Two Novel Semisynthetic Lipoglycopeptides Active against Staphylococcus aureus Biofilms and Cells in Late Stationary Growth Phase
Abstract
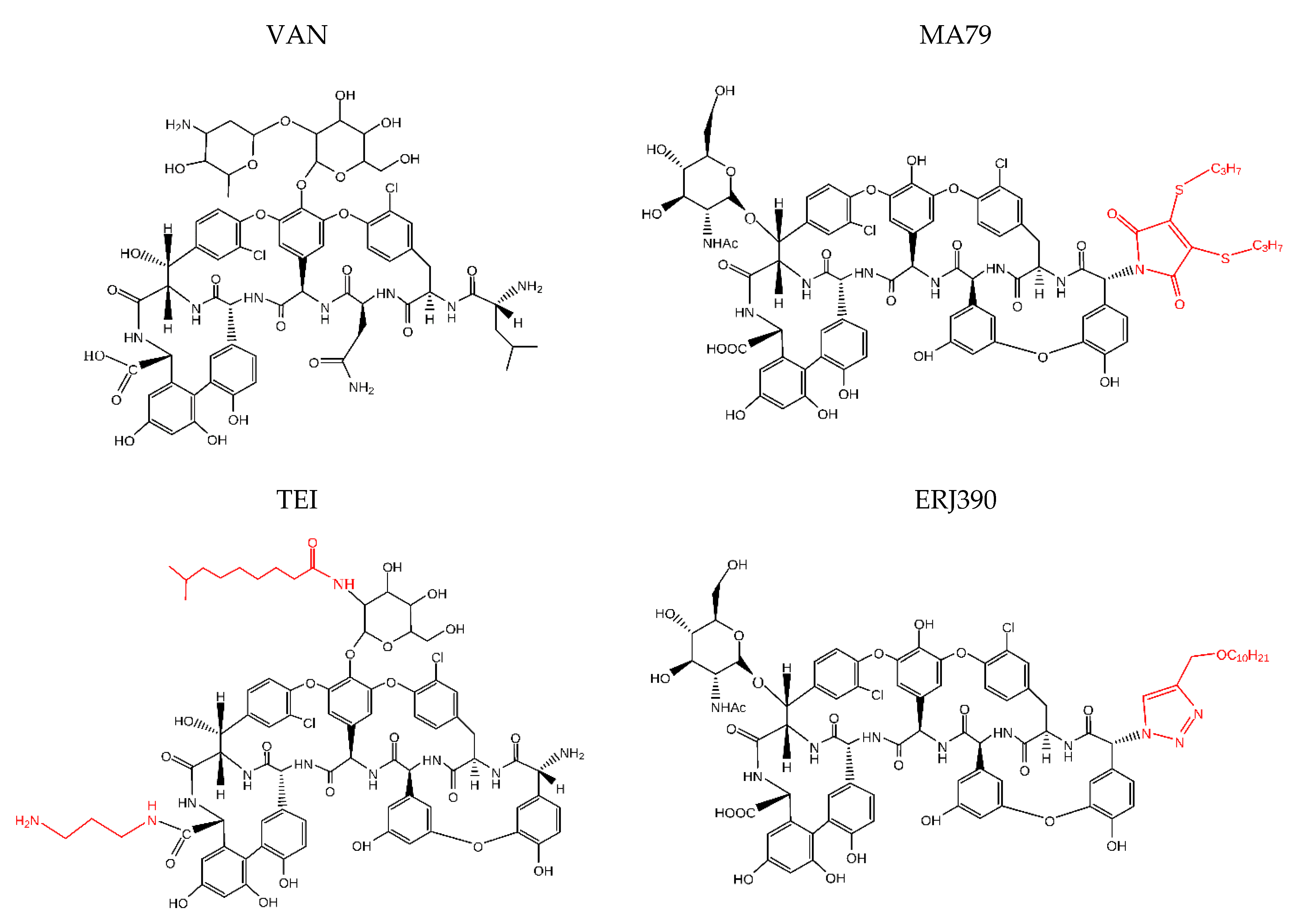
:1. Introduction
2. Results
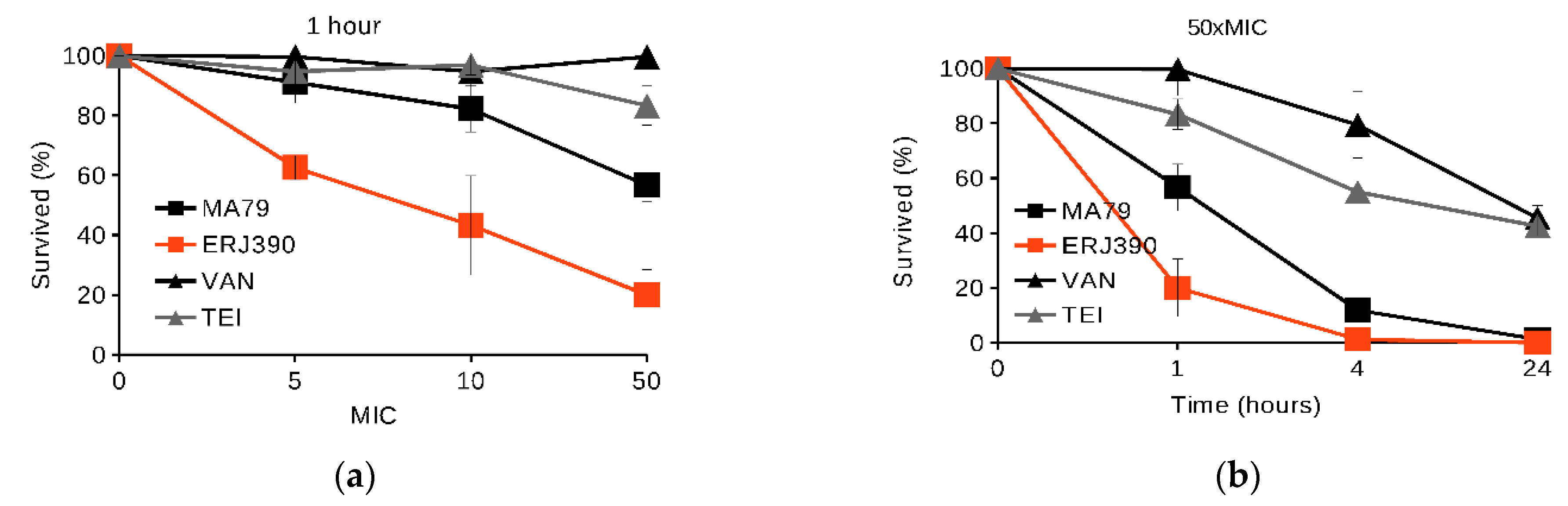
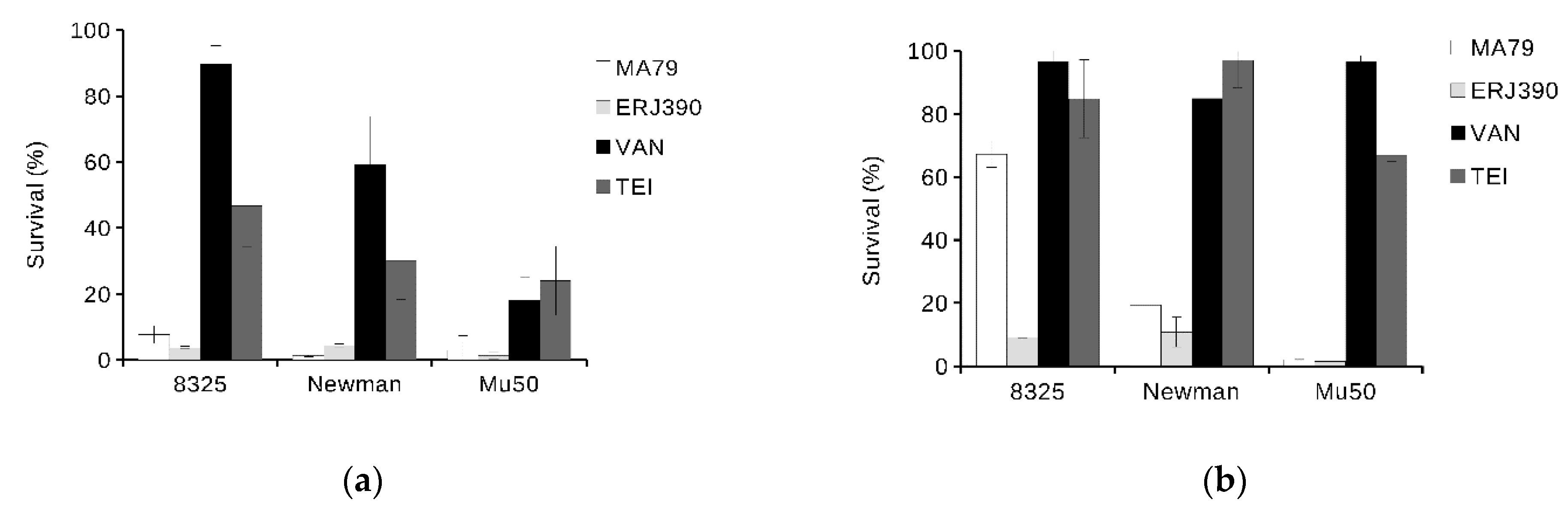
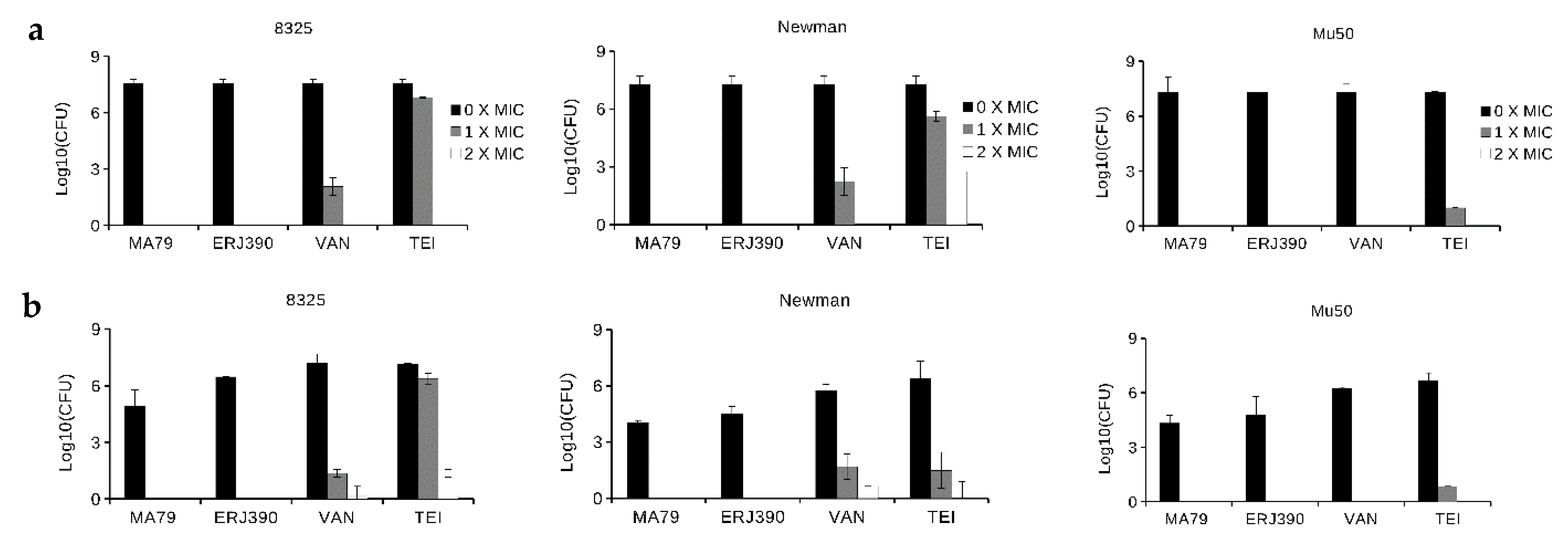
2.1. MA79 and ERJ390 Are Bactericidal against Exponential and Stationary-Phase S. aureus
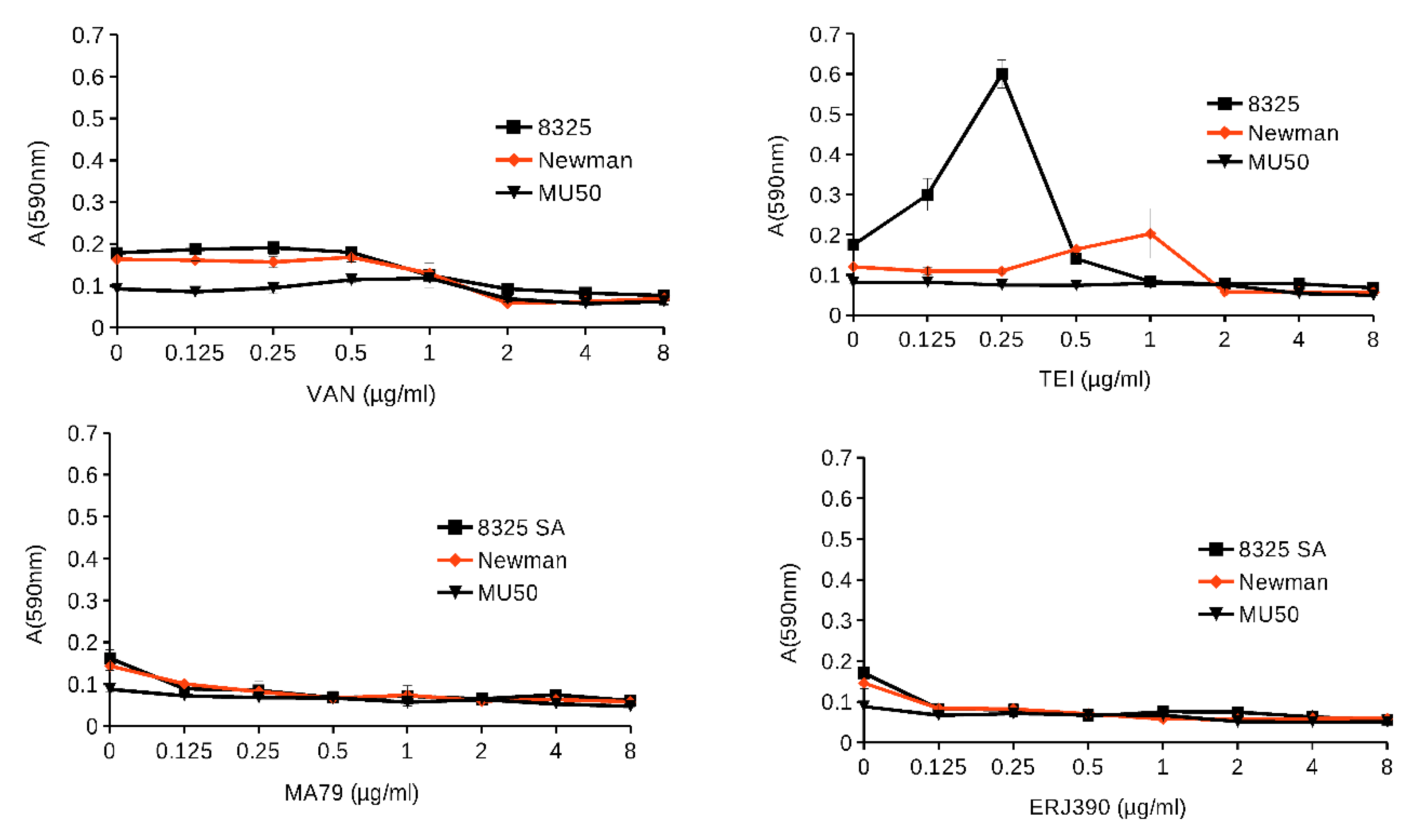
2.2. MA79 and ERJ390 Do Not Induce S. aureus Biofilm Formation
2.3. ERJ390 Eradicates S. aureus Biofilm
2.4. MA79 and ERJ390 Do Not Cause Resistance Selection
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Antibiotics
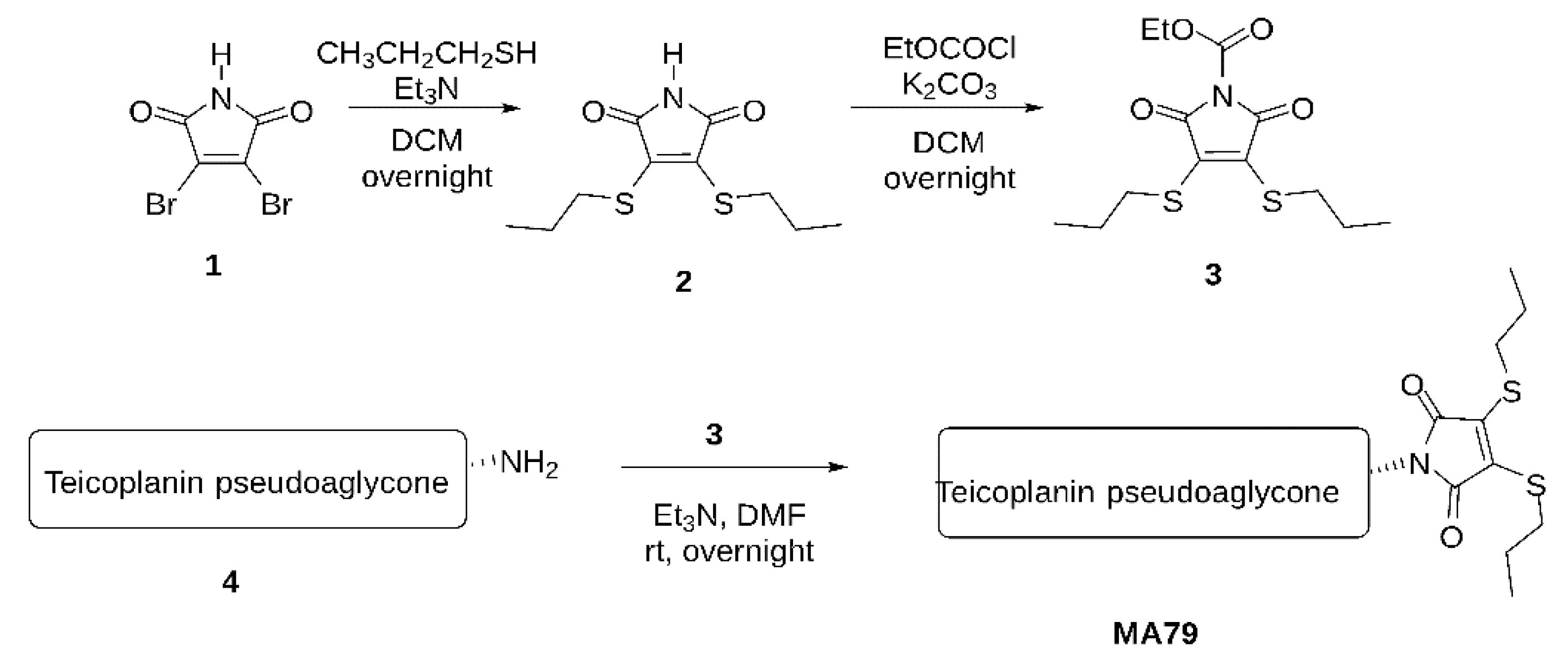
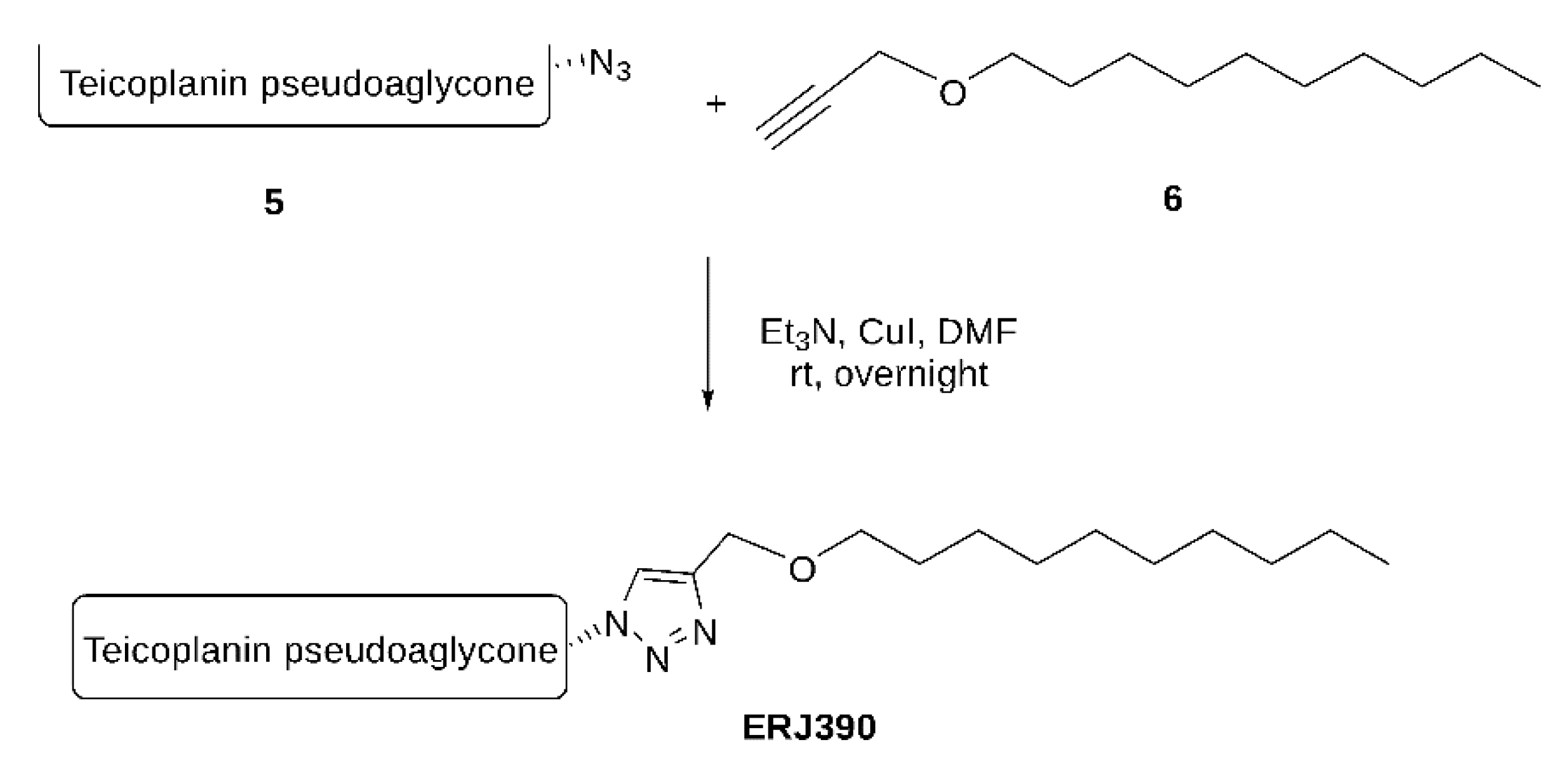
- A.
- Maleimide bis-n-propylsulfide (2)
- B.
- N-Ethoxycarbonyl maleimide bis-n-propylsulfide (3)
- C.
- MA79
4.3. Susceptibility Testing
4.4. S. aureus Killing in Exponential and Late Stationary Growth Phases by the Antibiotics
4.5. S. aureus Biofilm Formation in the Presence of Glycopeptide Antibiotics
4.6. Bacteria Killing in S. aureus Biofilm
