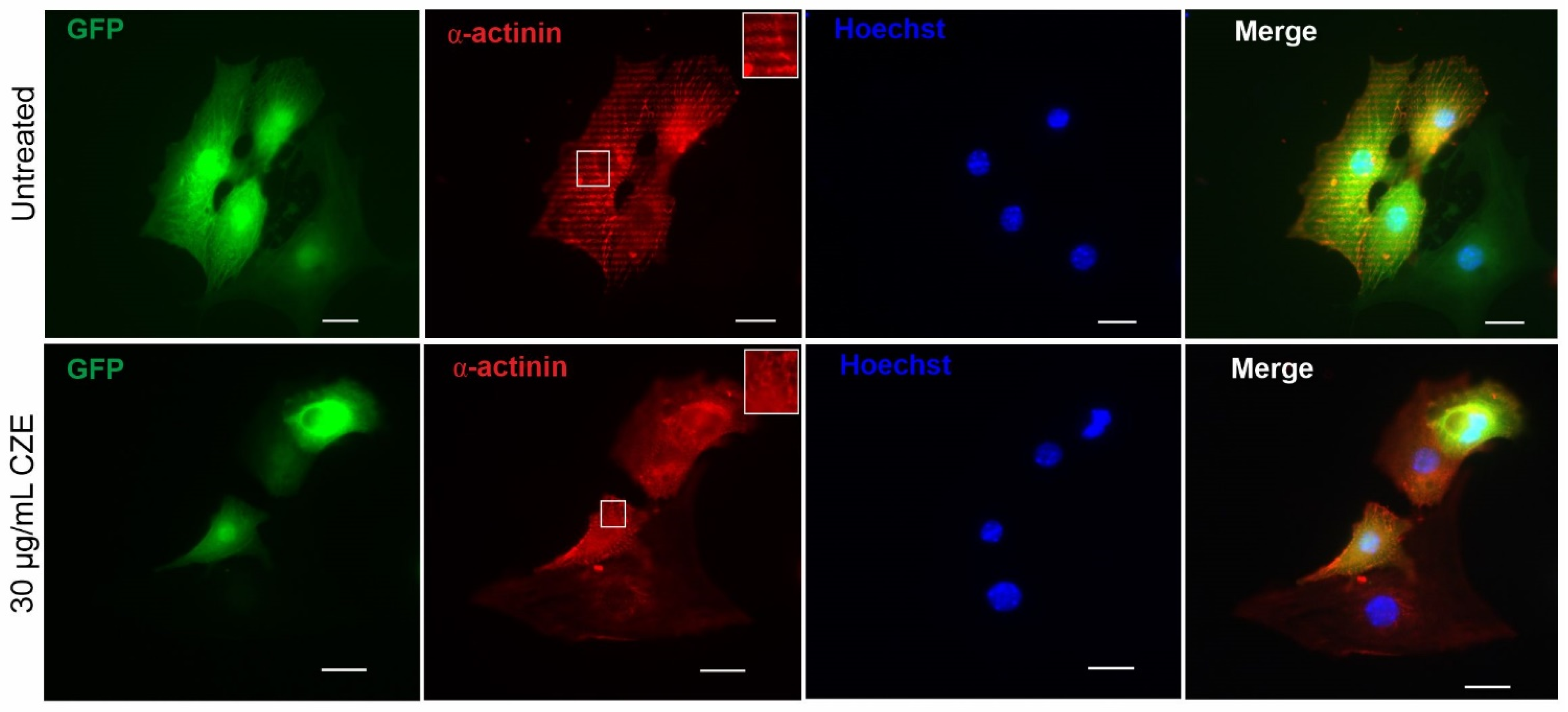
2.2. Effect of CZE on Cardiac Differentiation of miPS Cells
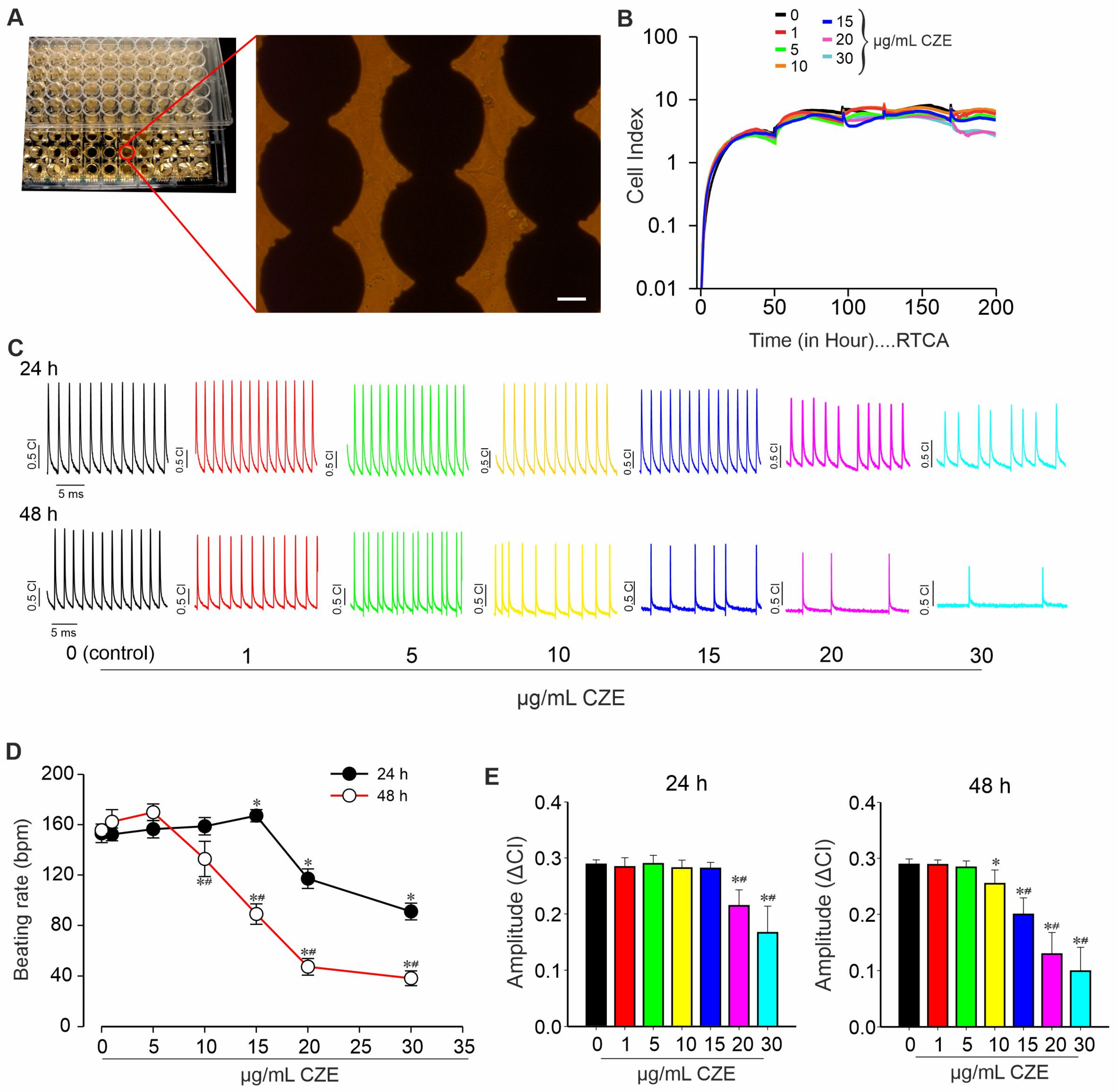
2.3. Effect of CZE on Spontaneous Contractile Activity of miPSCs-Derived CMs
3. Discussion
4. Conclusions
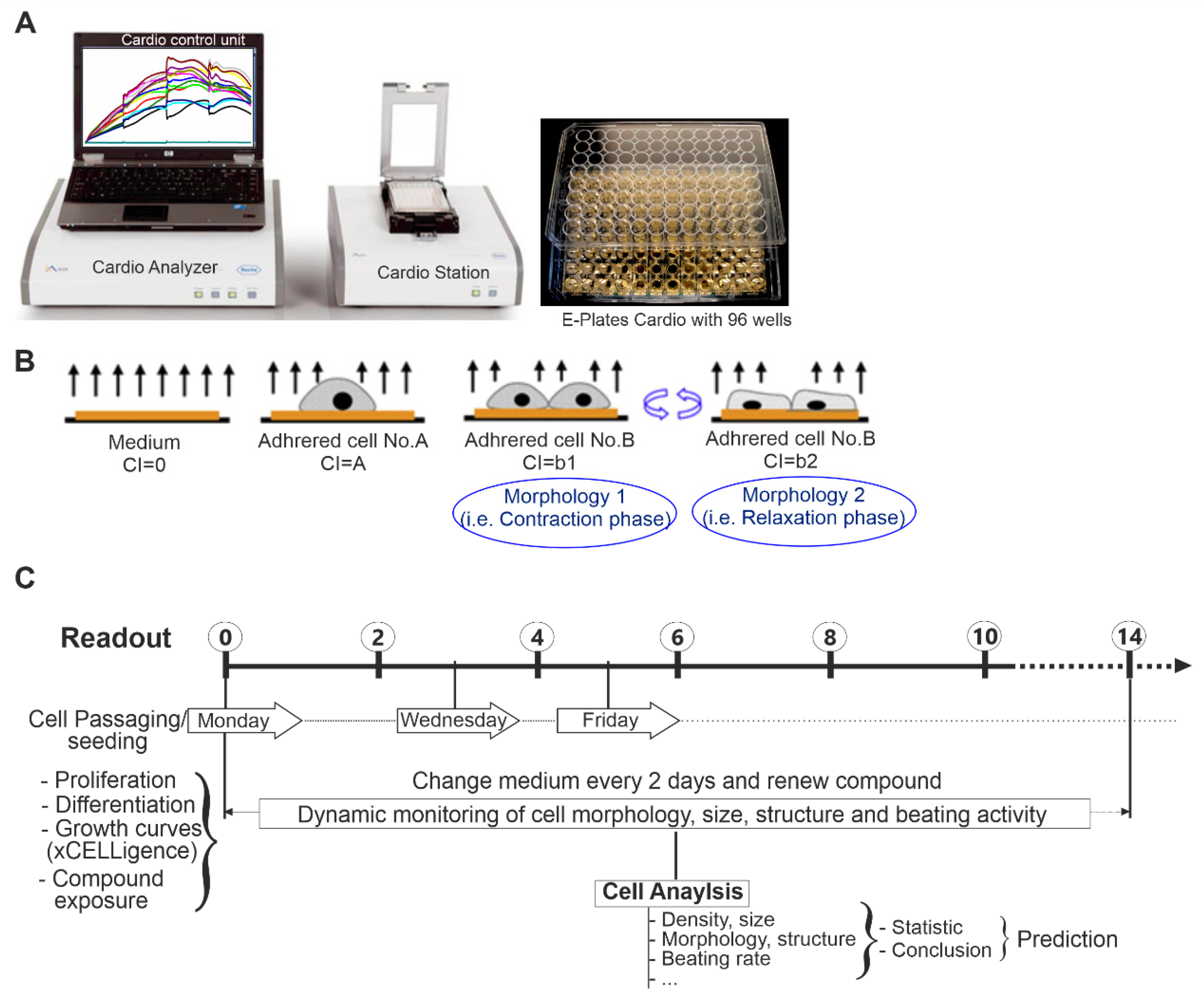
5. Materials and Methods
5.1. Plant Material and Extraction
5.2. Cell Culture and Differentiation
5.3. Dynamic Monitoring of the Effect of CZE on Cell Proliferation Using the xCELLigence RTCA System
5.4. Effect of CZE on Cell Growth and Cardiomyocytes Differentiation
5.5. Assessment of CZE Effects on Beating Parameters of Cardiomyocytes
5.6. Reactive Oxygen Species (ROS) Detection
5.7. Immunocytochemical Staining Analysis
5.8. Chemicals
5.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nembo, E.N.; Hescheler, J.; Nguemo, F. Stem cells in natural product and medicinal plant drug discovery-An overview of new screening approaches. Biomed. Pharmacother. 2020, 131, 110730. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kik, A.; Adamec, M.; Aikhenvald, A.Y.; Bajzekova, J.; Baro, N.; Bowern, C.; Colwell, R.K.; Drozd, P.; Duda, P.; Ibalim, S.; et al. Language and ethnobiological skills decline precipitously in Papua New Guinea, the world’s most linguistically diverse nation. Proc. Natl. Acad. Sci. USA 2021, 118, e2100096118. [Google Scholar] [CrossRef]
- Okaiyeto, K.; Oguntibeju, O.O. African Herbal Medicines: Adverse Effects and Cytotoxic Potentials with Different Therapeutic Applications. Int. J. Environ. Res. Public Health 2021, 18, 5988. [Google Scholar] [CrossRef]
