Medication Discrepancies and Regimen Complexity in Decompensated Cirrhosis: Implications for Medication Safety
Abstract
:1. Introduction
2. Results
2.1. Prevalence of Medication Discrepancies
2.2. Impact of Pharmacist Intervention on Medication Discrepancies
2.3. Medication Regimen Complexity
2.4. Medication-Related Outcomes
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Measures
4.2.1. Medication Discrepancies
4.2.2. Medication Regimen Complexity
4.2.3. Clinical and Demographic Information
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- If the medication was named by the patient but not the clinician, it is assumed that:
- The patient is taking the medication; and
- The clinician is not aware that the patient is taking the medication; and
- Overall risk is assessed on the premise that the patient continues to take the medication without monitoring for three months.
- 2.
- If the medication was named by the clinician but not the patient, it is assumed that:
- The clinician believes the patient is taking the medication; and
- The patient is not taking the medication; and
- Overall risk is assessed on the premise that the patient does not take the medication for three months.
- 3.
- If the medication is named by both the patient and clinician, but there is a discrepancy between dose/frequency, it is assumed that:
- The patient is taking the medication; and
- The clinician believes the patient is taking the medication; and
- Overall risk is assessed on the premise that the patient takes a ‘standard’ dose (for the patient’s indication) of the medication for three months, as opposed to the dose/s listed by the patient and/or clinician, with concurrent standard-of-care monitoring.
| SEVERITY | ||||||
|---|---|---|---|---|---|---|
| Negligible | Minor | Moderate | Severe | Catastrophic | ||
| LIKELIHOOD | Certain | 7 | 11 | 17 | 23 | 25 |
| Probable | 6 | 10 | 16 | 20 | 24 | |
| Possible | 3 | 9 | 15 | 18 | 22 | |
| Unlikely | 2 | 8 | 12 | 14 | 21 | |
| Rare | 1 | 4 | 5 | 13 | 19 | |
| Score 1–5: | Low risk |
| Score 6–14: | Medium risk |
| Score 15–22 | High risk |
| Score 23–25: | Very high risk |
| Negligible: | No harm |
| Minor: | Minimal harm, managed in general practice/outpatient setting |
| Moderate: | Temporary harm, including need for additional investigation, minor surgical intervention, or possible hospital admission without life-altering consequences |
| Severe: | Permanent harm requiring hospital admission with potential for life-altering consequences |
| Catastrophic: | Permanent harm, including preventable loss of life, intensive care unit admission, or probable life-altering consequences |
| Rare: | The harm would only occur in exceptional circumstances. |
| Unlikely: | The harm could occur at some time but is not expected (<3 0% of the time). |
| Possible: | The harm could be expected to occur at some time (30–60% of the time). |
| Probable: | The harm will likely occur at some time (60–90% of the time). |
| Certain: | The harm is either occurring or will likely occur in most circumstances (> 90% of the time). |
| Medication Group | NAME Discrepancy SEVERITY and LIKELIHOOD | Exceptions (Require Panel Review) | DOSE/FREQUENCY Discrepancy | |
|---|---|---|---|---|
| Patient Named Only | Clinician Named Only | |||
| Diuretics (‘Standard’ dose is spironolactone 100 mg daily/frusemide 40 mg daily) | Severe/Possible (e.g., electrolyte derangement, dehydration) | Moderate/Possible (e.g., admission to hospital with fluid overload) | Not prescribed for ascites/pleural effusion | If standard dose: Minor/Possible If non-standard dose: Panel review |
| Lactulose | Severe/Unlikely (e.g., electrolyte derangement, dehydration, but less likely than with diuretics because patients less likely to take excessively due to diarrhea) | Severe/Possible (e.g., admission to hospital with encephalopathy) | No history of encephalopathy | All doses: Panel review |
| Propranolol | Moderate/Possible (e.g., dizziness, hypotension, falls) | Catastrophic/Possible (e.g., variceal bleeding) | No history of varices/grade 1 only | All doses: Panel review |
| SBP Prophylaxis | Moderate/Possible | Severe/Possible | If standard dose: Minor/Unlikely If non-standard dose: Panel review | |