4.7. Selection of Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, D.A.; Williams, D.H.; Gwynn, M.N.; Knowles, D.J.C. Dimerization and membrane anchors in extracellular targeting of vancomycin group antibiotics. Antimicrob. Agents Chemother. 1995, 39, 781–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Economou, N.J.; Zentner, I.J.; Lazo, E.; Jakoncic, J.; Stojanoff, V.; Weeks, S.D.; Grasty, K.C.; Cocklin, S.; Loll, P.J. Structure of the complex between teicoplanin and a bacterial cell-wall peptide: Use of a carrier-protein approach. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved glycopeptide antibacterial drugs: Mechanism of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef] [Green Version]
- Cheung, A.L.; Bayer, A.S.; Zhang, G.; Gresham, H.; Xiong, Y.Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2004, 40, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pintér, G.; Batta, G.; Kéki, S.; Mándi, A.; Komáromi, I.; Takács-Novák, K.; Sztaricskai, F.; Roth, E.; Ostorházi, E.; Rozgonyi, F.; et al. Diazo transfer-click reaction route to new, lipophilic teicoplanin and ristocetin aglycon derivatives with high antibacterial and anti-influenza virus activity: An aggregation and receptor binding study. J. Med. Chem. 2009, 52, 6053–6061. [Google Scholar] [CrossRef] [PubMed]