- Liperoti, R.; Vetrano, D.L.; Bernabei, R.; Onder, G. Herbal Medications in Cardiovascular Medicine. J. Am. Coll. Cardiol. 2017, 69, 1188–1199. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Ndjenda Ii, M.K.; Nguelefack-Mbuyo, E.P.; Atsamo, A.D.; Fofie, C.K.; Fodem, C.; Nguemo, F.; Nguelefack, T.B. Antihypertensive Effects of the Methanol Extract and the Ethyl Acetate Fraction from Crinum zeylanicum (Amaryllidaceae) Leaves in L-NAME-Treated Rat. Evid.-Based Complement. Altern. Med. eCAM 2021, 2021, 2656249. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, R.J.; Engle, S.J. Human inducible pluripotent stem cells: Realization of initial promise in drug discovery. Cell Stem Cell 2021, 28, 1507–1515. [Google Scholar] [CrossRef]
- Berkov, S.; Romani, S.; Herrera, M.; Viladomat, F.; Codina, C.; Momekov, G.; Ionkova, I.; Bastida, J. Antiproliferative alkaloids from Crinum zeylanicum. Phytother. Res. PTR 2011, 25, 1686–1692. [Google Scholar] [CrossRef]
- Kuete, V.; Voukeng, I.K.; Tsobou, R.; Mbaveng, A.T.; Wiench, B.; Beng, V.P.; Efferth, T. Cytotoxicity of Elaoephorbia drupifera and other Cameroonian medicinal plants against drug sensitive and multidrug resistant cancer cells. BMC Complement. Altern. Med. 2013, 13, 250. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [Green Version]
- Patil, D.N.; Patil, S.A.; Sistla, S.; Jadhav, J.P. Comparative biophysical characterization: A screening tool for acetylcholinesterase inhibitors. PLoS ONE 2019, 14, e0215291. [Google Scholar] [CrossRef]
