NanoUPLC-QTOF-MS/MS Determination of Major Rosuvastatin Degradation Products Generated by Gamma Radiation in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
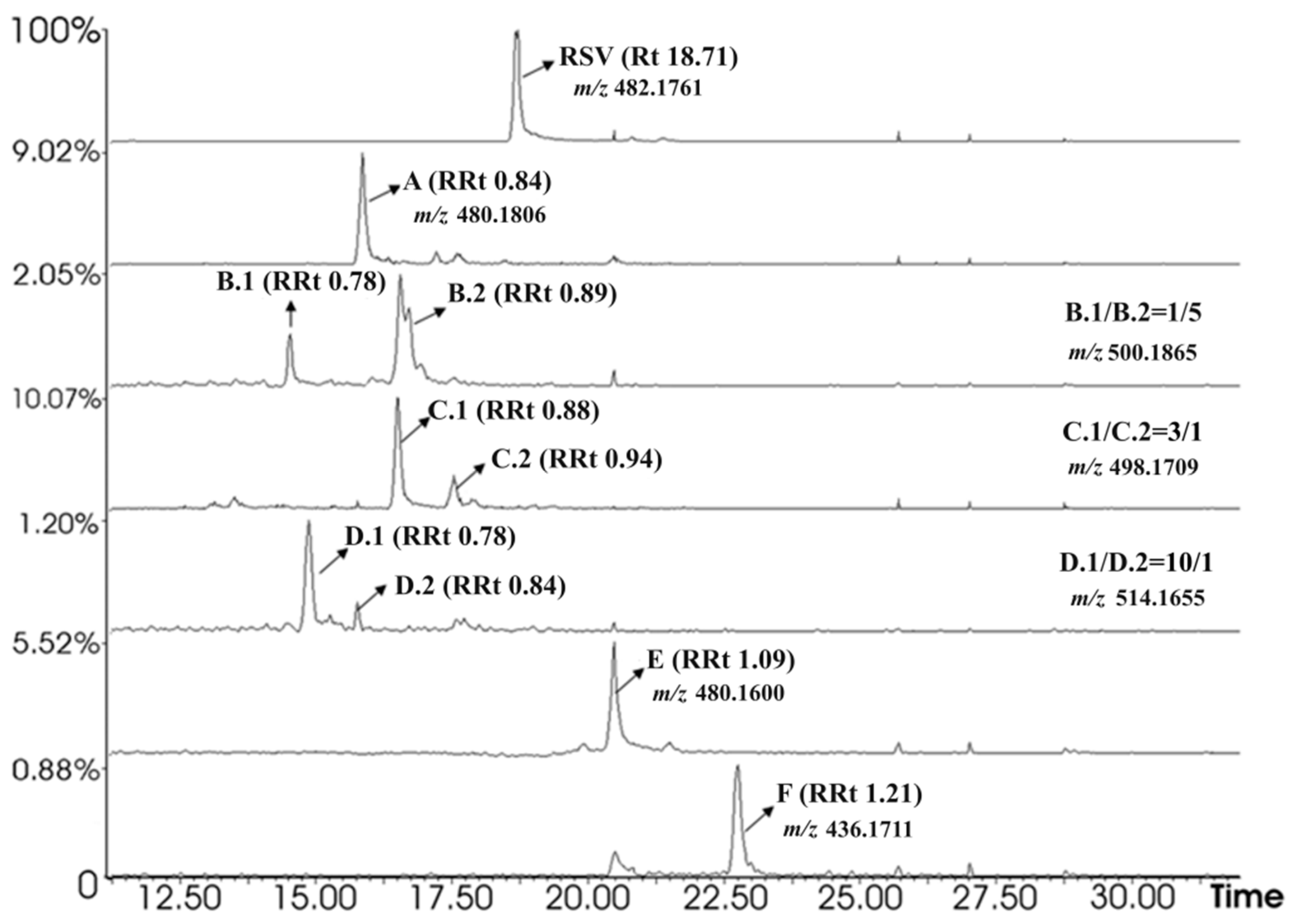
2.1. Chromatographic Analysis of Irradiated Samples
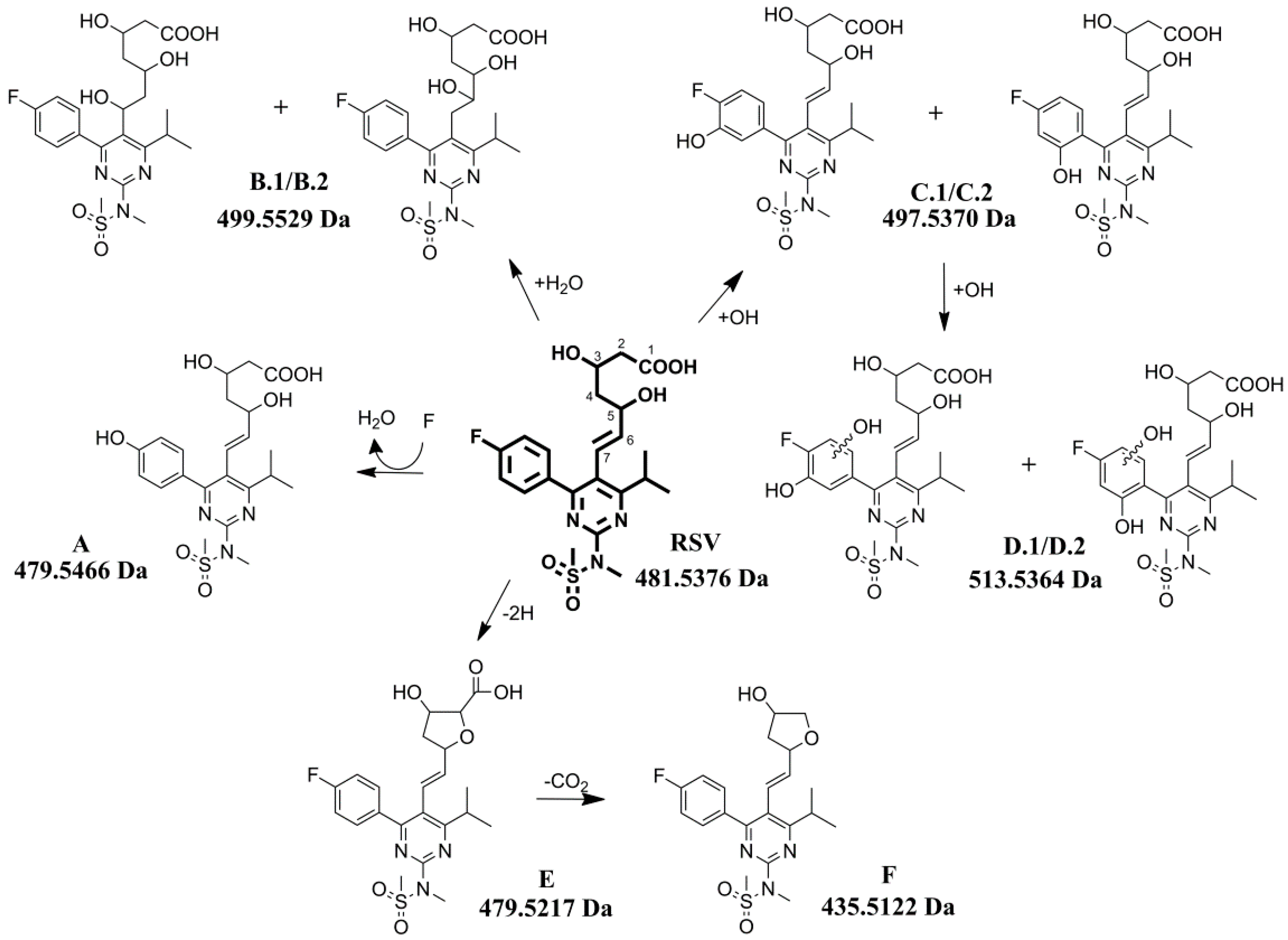
2.2. Fragmentation Pathway of RSV
2.3. Characterization of Degradation Products
2.3.1. Degradation Product A (m/z 480.1805)
2.3.2. Degradation Products B.1/B.2 (m/z 500.1867)
2.3.3. Degradation Products C.1/C.2 (m/z 498.1710)
2.3.4. Degradation Products D.1/D.2 (m/z 514.1659)
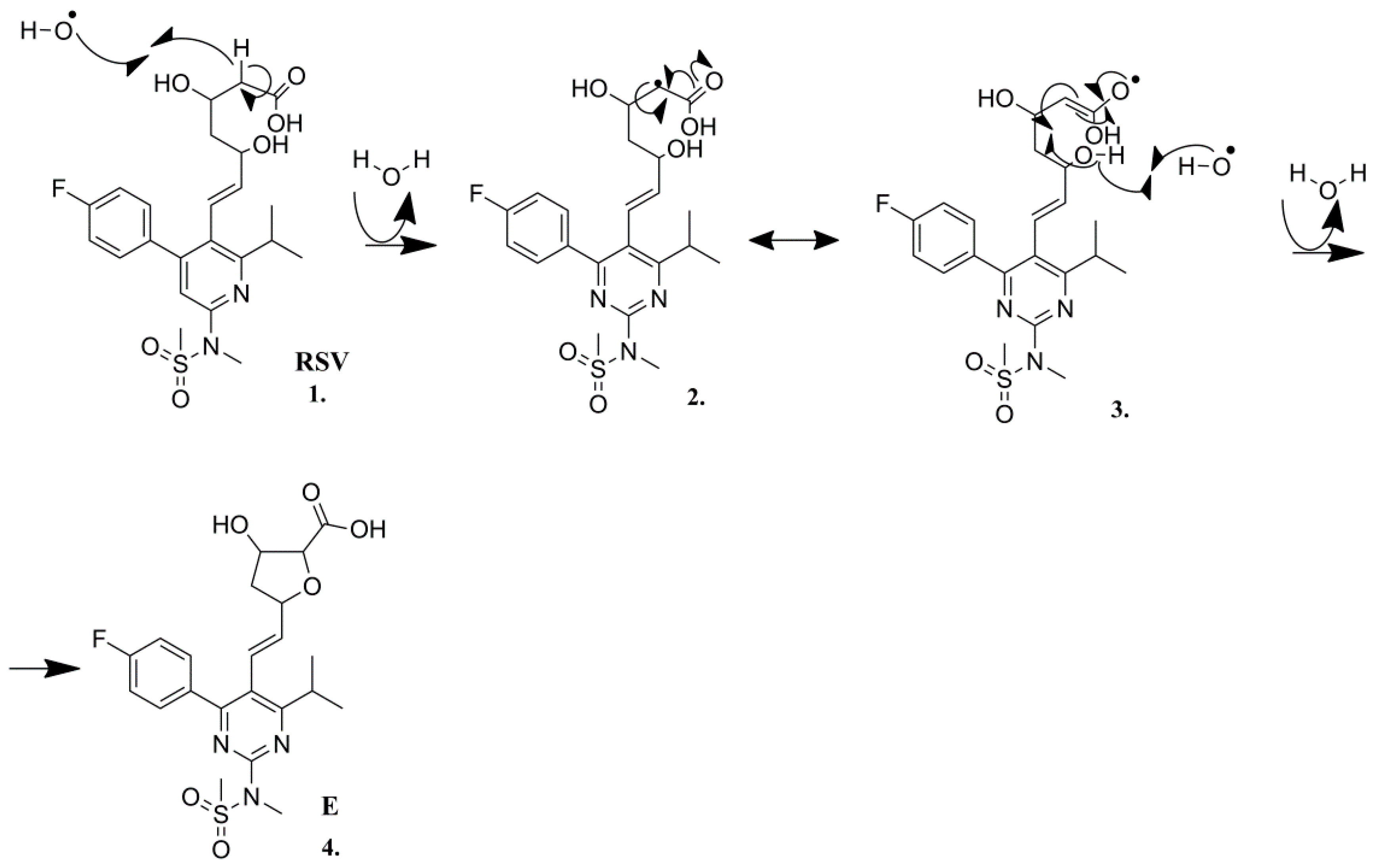
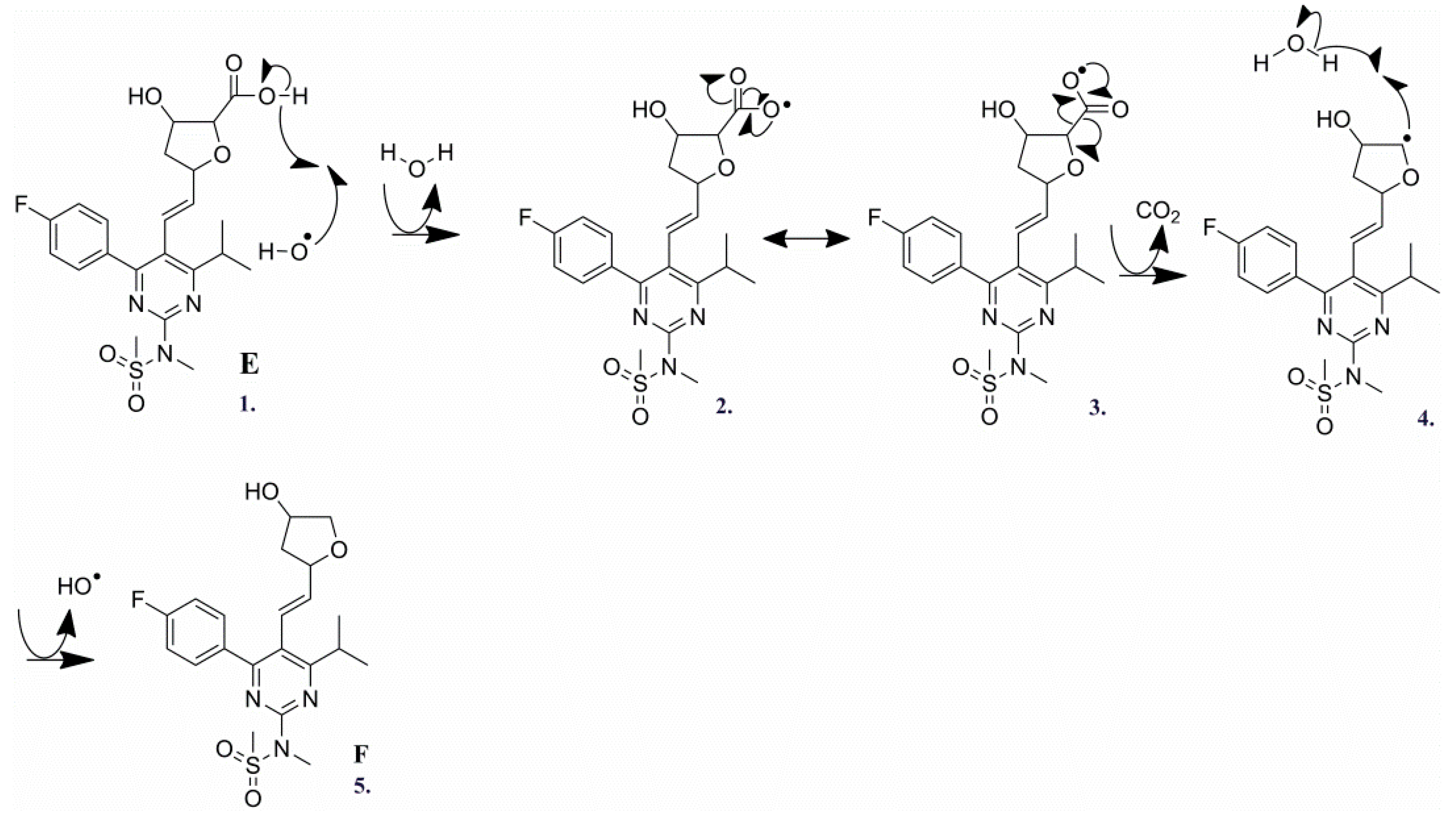
2.3.5. Degradation Product E (m/z 480.1605)
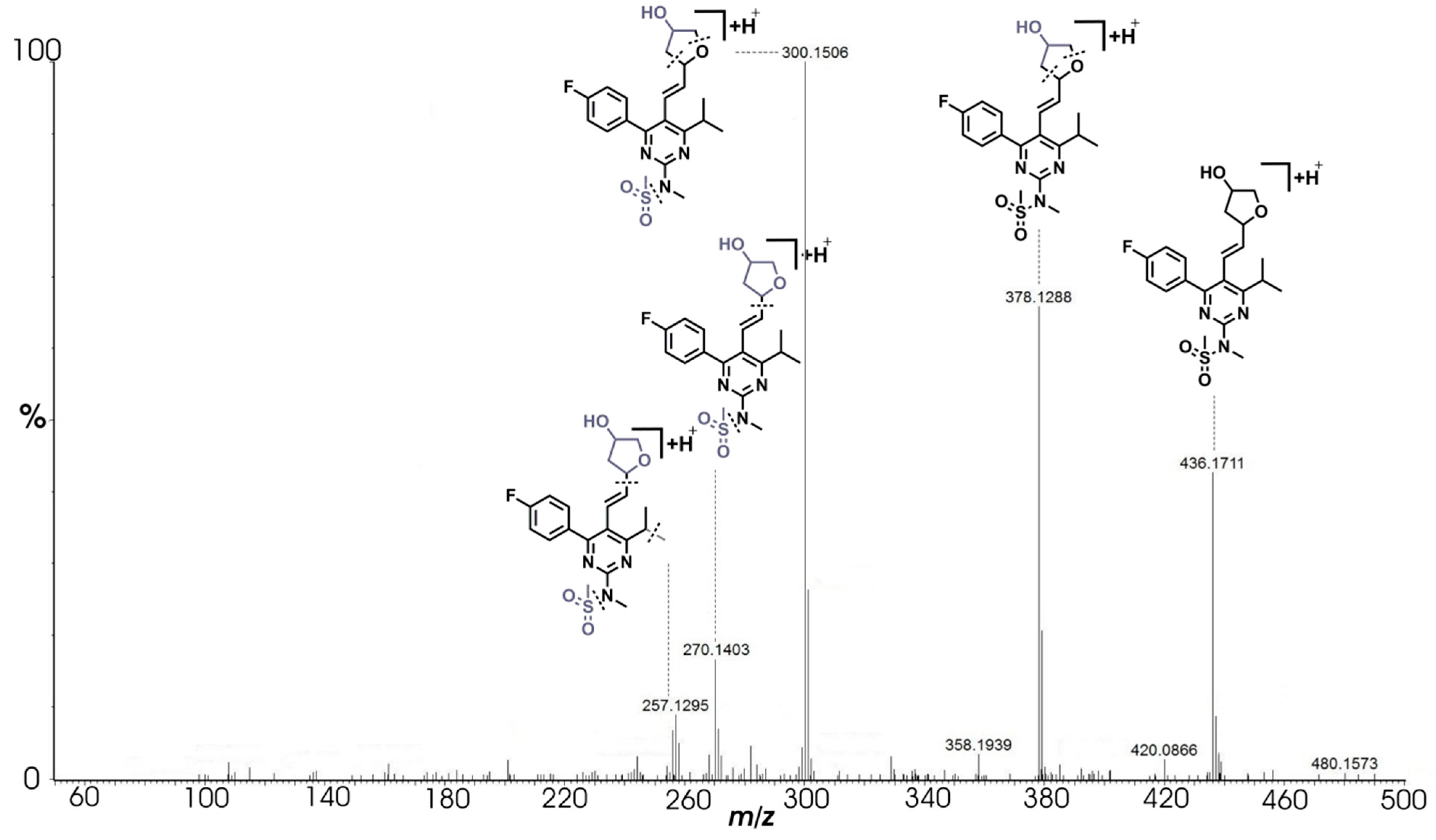
2.3.6. Degradation Product F (m/z 436.1706)
3. Materials and Methods
3.1. Raw Materials
3.2. Sample Preparation and Irradiation
3.3. NanoUPLC-NanoESI-QTOF Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khedr, A.; Belal, F.; Ibrahim, F.; Elawady, T. Analysis of rosuvastatin stress degradation behavior using liquid chromatography coupled to ultraviolet detection and electrospray ionization mass spectrometry. Anal. Methods 2013, 5, 6494–6502. [Google Scholar] [CrossRef]
- Carswell, C.I.; Plosker, G.L.; Jarvis, B. Rosuvastatin. Drugs 2002, 62, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current Perspectives on Statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef]
- Schupp, N.; Schmid, U.; Heidland, A.; Stopper, H. Rosuvastatin protects against oxidative stress and DNA damage in vitro via upregulation of glutathione synthesis. Atherosclerosis 2008, 199, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mahalwar, R.; Khanna, D. Pleiotropic antioxidant potential of rosuvastatin in preventing cardiovascular disorders. Eur. J. Pharmacol. 2013, 711, 57–62. [Google Scholar] [CrossRef]
- Zang, L.; He, H.; Xu, Q.; Yu, Y.; Zheng, N.; Liu, W.; Hayashi, T.; Tashiro, S.I.; Onodera, S.; Ikejima, T. Reactive oxygen species H2O2 and OH, but not O2− promote oridonin-induced phagocytosis of apoptotic cells by human histocytic lymphoma U937 cells. Int. Immunopharmacol. 2013, 15, 414–423. [Google Scholar] [CrossRef]
- Koksal, M.; Eren, M.A.; Turan, M.N.; Sabuncu, T. The effects of atorvastatin and rosuvastatin on oxidative stress in diabetic patients. Eur. J. Intern. Med. 2011, 22, 249–253. [Google Scholar] [CrossRef]
- Otto, A.; Fontaine, J.; Tschirhart, E.; Fontaine, D.; Berkenboom, G. Rosuvastatin treatment protects against nitrate-induced oxidative stress in eNOS knockout mice: Implication of the NAD(P)H oxidase pathway. Br. J. Pharmacol. 2006, 148, 544–552. [Google Scholar] [CrossRef]