- Csávás, M.; Miskovics, A.; Szűcs, Z.; Rőth, E.; Nagy, Z.L.; Bereczki, I.; Herczeg, M.; Batta, G.; Nemes-Nikodém, É.; Ostorházi, E.; et al. Synthesis and antibacterial evaluation of some teicoplanin pseudoaglycon derivatives containing alkyl-and arylthiosubstituted maleimides. J. Antibiot. 2015, 68, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Szűcs, Z.; Kelemen, V.; Le Thai, S.; Csávás, M.; Rőth, E.; Batta, G.; Stevaert, A.; Vanderlinden, E.; Naesens, L.; Herczegh, P.; et al. Structure-activity relationship studies of lipophilic teicoplanin pseudoaglycon derivatives as new anti-influenza virus agents. Eur. J. Med. Chem. 2018, 157, 1017–1030. [Google Scholar] [CrossRef]
- Vimberg, V.; Gazak, R.; Szűcs, Z.; Borbás, A.; Herczegh, P.; Cavanagh, J.P.; Zieglerova, L.; Závora, J.; Adámková, V.; Balikova Novotna, G. Fluorescence assay to predict activity of the glycopeptide antibiotics. J. Antibiot. 2019, 72, 114–117. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Jamil, N. Effect of sub-lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J. Basic Microbiol. 2011, 51, 191–195. [Google Scholar] [CrossRef]
- El-Azizi, M.; Rao, S.; Kanchanapoom, T.; Khardori, N. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzi, C.; Waters, E.M.; Rudkin, J.K.; Schaeffer, C.R.; Lohan, A.J.; Tong, P.; Loftus, B.J.; Pier, G.B.; Fey, P.D.; Massey, R.C.; et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog. 2012, 8, e1002626. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.; Wright, J.B.; Schultz, G.; Burrell, R.; Nadworny, P. Microbial biofilms and chronic wounds. Microorganisms 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Miao, J.; Lin, S.; Soteyome, T.; Peters, B.M.; Li, Y.; Chen, H.; Su, J.; Li, L.; Li, B.; Xu, Z.; et al. Biofilm Formation of Staphylococcus aureus under Food Heat Processing Conditions: First Report on CML Production within Biofilm. Sci. Rep. 2019, 9, 1312. [Google Scholar] [CrossRef] [PubMed]
- Gwynn, M.N.; Portnoy, A.; Rittenhouse, S.F.; Payne, D.J. Challenges of antibacterial discovery revisited. Ann. N. Y. Acad. Sci. 2010, 1213, 5–19. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in Glycopeptide Antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef] [Green Version]
- Mühlberg, E.; Umstätter, F.; Kleist, C.; Domhan, C.; Mier, W.; Uhl, P. Renaissance of vancomycin: Approaches for breaking antibiotic resistance in multidrug-resistant bacteria. Can. J. Microbiol. 2020, 66, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Belley, A.; Neesham-Grenon, E.; McKay, G.; Arhin, F.F.; Harris, R.; Beveridge, T.; Parr, T.R.; Moeck, G. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob. Agents Chemother. 2009, 53, 918–925. [Google Scholar] [CrossRef] [Green Version]