| Insulins | Severe/Probable (e.g., hypo/hyperglycemia) | Severe/Possible (e.g., Hyperglycemia, uncontrolled diabetes, risk of infection with elevated blood glucose) | All doses: Panel review | |
| Vitamin D (‘Standard’ dose is 25 mcg daily (one tablet)) | Minor/Rare | Severe/Rare (e.g., fracture, but rare likelihood of occurrence in three months in people WITHOUT osteoporosis) | Hx of osteoporosis/osteopenia | If standard dose: Negligible/Rare If non-standard dose: Minor/Unlikely |
| Proton pump inhibitors (‘Standard’ dose is pantoprazole 40 mg daily or equivalent) | Moderate/Unlikely | Minor/Possible if Rx for GORD Other indications: Panel review | If standard dose: Negligible/Rare If non-standard dose: Minor/Possible | |
| Paracetamol (‘Standard’ dose is ≤ 2 g daily) | Minor/Unlikely | Minor/Unlikely | Patient is taking non-standard dose | If standard dose: Negligible/Rare If non-standard dose: Panel review |
| Inhalers | Minor/Unlikely | Moderate/Possible | If standard dose: Negligible/Rare If non-standard dose: Panel review | |
| Topical (medicated) | Minor/Unlikely | Minor/Unlikely | All PRN doses: Minor/Unlikely All other doses: Panel review | |
References
- Coletti, D.J.; Stephanou, H.; Mazzola, N.; Conigliaro, J.; Gottridge, J.; Kane, J.M. Patterns and predictors of medication discrepancies in primary care. J. Eval. Clin. Pract. 2015, 21, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.L.; Lynch, K.J. Identifying discrepancies in electronic medical records through pharmacist medication reconciliation. J. Am. Pharm. Assoc. 2012, 52, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.L.; Valery, P.C.; Cottrell, W.N.; Irvine, K.M.; Horsfall, L.U.; Tallis, C.J.; Chachay, V.S.; Ruffin, B.J.; Martin, J.H.; Powell, E.E. Prevalence of medication discrepancies in patients with cirrhosis: A pilot study. BMC Gastroenterol. 2016, 16, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedell, S.E.; Jabbour, S.; Goldberg, R.; Glaser, H.; Gobble, S.; Young-Xu, Y.; Graboys, T.B.; Ravid, S. Discrepancies in the use of medications: Their extent and predictors in an outpatient practice. Arch. Intern. Med. 2000, 160, 2129–2134. [Google Scholar] [CrossRef] [Green Version]
- Franco, J.V.A.; Terrasa, S.A.; Kopitowski, K.S. Medication discrepancies and potentially inadequate prescriptions in elderly adults with polypharmacy in ambulatory care. J. Fam. Med. Prim. Care 2017, 6, 78–82. [Google Scholar] [CrossRef]
- Rose, O.; Jaehde, U.; Koberlein-Neu, J. Discrepancies between home medication and patient documentation in primary care. Res. Soc. Adm. Pharm. 2018, 14, 340–346. [Google Scholar] [CrossRef]
- Perry, T.D.; Nye, A.M.; Johnson, S.W. Medication discrepancy rates among Medicaid recipients at hospital discharge. J. Am. Pharm. Assoc. 2017, 57, 488–492. [Google Scholar] [CrossRef]
- Coleman, E.A.; Smith, J.D.; Raha, D.; Min, S.J. Posthospital medication discrepancies: Prevalence and contributing factors. Arch. Intern. Med. 2005, 165, 1842–1847. [Google Scholar] [CrossRef]
- Neumiller, J.J.; Setter, S.M.; White, A.M.; Corbett, C.F.; Weeks, D.L.; Daratha, K.B.; Collins, J.B. Medication Discrepancies and Potential Adverse Drug Events during Transfer of Care from Hospital to Home; Battles, J., Azam, I., Reback, K., Grady, M., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. [Google Scholar]
- Hayward, K.L.; Weersink, R.A. Improving medication-related outcomes in chronic liver disease. Hepatol. Commun. 2020, 4, 1562–1577. [Google Scholar] [CrossRef]
- World Health Organisation. The High 5s Project. In Assuring Medication Accuracy at Transitions of Care: Medication Reconciliation; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Hayward, K.L.; Patel, P.J.; Valery, P.C.; Horsfall, L.U.; Li, C.Y.; Wright, P.L.; Tallis, C.J.; Stuart, K.A.; Irvine, K.M.; Cottrell, W.N.; et al. Medication-related problems in outpatients with decompensated cirrhosis: Opportunities for harm prevention. Hepatol. Commun. 2019, 3, 620–631. [Google Scholar] [CrossRef] [Green Version]
- Australian Bureau of Statistics. Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia. 2011. Available online: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/2033.0.55.0012011?OpenDocument (accessed on 10 October 2021).
