Use of Stingless Bee Propolis and Geopropolis against Cancer—A Literature Review of Preclinical Studies
Abstract
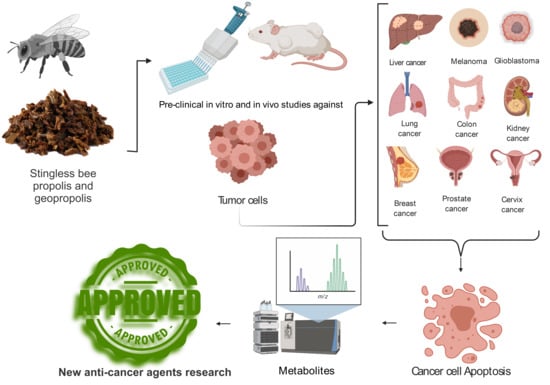
1. Introduction
2. Results and Discussion
| Bee Species | Place of Origin | Product | Type of Preparation | Tumor Cells | Result | Type of Test | Chemical Identification | Ref. |
|---|---|---|---|---|---|---|---|---|
| Melipona fasciculata (Smith 1854) | Maranhão, Brazil | Geopropolis | Hydroethanolic extract | Canine osteosarcoma (OSA) | Dose- and time-dependent cytotoxicity | In vitro | No | [23] |
| Human epidermoid laryngeal carcinoma (HEp-2) | Decrease in cell viability from 25 to 100 μg/mL | Yes a | [30] | |||||
| Human epidermoid laryngeal carcinoma (HEp-2) | Inhibition of cell proliferation and migration | No | [25] | |||||
| Lung cancer (A549 and H460) and ovarian cancer (ES2 and A2780) | Dose- and time-dependent cytotoxicity | Yes a | [8] | |||||
| Melipona scutellaris (Latreille 1811) | Bahia, Brazil | Ethanolic extract | Glioma (U251), melanoma (UACC-62), breast (MCF-7), multidrug-resistant ovarian (NCI-ADR/RES), kidney (786-0), lung (NCI-H460), prostate (PC-3), and ovary (OVCAR-03) | Anti-proliferative activity | Yes a | [24] | ||
| Melipona mondury (Smith 1863) | Bahia, Brazil | Hydroethanolic extract | B16-F10 (melanoma murine), HepG2 (human hepatocellular carcinoma), K562 (human chronic myeloid leukemia), and HL-60 (human promyelocytic leukemia) | IC50 24.2 to 46.6 μg/mL | Partially | [15] | ||
| Melipona quadrifasciata quadrifasciata (Lepeletier 1836) | Paraná, Brazil | Propolis | Ethanolic extract | MDA-MB-231 (triple-negative human breast adenocarcinoma), MCF-7 (human breast adenocarcinoma), HeLa (human cervical adenocarcinoma), HepG2 (human hepatocellular carcinoma), HRT-18 (human colorectal adenocarcinoma) | IC50 97.53 to 155.1 μg/mL | In vitro | Yes a | [17] |
| Melipona quadrifasciata anthidioides (Lepeletier 1836) | Mato Grosso do Sul, Brazil | Propolis | Ethanolic extract | Erythroleukemia cell line (K562) | Decrease in cell growth to 21.2% ± 4.1% at 500 µg/mL | In vitro | Yes a | [31] |
| Santa Catarina, Brazil | Ethanolic extract | Human melanoma (SK-MEL-28) | Decreased migration and invasion of melanoma cells | Yes a | [32] | |||
| Melipona orbignyi (Guérin-Méneville 1844) | Mato Grosso do Sul, Brazil | Ethanolic extract | Erythroleukemia cell line (K562) | Decrease in cell viability to less than 25% at 500 µg/mL | Yes b | [33] | ||
| Trigona spp. | Maharashtra, India | Hydroethanolic extract | Human breast adenocarcinoma (MCF-7), human colon adenocarcinoma (HT-29), human epithelial colorectal adenocarcinoma (CaCo-2), and murine melanoma cell lines (B16F1). | Time- and dose-dependent cytotoxicity IC50 250 µg/mL | No | [26] | ||
| Indonesia | Hydroethanolic extract | Breast (MCF-7) | Decrease in cell growth to 47.71% | Partially | [34,35] | |||
| Trigona sirindhornae (Michener and Boongird 2004) | Chantaburi, Thailand | Dichloromethane extract | Primary lesions of the pharynx (HN30) and lymph node metastases (HN31) | Dose-dependent cytotoxicity | No | [36] | ||