- Belley, A.; Lalonde Seguin, D.; Arhin, F.; Moeck, G. Comparative in vitro activities of oritavancin, dalbavancin, and vancomycin against methicillin-resistant Staphylococcus aureus isolates in a nondividing state. Antimicrob. Agents Chemother. 2016, 60, 4342–4345. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.; Hardin, T.C.; Smart, J.I. A review of telavancin activity in in vitro biofilms and animal models of biofilm-associated infections. Future Microbiol. 2015, 10, 1325–1338. [Google Scholar] [CrossRef] [PubMed]
- Meeker, D.G.; Beenken, K.E.; Mills, W.B.; Loughran, A.J.; Spencer, H.J.; Lynn, W.B.; Smeltzer, M.S. Evaluation Of Antibiotics Active Against methicillin-resistant Staphylococcus aureus based on activity in an established biofilm. Antimicrob. Agents Chemother. 2016, 60, 5688–5694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhanel, G.G.; Calic, D.; Schweizer, F.; Zelenitsky, S.; Adam, H.; Lagac-Wiens, P.R.S.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; Karlowsky, J.A. New lipoglycopeptides: A comparative review of dalbavancin, oritavancin and telavancin. Drugs 2010, 70, 859–886. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Schweizer, F.; Karlowsky, J.A. Oritavancin: Mechanism of action. Clin. Infect. Dis. 2012, 54, S214–S219. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Lunde, C.S.; Benton, B.M.; Wilkinson, B.J. Further insights into the mode of action of the lipoglycopeptide telavancin through global gene expression studies. Antimicrob. Agents Chemother. 2012, 56, 3157–3164. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Ziora, Z.M.; Hansford, K.A.; Blaskovich, M.A.; Butler, M.S.; Cooper, M.A. Anti-cooperative ligand binding and dimerisation in the glycopeptide antibiotic dalbavancin. Org. Biomol. Chem. 2014, 12, 2568–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berscheid, A.; François, P.; Strittmatter, A.; Gottschalk, G.; Schrenzel, J.; Sass, P.; Bierbaum, G. Generation of a vancomycin-intermediate Staphylococcus aureus (VISA) strain by two amino acid exchanges in VraS. J. Antimicrob. Chemother. 2014, 69, 3190–3198. [Google Scholar] [CrossRef] [Green Version]
- Werth, B.J.; Jain, R.; Hahn, A.; Cummings, L.; Weaver, T.; Waalkes, A.; Sengupta, D.; Salipante, S.J.; Rakita, R.M.; Butler-Wu, S.M. Emergence of dalbavancin non-susceptible, vancomycin-intermediate Staphylococcus aureus (VISA) after treatment of MRSA central line-associated bloodstream infection with a dalbavancin- and vancomycin-containing regimen. Clin. Microbiol. Infect. 2018, 24, 429.e1–429.e5. [Google Scholar] [CrossRef] [Green Version]