- Deng, J.; Wu, G.; Yang, C.; Li, Y.; Jing, Q.; Han, Y. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Umeda, R.; Takanari, H.; Ogata, K.; Matsumoto, S.; Kitano, T.; Ono, K.; Tokumaru, O. Direct free radical scavenging effects of water-soluble HMG-CoA reductase inhibitors. J. Clin. Biochem. Nutr. 2019, 64, 20–26. [Google Scholar] [CrossRef]
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Song, W.; Xu, T.; Cooper, W.J.; Dionysiou, D.D.; De La Cruz, A.A.; O’Shea, K.E. Radiolysis studies on the destruction of microcystin-LR in aqueous solution by hydroxyl radicals. Environ. Sci. Technol. 2009, 43, 1487–1492. [Google Scholar] [CrossRef]
- Yakabuskie, P.A.; Joseph, J.M.; Stuart, C.R.; Wren, J.C. Long-term γ-radiolysis kinetics of NO3− and NO2− solutions. J. Phys. Chem. A 2011, 115, 4270–4278. [Google Scholar] [CrossRef] [PubMed]
- Khouri, H.; Collin, F.; Bonnefont-Rousselot, D.; Legrand, A.; Jore, D.; Gardes-Albert, M. Radical-induced oxidation of metformin. Eur. J. Biochem. 2004, 271, 4745–4752. [Google Scholar] [CrossRef] [PubMed]
- Collin, F.; Khoury, H.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Jore, D.; Gardès-Albert, M. Liquid chromatographic/electrospray ionization mass spectrometric identification of the oxidation end-products of metformin in aqueous solutions. J. Mass Spectrom. 2004, 39, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Abdel Rahman, R.O.; Hung, Y.-T. Application of Ionizing Radiation in Wastewater Treatment: An Overview. Water 2019, 12, 19. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Lee, H.B.; Peart, T.E.; Lewina Svoboda, M.; Backus, S. Occurrence and fate of rosuvastatin, rosuvastatin lactone, and atorvastatin in Canadian sewage and surface water samples. Chemosphere 2009, 77, 1285–1291. [Google Scholar] [CrossRef]
- Ashfaq, M.; Nawaz Khan, K.; Saif Ur Rehman, M.; Mustafa, G.; Faizan Nazar, M.; Sun, Q.; Iqbal, J.; Mulla, S.I.; Yu, C.P. Ecological risk assessment of pharmaceuticals in the receiving environment of pharmaceutical wastewater in Pakistan. Ecotoxicol. Environ. Saf. 2017, 136, 31–39. [Google Scholar] [CrossRef]