- Tenfen, A.; Vechi, G.; Cechinel-Zanchett, C.C.; Lorenzett, T.S.; Reginato-Couto, C.E.; Siebert, D.A.; Vitali, L.; Micke, G.; Klein-Junior, L.C.; Cechinel Filho, V. Phenolic profile by HPLC-ESI-MS/MS of six Brazilian Eugenia species and their potential as cholinesterase inhibitors. Nat. Prod. Res. 2021, 35, 2608–2611. [Google Scholar] [CrossRef] [PubMed]
- ten Berge, D.; Koole, W.; Fuerer, C.; Fish, M.; Eroglu, E.; Nusse, R. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 2008, 3, 508–518. [Google Scholar] [CrossRef] [Green Version]
- Nembo, E.N.; Dimo, T.; Bopda, O.S.; Hescheler, J.; Nguemo, F. The proliferative and chronotropic effects of Brillantaisia nitens Lindau (Acanthaceae) extracts on pluripotent stem cells and their cardiomyocytes derivatives. J. Ethnopharmacol. 2014, 156, 73–81. [Google Scholar] [CrossRef]
- Zeng, D.; Ou, D.B.; Wei, T.; Ding, L.; Liu, X.T.; Hu, X.L.; Li, X.; Zheng, Q.S. Collagen/beta(1) integrin interaction is required for embryoid body formation during cardiogenesis from murine induced pluripotent stem cells. BMC Cell Biol. 2013, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Focaccetti, C.; Bruno, A.; Magnani, E.; Bartolini, D.; Principi, E.; Dallaglio, K.; Bucci, E.O.; Finzi, G.; Sessa, F.; Noonan, D.M.; et al. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS ONE 2015, 10, e0115686. [Google Scholar] [CrossRef]
- Dai, Y.; Amenov, A.; Ignatyeva, N.; Koschinski, A.; Xu, H.; Soong, P.L.; Tiburcy, M.; Linke, W.A.; Zaccolo, M.; Hasenfuss, G.; et al. Troponin destabilization impairs sarcomere-cytoskeleton interactions in iPSC-derived cardiomyocytes from dilated cardiomyopathy patients. Sci. Rep. 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, J.J.; van Staden, J. Antiprotozoal alkaloid principles of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2019, 29, 126642. [Google Scholar] [CrossRef] [PubMed]