- Vimberg, V.; Zieglerová, L.; Buriánková, K.; Branny, P.; Balíková Novotná, G. VanZ Reduces the Binding of Lipoglycopeptide Antibiotics to Staphylococcus aureus and Streptococcus pneumoniae Cells. Front. Microbiol. 2020, 11, 566. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Gillaspy, A.F.; Worrell, V.; Orvis, J.; Roe, B.A.; Dyer, D.W.; Iandolo, J.J. The Staphylococcus aureus NCTC 8325 Genome. In Gram-Positive Pathogens, 2nd ed.; American Society of Microbiology: Washington, DC, USA, 2006; pp. 381–412. [Google Scholar]
- Hiramatsu, K. The emergence of Staphylococcus aureus with reduced susceptibility to vancomycin in Japan. Am. J. Med. 1998, 104, 7S–10S. [Google Scholar] [CrossRef]
- Baba, T.; Bae, T.; Schneewind, O.; Takeuchi, F.; Hiramatsu, K. Genome sequence of Staphylococcus aureus strain newman and comparative analysis of staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 2008, 190, 300–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimberg, V.; Kuzma, M.; Stodůlková, E.; Novák, P.; Bednárová, L.; Šulc, M.; Gažák, R. Hydnocarpin-Type Flavonolignans: Semisynthesis and Inhibitory Effects on Staphylococcus aureus Biofilm Formation. J. Nat. Prod. 2015, 78, 2095–2103. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vimberg, V.; Zieglerova, L.; Mazumdar, A.; Szűcs, Z.; Borbás, A.; Herczegh, P.; Novotna, G.B. Two Novel Semisynthetic Lipoglycopeptides Active against Staphylococcus aureus Biofilms and Cells in Late Stationary Growth Phase. Pharmaceuticals 2021, 14, 1182. https://doi.org/10.3390/ph14111182
Vimberg V, Zieglerova L, Mazumdar A, Szűcs Z, Borbás A, Herczegh P, Novotna GB. Two Novel Semisynthetic Lipoglycopeptides Active against Staphylococcus aureus Biofilms and Cells in Late Stationary Growth Phase. Pharmaceuticals. 2021; 14(11):1182. https://doi.org/10.3390/ph14111182
Chicago/Turabian StyleVimberg, Vladimir, Leona Zieglerova, Aninda Mazumdar, Zsolt Szűcs, Aniko Borbás, Pál Herczegh, and Gabriela Balikova Novotna. 2021. "Two Novel Semisynthetic Lipoglycopeptides Active against Staphylococcus aureus Biofilms and Cells in Late Stationary Growth Phase" Pharmaceuticals 14, no. 11: 1182. https://doi.org/10.3390/ph14111182
APA StyleVimberg, V., Zieglerova, L., Mazumdar, A., Szűcs, Z., Borbás, A., Herczegh, P., & Novotna, G. B. (2021). Two Novel Semisynthetic Lipoglycopeptides Active against Staphylococcus aureus Biofilms and Cells in Late Stationary Growth Phase. Pharmaceuticals, 14(11), 1182. https://doi.org/10.3390/ph14111182