- Golovko, O.; Kumar, V.; Fedorova, G.; Randak, T.; Grabic, R. Seasonal changes in antibiotics, antidepressants/psychiatric drugs, antihistamines and lipid regulators in a wastewater treatment plant. Chemosphere 2014, 111, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Buchberger, W.; Alonso, E.; Himmelsbach, M.; Aparicio, I. Comparison of different extraction methods for the determination of statin drugs in wastewater and river water by HPLC/Q-TOF-MS. Talanta 2011, 85, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.; Song, W.; Santoke, H.; Cooper, W.J. Treatment of statin compounds by advanced oxidation processes: Kinetic considerations and destruction mechanisms. Radiat. Phys. Chem. 2011, 80, 453–461. [Google Scholar] [CrossRef]
- Zakrajšek, J.; Bevc-Černilec, K.; Bohanec, S.; Urleb, U. Optimization of UPLC method for simultaneous determination of rosuvastatin and rosuvastatin degradation products. Acta Chim. Slov. 2017, 64, 968–979. [Google Scholar] [CrossRef]
- Mostafa, N.M.; Badawey, A.M.; Lamie, N.T.; Abd El-Aleem, A.E.A.B. Selective chromatographic methods for the determination of Rosuvastatin calcium in the presence of its acid degradation products. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 2182–2196. [Google Scholar] [CrossRef]
- Mehta, T.N.; Patel, A.K.; Kulkarini, G.M.; Suubbaiah, G. Determination of rosuvastatin in the presence of its degradation products by a stability-indicating LC method. J. AOAC Int. 2005, 88, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.K.; Patel, M.C. Development and validation of a stability indicating RP-UPLC method for determination of quetiapine in pharmaceutical dosage form. Sci. Pharm. 2011, 79, 97–111. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Liu, X.; Wei, C.; Zhang, R.; Song, H.; Yao, H.; Yuan, G.; Wang, B.; Guo, R. Validated LC-MS/MS Method for the Determination of Rosuvastatin in Human Plasma: Application to a Bioequivalence Study in Chinese Volunteers. Pharmacol. Pharm. 2011, 02, 341–346. [Google Scholar] [CrossRef]
- Segalin, J.; Sirtori, C.; Jank, L.; Lima, M.F.S.; Livotto, P.R.; Machado, T.C.; Lansarin, M.A.; Pizzolato, T.M. Identification of transformation products of rosuvastatin in water during ZnO photocatalytic degradation through the use of associated LC-QTOF-MS to computational chemistry. J. Hazard. Mater. 2015, 299, 78–85. [Google Scholar] [CrossRef]
- Shah, R.P.; Sahu, A.; Singh, S. LC-MS/TOF, LC-MSn, on-line H/D exchange and LC-NMR studies on rosuvastatin degradation and in silico determination of toxicity of its degradation products: A comprehensive approach during drug development. Anal. Bioanal. Chem. 2013, 405, 3215–3231. [Google Scholar] [CrossRef]
- Gama, M.R.; Collins, C.H.; Bottoli, C.B.G. Nano-Liquid Chromatography in Pharmaceutical and Biomedical Research. J. Chromatogr. Sci. 2013, 51, 694–703. [Google Scholar] [CrossRef] [PubMed]