- Nguemo, F.; Nembo, E.N.; Kamga Kapchoup, M.V.; Enzmann, F.; Hescheler, J. QuinoMit Q10-Fluid attenuates hydrogen peroxide-induced irregular beating in mouse pluripotent stem cell-derived cardiomyocytes. Biomed. Pharmacother. 2021, 142, 112089. [Google Scholar] [CrossRef] [PubMed]
- Ezenyi, I.C.; Okpoko, C.K.; Ufondu, C.A.; Okhale, S.E.; Adzu, B. Antiplasmodial, antinociceptive and antipyretic potential of the stem bark extract of Burkea africana and identification of its antiplasmodial-active fraction. J. Tradit. Complement. Med. 2021, 11, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rubach, M.; Adelmann, R.; Haustein, M.; Drey, F.; Pfannkuche, K.; Xiao, B.; Koester, A.; Udink ten Cate, F.E.; Choi, Y.H.; Neef, K.; et al. Mesenchymal stem cells and their conditioned medium improve integration of purified induced pluripotent stem cell-derived cardiomyocyte clusters into myocardial tissue. Stem Cells Dev. 2014, 23, 643–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abassi, Y.A.; Xi, B.; Li, N.; Ouyang, W.; Seiler, A.; Watzele, M.; Kettenhofen, R.; Bohlen, H.; Ehlich, A.; Kolossov, E.; et al. Dynamic Monitoring of Beating Periodicity of Stem Cell-Derived Cardiomyocytes as a Predictive Tool for Preclinical Safety Assessment. Br. J. Pharmacol. 2012, 165, 1424–1441. [Google Scholar] [CrossRef] [Green Version]