- Australian Institute of Health and Welfare. Rural, Regional and Remote Health: A Guide to Remoteness Classifications. Available online: http://www.aihw.gov.au/publication-detail/?id=6442467589 (accessed on 10 October 2021).
- Lee, K.P.; Nishimura, K.; Ngu, B.; Tieu, L.; Auerbach, A.D. Predictors of completeness of patients’ self-reported personal medication lists and discrepancies with clinic medication lists. Ann. Pharm. 2014, 48, 168–177. [Google Scholar] [CrossRef]
- Weersink, R.A.; Taxis, K.; Drenth, J.P.H.; Houben, E.; Metselaar, H.J.; Borgsteede, S.D. Prevalence of drug prescriptions and potential safety in patients with cirrhosis: A retrospective real-world study. Drug Saf. 2019, 42, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, J.K.; Fouts, M.M.; Kotabe, S.E.; Lo, E. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am. J. Geriatr. Pharm. 2006, 4, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Schoonover, H.; Corbett, C.F.; Weeks, D.L.; Willson, M.N.; Setter, S.M. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J. Patient Saf. 2014, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.M.; Libby, A.M.; Metz, K.R.; Page, R.L., 2nd; Ambardekar, A.V.; Lindenfeld, J.; Aquilante, C.L. Evaluating patient-level medication regimen complexity over time in heart transplant recipients. Ann. Pharm. 2016, 50, 926–934. [Google Scholar] [CrossRef]
- Metz, K.R.; Fish, D.N.; Hosokawa, P.W.; Hirsch, J.D.; Libby, A.M. Patient-level medication regimen complexity in patients with hiv. Ann. Pharm. 2014, 48, 1129–1137. [Google Scholar] [CrossRef]
- Libby, A.M.; Fish, D.N.; Hosokawa, P.W.; Linnebur, S.A.; Metz, K.R.; Nair, K.V.; Saseen, J.J.; Vande Griend, J.P.; Vu, S.P.; Hirsch, J.D. Patient-level medication regimen complexity across populations with chronic disease. Clin. Ther. 2013, 35, 385–398.e381. [Google Scholar] [CrossRef] [Green Version]