- De Vijlder, T.; Valkenborg, D.; Lemière, F.; Romijn, E.P.; Laukens, K.; Cuyckens, F. A tutorial in small molecule identification via electrospray ionization-mass spectrometry: The practical art of structural elucidation. Mass Spectrom. Rev. 2018, 37, 607–629. [Google Scholar] [CrossRef] [PubMed]
- Gathungu, R.M.; Kautz, R.; Kristal, B.S.; Bird, S.S.; Vouros, P. The integration of LC-MS and NMR for the analysis of low molecular weight trace analytes in complex matrices. Mass Spectrom. Rev. 2020, 39, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Machado, T.C.; Pizzolato, T.M.; Arenzon, A.; Segalin, J.; Lansarin, M.A. Photocatalytic degradation of rosuvastatin: Analytical studies and toxicity evaluations. Sci. Total Environ. 2015, 502, 571–577. [Google Scholar] [CrossRef]
- Jinga, L.I.; Popescu-Pelin, G.; Socol, G.; Mocanu, S.; Tudose, M.; Culita, D.C.; Kuncser, A.; Ionita, P. Chemical Degradation of Methylene Blue Dye Using TiO2/Au Nanoparticles. Nanomaterials 2021, 11, 1605. [Google Scholar] [CrossRef] [PubMed]
- Saquib, M.; Abu Tariq, M.; Haque, M.M.; Muneer, M. Photocatalytic degradation of disperse blue 1 using UV/TiO2/H2O2 process. J. Environ. Manag. 2008, 88, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cristoni, S.; Brioschi, M.; Rizzi, A.; Sironi, L.; Gelosa, P.; Tremoli, E.; Bernardi, L.R.; Banfi, C. Analysis of rosuvatatin by imaging mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 3483–3487. [Google Scholar] [CrossRef] [PubMed]
- Kishore, C.R.P.; Mohan, G.V.K. Structural identification and estimation of Rosuvastatin calcium related impurities in Rosuvastatin calcium tablet dosage form. Anal. Chem. Res. 2017, 12, 17–27. [Google Scholar] [CrossRef]
- Reddy, G.V.R.; Reddy, B.V.; Haque, S.W.; Gautam, H.D.; Kumar, P.; Kumar, A.P.; Park, J.H. Development and validation of a stability-indicating uplc method for rosuvastatin and its related impurities in pharmaceutical dosage forms. Quim. Nova 2011, 34, 250–255. [Google Scholar] [CrossRef][Green Version]
- Choure, S.C.; Bamatraf, M.M.M.; Rao, B.S.M.; Das, R.; Mohan, H.; Mittal, J.P. Hydroxylation of chlorotoluenes and cresols: A pulse radiolysis, laser flash photolysis, and product analysis study. J. Phys. Chem. A 1997, 101, 9837–9845. [Google Scholar] [CrossRef]
- Dončević, L.; Svetličić, E.; Hozić, A.; Cindrić, M. Dataset for: Determination of Rosuvastatin Degradation Products. 2020. Available online: https://data.mendeley.com/datasets/cg3hmww4t3/1 (accessed on 9 November 2021).