- Abassi, Y.A.; Xi, B.; Zhang, W.; Ye, P.; Kirstein, S.L.; Gaylord, M.R.; Feinstein, S.C.; Wang, X.; Xu, X. Kinetic cell-based morphological screening: Prediction of mechanism of compound action and off-target effects. Chem. Biol. 2009, 16, 712–723. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndjenda II, M.K.; Nguelefack-Mbuyo, E.P.; Hescheler, J.; Nguelefack, T.B.; Nguemo, F. Assessment of the In Vitro Cytotoxicity Effects of the Leaf Methanol Extract of Crinum zeylanicum on Mouse Induced Pluripotent Stem Cells and Their Cardiomyocytes Derivatives. Pharmaceuticals 2021, 14, 1208. https://doi.org/10.3390/ph14121208
Ndjenda II MK, Nguelefack-Mbuyo EP, Hescheler J, Nguelefack TB, Nguemo F. Assessment of the In Vitro Cytotoxicity Effects of the Leaf Methanol Extract of Crinum zeylanicum on Mouse Induced Pluripotent Stem Cells and Their Cardiomyocytes Derivatives. Pharmaceuticals. 2021; 14(12):1208. https://doi.org/10.3390/ph14121208
Chicago/Turabian StyleNdjenda II, Magloire Kanyou, Elvine Pami Nguelefack-Mbuyo, Jürgen Hescheler, Télesphore Benoît Nguelefack, and Filomain Nguemo. 2021. "Assessment of the In Vitro Cytotoxicity Effects of the Leaf Methanol Extract of Crinum zeylanicum on Mouse Induced Pluripotent Stem Cells and Their Cardiomyocytes Derivatives" Pharmaceuticals 14, no. 12: 1208. https://doi.org/10.3390/ph14121208
APA StyleNdjenda II, M. K., Nguelefack-Mbuyo, E. P., Hescheler, J., Nguelefack, T. B., & Nguemo, F. (2021). Assessment of the In Vitro Cytotoxicity Effects of the Leaf Methanol Extract of Crinum zeylanicum on Mouse Induced Pluripotent Stem Cells and Their Cardiomyocytes Derivatives. Pharmaceuticals, 14(12), 1208. https://doi.org/10.3390/ph14121208