- Cobretti, M.R.; Page, M.R., II; Linnebur, S.A.; Deininger, K.M.; Ambardekar, A.V.; Lindenfeld, J.; Aquilante, C.L. Medication regimen complexity in ambulatory older adults with heart failure. Clin. Interv. Ageing 2017, 12, 679–868. [Google Scholar] [CrossRef] [Green Version]
- Thomson, M.J.; Lok, A.S.F.; Tapper, E.B. Appropriate and potentially inappropriate medication use in decompensated cirrhosis. Hepatology 2021, 73, 2429–2440. [Google Scholar] [CrossRef]
- Weersink, R.A.; Burger, D.M.; Hayward, K.L.; Taxis, K.; Drenth, J.P.H.; Borgsteede, S.D. Safe use of medication in patients with cirrhosis: Pharmacokinetic and pharmacodynamic considerations. Expert Opin. Drug Metab. Toxicol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Williams, S.; Louissaint, J.; Nikirk, S.; Bajaj, J.S.; Tapper, E.B. Deprescribing medications that may increase the risk of hepatic encephalopathy: A qualitative study of patients with cirrhosis and their doctors. United Eur. Gastroenterol. J. 2021, 9, 193–202. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Phun, Y.T.; Bailey, M.J.; Kong, D.C.; Stewart, K. Development and validation of the medication regimen complexity index. Ann. Pharm. 2004, 38, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
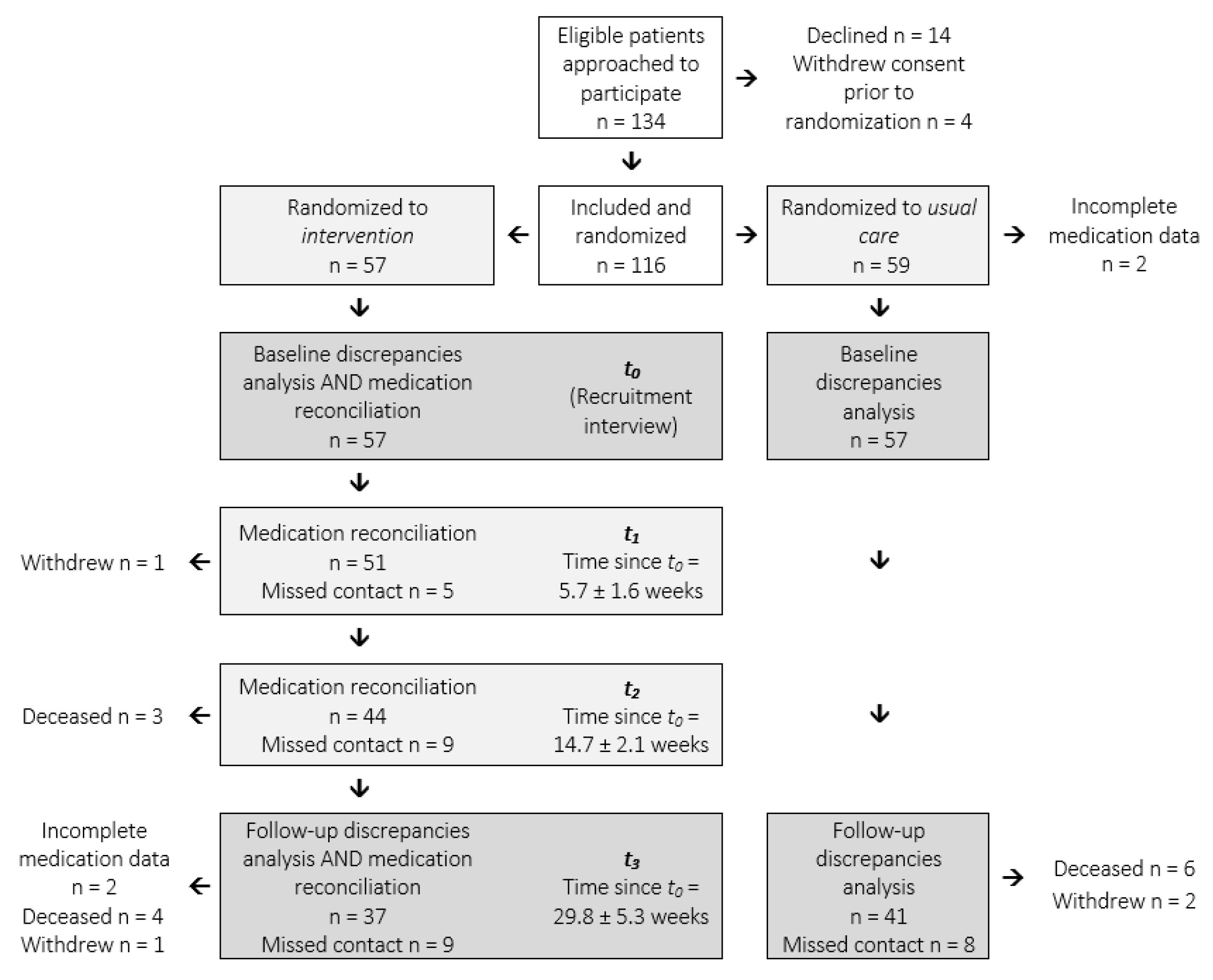

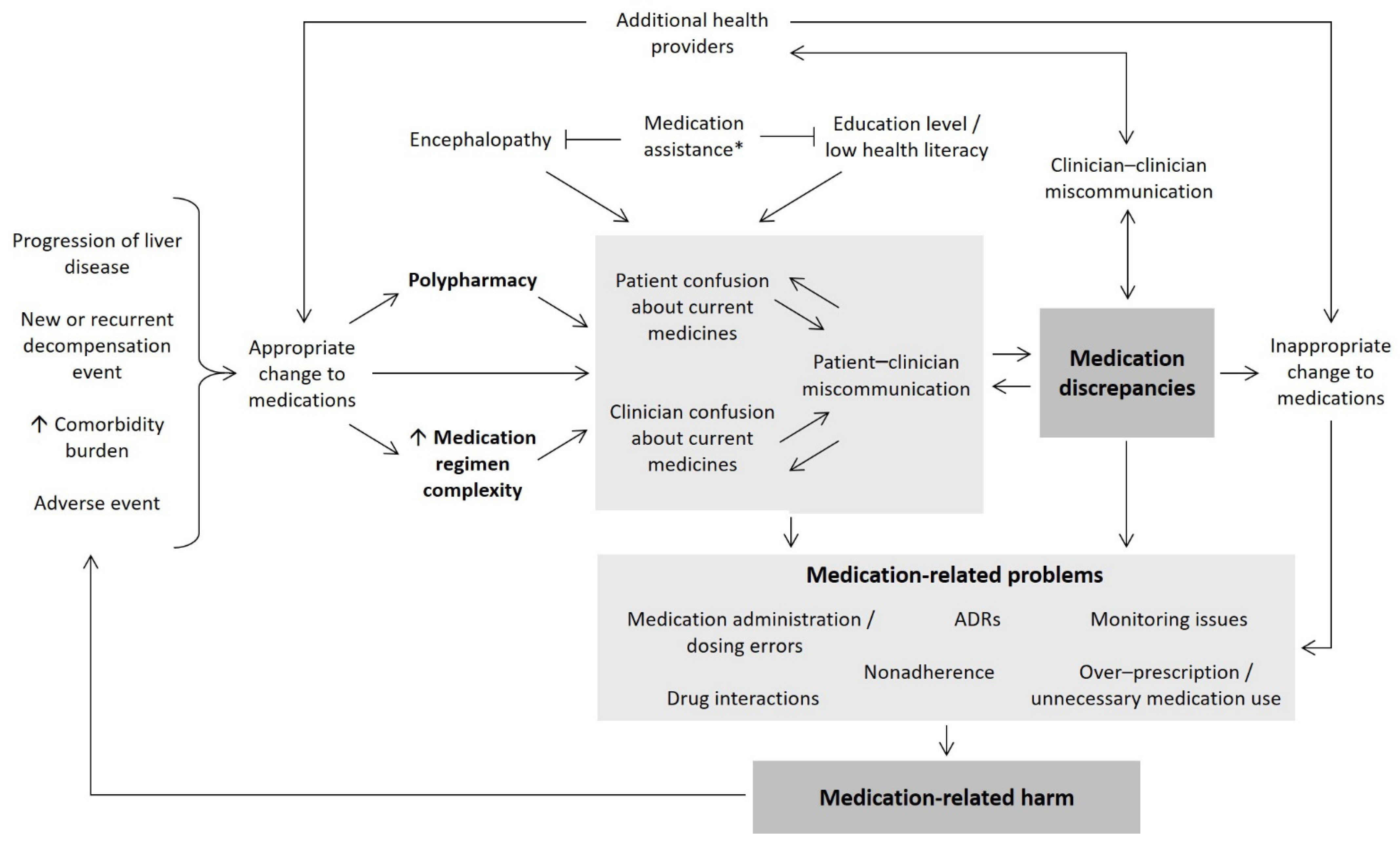
| Clinical and Demographic Characteristics | Intervention n = 57 | Usual Care n = 57 | p | |
|---|---|---|---|---|
| Age (mean ± SD) | 58.1 ± 10.0 | 59.5 ± 10.4 | 0.471 1 | |
| Male gender | 39 (68.4%) | 36 (63.2%) | 0.554 2 | |
| Liver disease etiology | Alcohol-related liver disease | 22 (38.6%) | 32 (56.1%) | 0.220 3 |
| Hepatitis C | 21 (36.8%) | 17 (29.8%) | ||
| Non-alcoholic fatty liver disease | 8 (14.0%) | 6 (10.5%) | ||
| Other | 6 (10.5%) | 2 (3.5%) | ||
| MELD ^ score (median [IQR]) | 14.5 [10.5–18.0] | 13.0 [10.0–16.0] | 0.189 4 | |