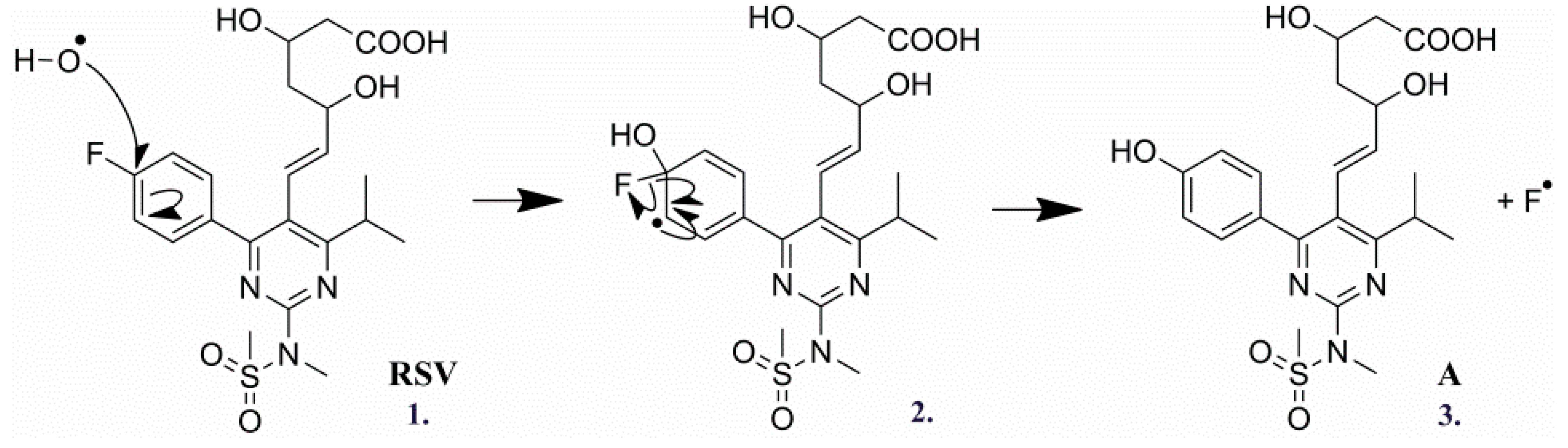

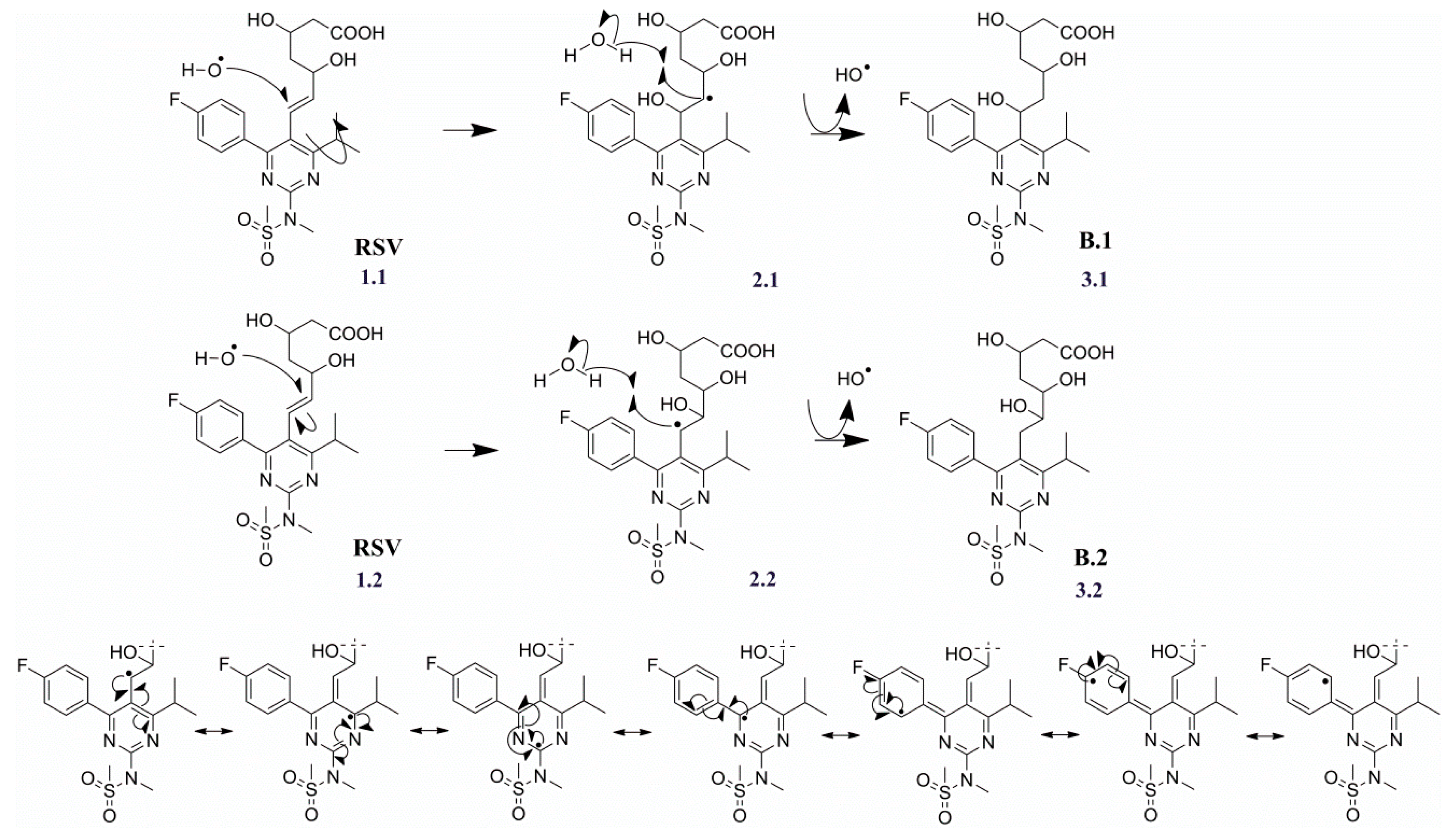

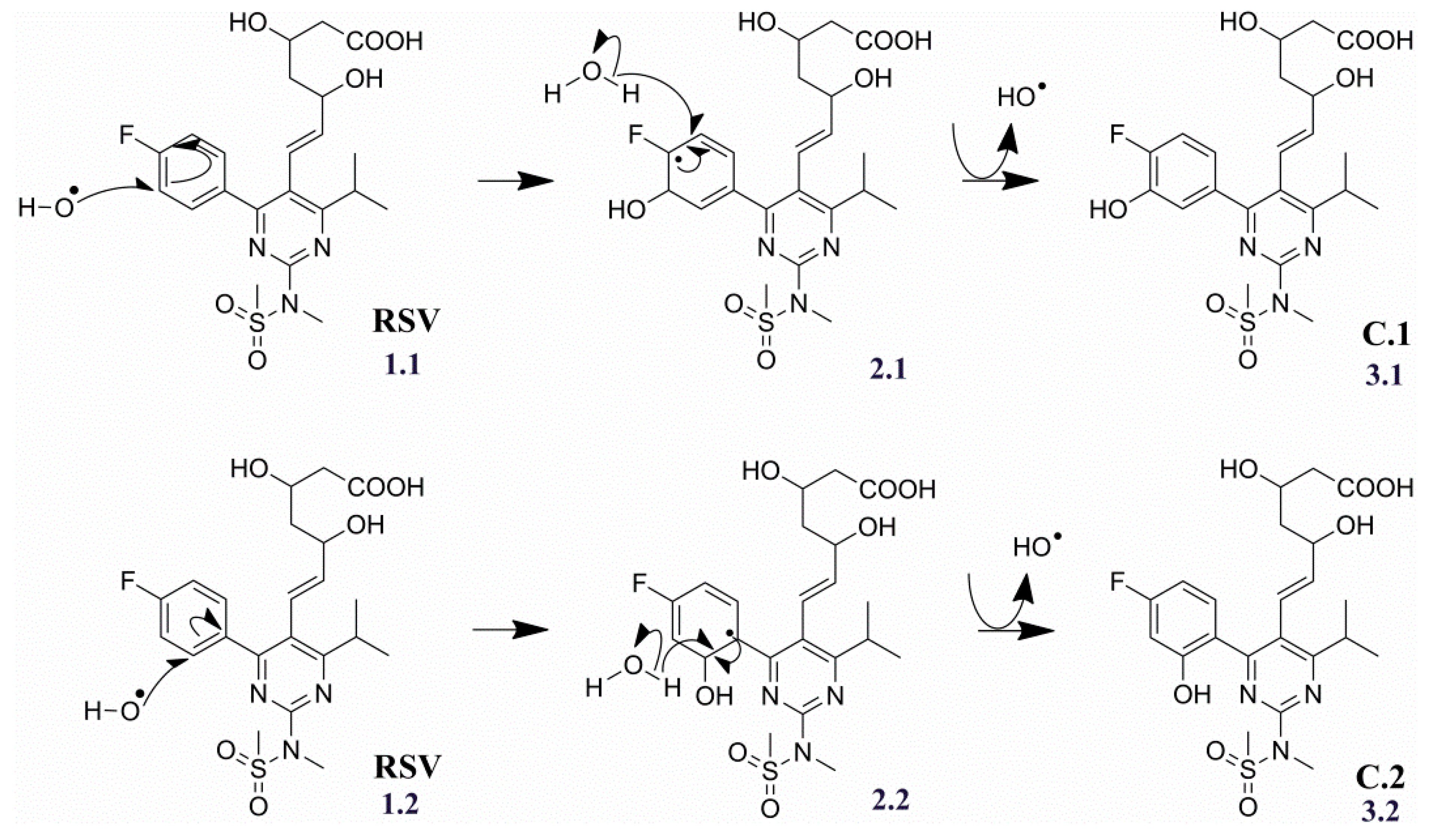
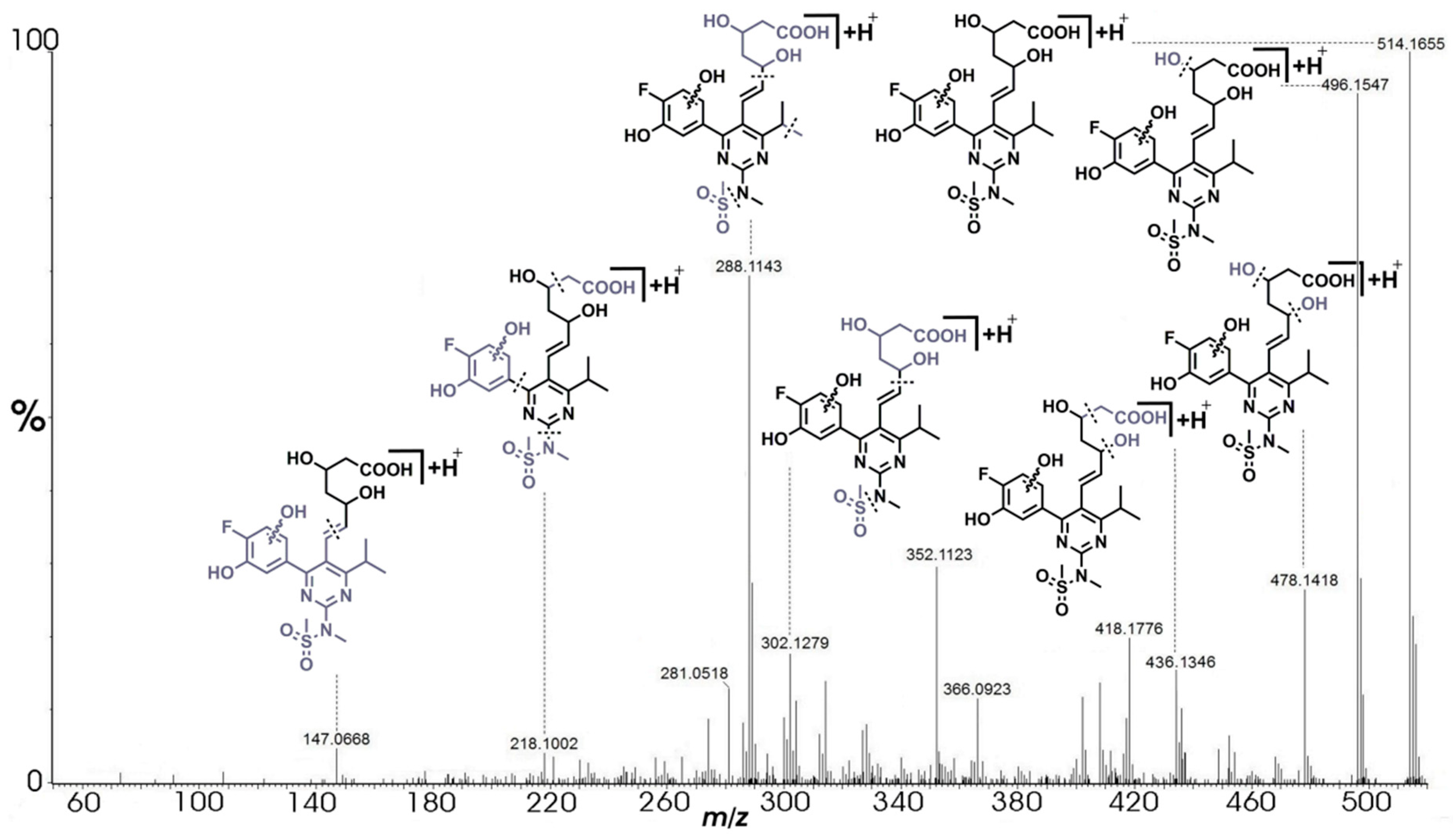

| Products | Molecular Formulae | Experimental Mass (Da) | Theoretical Mass (Da) | Mass Error (ppm) | RDB | Major Fragments (Chemical Formula) |
|---|---|---|---|---|---|---|
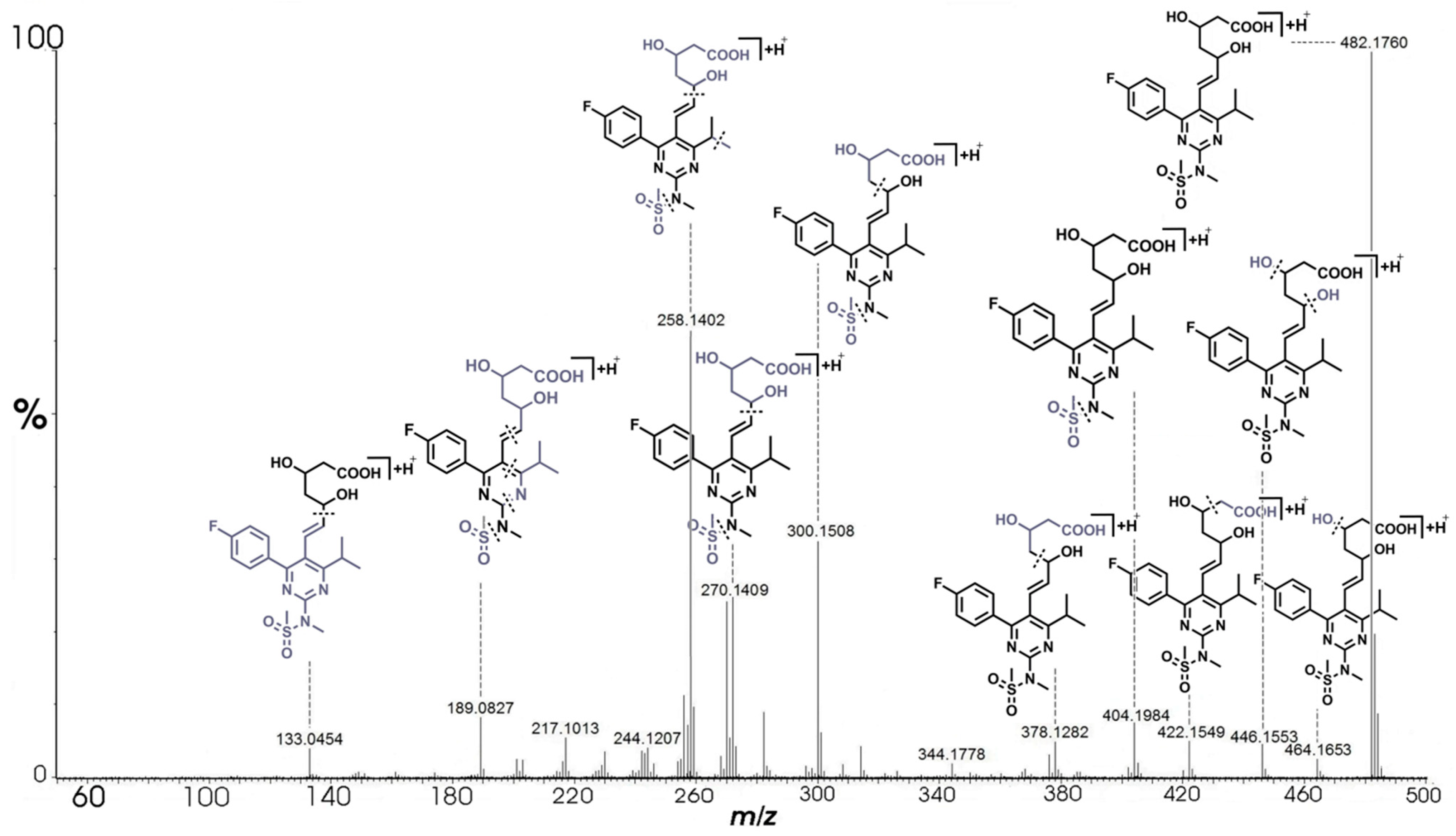
| RSV | C22H29N3O6FS+ | 482.1760 | 482.1761 | −0.21 | 9.5 | 464.1653 (C22H27N3O5FS+), 446.1553 (C22H25N3O4FS+), 422.1549 (C20H25N3O4FS+), 404.1984 (C21H27N3O4F+), 378.1282 (C18H21N3O3FS+), 376.1484 (C19H23N3O2FS+), 314.1665 (C18H21N3OF+) 300.1508 (C17H19N3OF+), 282.1403 (C17H17N3F+), 272.1559 (C16H19N3F+), 270.1409 (C16H17N3F+), 258.1402 (C15H17N3F+), 256.1242 (C15H15N3F+), 230.1093 (C13H13N3F+), 189.0827 (C11H10N2F+), 133.0454 (C5H9O4+) |
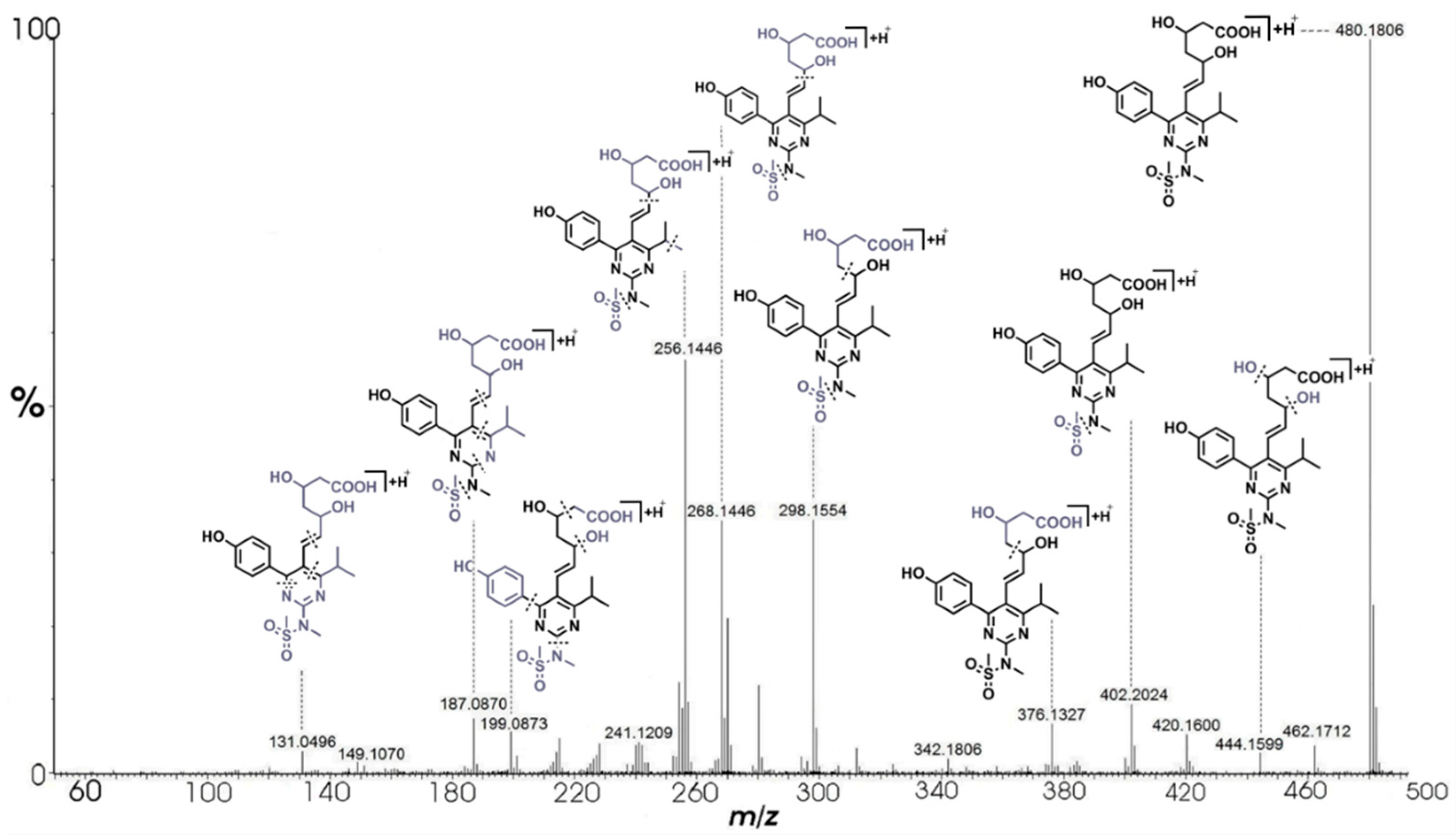
| A | C22H30N3O7S+ | 480.1806 | 480.1805 | 0.21 | 9.5 | 462.1712 (C22H28N3O6S+), 444.1599 (C22H26N3O5S+), 420.1600 (C20H26N3O5S+), 402.2024 (C21H28N3O5+), 376.1327 (C18H22N3O4S+), 312.1708 (C18H22N3O2+), 298.1554 (C17H20N3O2+), 280.1446 (C17H18N3O+), 270.1600 (C16H20N3O+), 268.1446 (C16H18N3O+), 256.1446 (C15H18N3O+), 254.1293 (C15H16N3O+), 199.0873 (C12H11N2O+), 187.0870 (C11H11N2O+), 131.0496 (C9H7O+) |
| B.1/B.2 | C22H31N3O7FS+ | 500.1865 | 500.1867 | −0.40 | 8.5 | 482.1767 (C22H29N3O6FS+), 464.1646 (C22H27N3O5FS+), 446.1538 (C22H25N3O4FS+), 422.1540 (C20H25N3O4FS+), 404.1974 (C21H27N3O4F+), 378.1268 (C18H21N3O3FS+), 376.1470 (C19H23N3O2FS+), 350.1330 (C17H21N3O2FS+), 300.1502 (C17H19N3OF+), 288.1495 (C16H19N3OF+), 281.0492 (C11H11N3O4S+), 270.1400 (C16H17N3F+), 258.1398 (C15H17N3F+), 256.1243 (C15H15N3F+), 228.0807 (C9H14N3O2S+), 201.0836 (C12H10N2F+), 189.0823 (C11H10N2F+), 175.0736 * (C9H9N3O+), 147.0663 * (C6H11O4+), 133.0459 (C5H9O4+) |
| C.1/C.2 | C22H29N3O7FS+ | 498.1709 | 498.1710 | −0.20 | 9.5 | 480.1595 (C22H27N3O6FS+), 462.1502 (C22H25N3O5FS+), 438.1485 (C20H25N3O5FS+), 420.1923 (C21H27N3O5F+), 394.1230 (C18H21N3O4FS+), 342.1599 (C19H21N3O2F+), 317.1476 (C17H20N3O2F+), 316.1459 (C17H19N3O2F+), 298.1350 (C17H17N3OF+), 296.1195 (C17H15N3OF+), 288.1506 (C16H19N3OF+), 287.1384 (C16H18N3OF+), 286.1352 (C16H17N3OF+), 274.1350 (C15H17N3OF+), 273.1260 (C15H16N3OF+), 272.1192 (C15H15N3OF+), 258.1397 (C15H17N3F+), 246.1033 (C13H13N3OF+), 217.0767 (C12H10N2OF+), 189.0838 (C10H11N3O+) |