| Child-Pugh ^ score (median [IQR]) | 8.0 [7.0–9.0] | 8.0 [6.0–9.0] | 0.088 4 | |
| Ascites at t0 (including suppressed by medication) | 45 (78.9%) | 44 (77.2%) | 0.821 2 | |
| Encephalopathy at t0 (including suppressed by medication) | 23 (40.4%) | 17 (29.8%) | 0.239 2 | |
| Variceal bleeding (in the preceding two years) | 7 (12.3%) | 11 (19.3%) | 0.304 2 | |
| Hepatocellular carcinoma | 4 (7.0%) | 11 (19.3%) | 0.094 3 | |
| Number of medications (median [IQR]) | Total | 10.0 [7.0–12.0] | 8.0 [6.0–9.0] | 0.006 4 |
| CLD | 3.0 [2.0–4.0] | 2.0 [1.0–3.0] | 0.014 4 | |
| Non-CLD | 7.0 [4.0–9.0] | 6.0 [4.0–7.0] | 0.061 4 | |
| Medication management * | Self-managed | 34 (59.6%) | 44 (77.2%) | 0.144 3 |
| Professional caregiver and/or professionally-packed dosage administration aid | 9 (15.8%) | 4 (7.0%) | ||
| Partner, family or another caregiver helps | 14 (24.6%) | 9 (15.8%) | ||
| Charlson Comorbidity Index (median [IQR]) | 4.0 [4.0–5.0] | 4.0 [3.0–9.0] | 0.566 4 | |
| Highest level of education † | Nil, primary, middle school | 26 (53.1%) | 18 (32.7%) | 0.036 2 |
| Completed high school and/ or additional education | 23 (46.9%) | 37 (67.3%) | ||
| Employment status ‡ | Employed | 11 (21.6%) | 8 (14.3%) | 0.325 2 |
| Government welfare | 37 (72.5%) | 45 (80.4%) | 0.340 2 | |
| No active income | 4 (7.8%) | 4 (7.1%) | 1.000 3 | |
| ARIA | Living in “highly accessible” areas | 53 (93.0%) | 47 (82.5%) | 0.152 3 |
| Living in “accessible” to “remote” areas | 4 (7.0%) | 10 (17.5%) | ||
| IRSD | Living in “most disadvantaged” areas | 18 (31.6%) | 20 (35.1%) | 0.691 2 |
| Living in areas of “low” to “moderate” disadvantage | 39 (68.4%) | 37 (64.9%) | ||
| Variables | Total Discrepancies | p | Total ‘High’ Risk Discrepancies | p |
|---|---|---|---|---|
| IRR (95%CI) | IRR (95%CI) | |||
| Number of medications | 1.12 (1.06–1.18) | <0.001 | 1.10 (1.04–1.17) | 0.001 |