| D.1/D.2 | C22H29N3O8FS+ | 514.1655 | 514.1659 | −0.78 | 9.5 | 496.1547 (C22H27N3O7FS+), 478.1418 (C22H25N3O6FS+), 452.1666 (C21H27N3O5FS+), 436.1880 (C21H27N3O6F+), 436.1346 (C20H23N3O5FS+), 418.1776 (C21H25N3O5F+), 408.1402 (C19H23N3O4FS+), 402.1820 (C21H25N3O4F+), 400.1643 (C21H23N3O4F+), 366.0923 (C16H17N3O4FS+), 352.1123 (C16H19N3O3FS+), 328.1451 (C18H19N3O2F+), 314.1311 (C17H17N3O2F+), 302.1279 (C16H17N3O2F+), 289.1178 (C15H16N3O2F+), 288.1143 (C15H15N3O2F+), 281.0518 (C11H11N3O4S+), 274.1363 (C15H17N3OF+), 265.0165 (C10H7N3O4S+), 218.1002 (C12H14N2O2+), 147.0668 (C6H11O4+) |
| E | C22H27N3O6FS+ | 480.1600 | 480.1605 | −1.04 | 10.5 | 462.1492 (C22H25N3O5FS+), 436.1700 (C21H27N3O4FS+), 420.1756 (C21H27N3O3FS+), 392.1440 (C19H23N3O3FS+), 378.1284 (C18H21N3O3FS+), 358.1205 (C18H17N3O4F+), 340.1087 (C18H15N3O3F+), 314.1665 (C18H21N3OF +), 300.1505 (C17H19N3OF+), 298.1350 (C17H17N3OF+), 282.1402 (C17H17N3F+), 270.1403 (C16H17N3F+), 270.1042 (C15H13N3OF+), 256.1244 (C15H15N3F+), 201.0826 (C12H10N2F+), 189.0830 (C11H10N2F+), 189.0830 (C12H13O2+), 177.1034 (C10H13N2O+), 149.0713 (C10H10F+) |
| F | C21H27N3O4FS+ | 436.1711 | 436.1707 | 0.92 | 9.5 | 378.1288 (C18H21N3O3FS+), 300.1506 (C17H19N3OF+), 270.1403 (C16H17N3F+), 257.1295 (C15H16N3F+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dončević, L.; Svetličić, E.; Hozić, A.; Mihaljević, B.; Jarmużek, D.; Tartaro Bujak, I.; Pluskota-Karwatka, D.; Ozdanovac, L.; Džeba, I.; Cindrić, M. NanoUPLC-QTOF-MS/MS Determination of Major Rosuvastatin Degradation Products Generated by Gamma Radiation in Aqueous Solution. Pharmaceuticals 2021, 14, 1160. https://doi.org/10.3390/ph14111160
Dončević L, Svetličić E, Hozić A, Mihaljević B, Jarmużek D, Tartaro Bujak I, Pluskota-Karwatka D, Ozdanovac L, Džeba I, Cindrić M. NanoUPLC-QTOF-MS/MS Determination of Major Rosuvastatin Degradation Products Generated by Gamma Radiation in Aqueous Solution. Pharmaceuticals. 2021; 14(11):1160. https://doi.org/10.3390/ph14111160
Chicago/Turabian StyleDončević, Lucija, Ema Svetličić, Amela Hozić, Branka Mihaljević, Dorota Jarmużek, Ivana Tartaro Bujak, Donata Pluskota-Karwatka, Luka Ozdanovac, Iva Džeba, and Mario Cindrić. 2021. "NanoUPLC-QTOF-MS/MS Determination of Major Rosuvastatin Degradation Products Generated by Gamma Radiation in Aqueous Solution" Pharmaceuticals 14, no. 11: 1160. https://doi.org/10.3390/ph14111160
APA StyleDončević, L., Svetličić, E., Hozić, A., Mihaljević, B., Jarmużek, D., Tartaro Bujak, I., Pluskota-Karwatka, D., Ozdanovac, L., Džeba, I., & Cindrić, M. (2021). NanoUPLC-QTOF-MS/MS Determination of Major Rosuvastatin Degradation Products Generated by Gamma Radiation in Aqueous Solution. Pharmaceuticals, 14(11), 1160. https://doi.org/10.3390/ph14111160