| Self-managing medications | 0.67 (0.44–1.02) | 0.062 | 0.67 (0.42–1.09) | 0.106 |
| Child–Pugh score | 1.05 (0.94–1.17) | 0.377 | 1.09 (0.96–1.23) | 0.200 |
| Ascites at t0 | 1.41 (0.87–2.28) | 0.162 | 1.70 (0.95–3.06) | 0.074 |
| HE at t0 | 1.37 (0.91–2.06) | 0.132 | 1.61 (1.01–2.57) | 0.045 |
| Medication and Discrepancy Types | Baseline | Follow-Up | |||||
|---|---|---|---|---|---|---|---|
| Usual Care | Intervention | p | Usual Care | Intervention | p | ||
| Total n medications 1 | n = 451 | n = 468 | n = 310 | n = 326 | |||
| All discrepancies | Name level | 45.0% | 45.9% | 0.777 | 59.0% | 42.6% | < 0.001 |
| Total | 81.8% | 77.1% | 0.079 | 87.4% | 67.5% | < 0.001 | |
| ‘High’ risk discrepancies | Name level | 15.3% | 16.5% | 0.632 | 23.3% | 14.7% | 0.005 |
| Total | 24.4% | 23.3% | 0.696 | 28.6% | 18.7% | 0.003 | |
| CLD medications 2 | n = 118 | n = 143 | n = 82 | n = 96 | |||
| CLD discrepancies | Name level | 38.1% | 34.3% | 0.517 | 52.4% | 36.5% | 0.032 |
| Total | 71.2% | 65.0% | 0.290 | 79.3% | 57.3% | 0.002 | |
| Non-CLD medications 3 | n = 333 | n = 325 | n = 228 | n = 230 | |||
| Non-CLD discrepancies | Name level | 47.4% | 51.1% | 0.352 | 61.4% | 45.2% | 0.001 |
| Total | 85.6% | 82.5% | 0.274 | 90.4% | 71.7% | < 0.001 | |
| MRCI Score | t0 (n = 57) | t1 (n = 51) | t2 (n = 44) | t3 (n = 39) |
|---|---|---|---|---|
| Time since t0 (weeks) | - | 5.7 ± 1.6 | 14.7 ± 2.1 | 29.8 ± 5.3 |
| Total MRCI score | 25.59 ± 13.49 | 25.50 ± 13.57 | 23.42 ± 11.83 | 24.98 ± 11.70 |
| Section A score | 5.12 ± 3.80 | 5.27 ± 4.53 | 5.00 ± 4.32 | 4.77 ± 4.19 |
| Section B score | 10.89 ± 5.73 | 10.95 ± 6.15 | 9.97 ± 5.53 | 10.90 ± 5.47 |
| CLD medicines | 3.38 ± 2.40 | 3.65 ± 2.57 | 3.66 ± 2.76 | 3.62 ± 2.49 |
| Non-CLD medicines | 7.51 ± 4.98 | 7.30 ± 5.11 | 6.31 ± 4.65 | 7.28 ± 4.63 |
| Section C score | 9.58 ± 5.56 | 9.27 ± 5.08 | 8.45 ± 4.35 | 9.21 ± 4.29 |
| CLD medicines | 2.81 ± 2.33 | 2.78 ± 2.22 | 2.95 ± 2.62 | 2.79 ± 1.98 |
| Non-CLD medicines | 6.77 ± 4.70 | 6.49 ± 4.44 | 5.50 ± 3.50 | 6.41 ± 3.74 |
| Variable | Adj-OR 1 (95%CI) | p |
|---|---|---|
| Number of ‘high’ risk discrepancies at t0 | 1.25 (0.97–1.63) | 0.088 |
| Child–Pugh score | 1.35 (1.04–1.75) | 0.024 |
| Variceal bleeding | 4.85 (1.54–15.28) | 0.007 |
| Randomization: intervention | 0.79 (0.32–1.99) | 0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayward, K.L.; Valery, P.C.; Patel, P.J.; Li, C.; Horsfall, L.U.; Wright, P.L.; Tallis, C.J.; Stuart, K.A.; David, M.; Irvine, K.M.; et al. Medication Discrepancies and Regimen Complexity in Decompensated Cirrhosis: Implications for Medication Safety. Pharmaceuticals 2021, 14, 1207. https://doi.org/10.3390/ph14121207
Hayward KL, Valery PC, Patel PJ, Li C, Horsfall LU, Wright PL, Tallis CJ, Stuart KA, David M, Irvine KM, et al. Medication Discrepancies and Regimen Complexity in Decompensated Cirrhosis: Implications for Medication Safety. Pharmaceuticals. 2021; 14(12):1207. https://doi.org/10.3390/ph14121207
Chicago/Turabian StyleHayward, Kelly L., Patricia C. Valery, Preya J. Patel, Catherine Li, Leigh U. Horsfall, Penny L. Wright, Caroline J. Tallis, Katherine A. Stuart, Michael David, Katharine M. Irvine, and et al. 2021. "Medication Discrepancies and Regimen Complexity in Decompensated Cirrhosis: Implications for Medication Safety" Pharmaceuticals 14, no. 12: 1207. https://doi.org/10.3390/ph14121207
APA StyleHayward, K. L., Valery, P. C., Patel, P. J., Li, C., Horsfall, L. U., Wright, P. L., Tallis, C. J., Stuart, K. A., David, M., Irvine, K. M., Cottrell, N., Martin, J. H., & Powell, E. E. (2021). Medication Discrepancies and Regimen Complexity in Decompensated Cirrhosis: Implications for Medication Safety. Pharmaceuticals, 14(12), 1207. https://doi.org/10.3390/ph14121207