| Tetragonula pagdeni (Schwarz 1939) | Chanthaburi, Thailand | Propolis | Methanolic extract | Squamous cell carcinoma of the mouth (KB), hepatocellular carcinoma (HepG2), colon adenocarcinoma (CaCo-2), and melanoma (SK-MEL-28) | Cytotoxicity IC50 33.38 to 80.81 μg/mL | In vitro | Yes b | [27] |
| Tetragonula testaceitarsis (Cameron 1901) | Kalimantan, Indonesia | Ethanolic extract | Human breast cancer (MCF-7), human cervical adenocarcinoma (HeLa), and human colon cancer (CaCo-2) | Moderate decrease in cell viability to 75 μg/mL | No | [37] | ||
| Tetragonula sarawakensis (Schwarz 1939) | No | |||||||
| Tetragonula fuscobalteata (Cameron 1908) | No | |||||||
| Tetragonula laeviceps (Smith 1857) | No | |||||||
| Tetragonisca fiebrigi (Schwarz 1938) | Mato Grosso do Sul, Brazil | Propolis | Ethanolic extract | Erythroleukemia cell line (K562) | Dose-dependent cytotoxicity | Yes a | [20] | |
| Trigona incisa (Sakagami and Inoue 1989) | Kalimantan, Indonesia | Propolis | Methanolic extract | Colon (SW620), liver (HepG2), stomach (KATO-III), lung (ChaGo-1), and breast (BT-474) | Anti-proliferative activity | In vitro | Yes b | [38,39,40] |
| Trigona apicalis (Smith 1857) | No | [38] | ||||||
| Trigona fuscobalteata (Cameron 1908) | No | |||||||
| Trigona fuscibisca (Friese 1900) | ||||||||
| Heterotrigona itama (Cockerell 1918) | Ethanolic extract | Human breast cancer (MCF-7), human cervical adenocarcinoma (HeLa), and human colon cancer (CaCo-2) | Moderate decrease in cell viability to 75 μg/mL | In vitro | No | [37] | ||
| Heterotrigona bakeri (Cockerell 1919) | No | |||||||
| Homotrigona fimbriata (Smith 1857) | Yes b | |||||||
| Lepidotrigona terminata (Smith 1878) | Chanthaburi, Thailand | Propolis | Methanolic extract | Squamous cell carcinoma of the mouth (KB), hepatocellular carcinoma (HepG2), colon adenocarcinoma (CaCo-2), and melanoma (SK-MEL-28) | Cytotoxicity IC50 74.30 to 264.78 μg/mL | In vitro | No | [27] |
| Trigona laeviceps (Smith 1857) | Samut Songkram, Thailand | Aqueous extract | Colon (SW620) | Decrease of cell viability to 23% | No | [41] | ||
| Ethanolic extract | Colon (SW620), breast (BT-474), liver (HepG2), lung (ChaGo), and stomach (KATO-III) | Anti-proliferative activity IC50 19.9 to 36.19 μg/mL | No | [42] | ||||
| Lepidotrigona ventralis (Smith 1857) | Chanthaburi, Thailand | Methanolic extract | Squamous cell carcinoma of the mouth (KB), hepatocellular carcinoma (HepG2), colon adenocarcinoma (CaCo-2), and melanoma (SK-MEL-28). | Cytotoxicity IC50 96.58 to 565.19 μg/mL | No | [27] | ||
| Geniotrigona thoracica (Smith 1857) | Perak, Malaysia | Ethanolic extract | Human breast adenocarcinoma (MCF-7) | Growth inhibition IC50 38.9 μg/mL | No | [43] | ||
| Plebeia remota (Holmberg 1903) | Paraná, Brazil | Ethanolic extract | MDA-MB-231 (triple-negative human breast adenocarcinoma), MCF-7 (human breast adenocarcinoma), HeLa (human cervical adenocarcinoma), HepG2 (human hepatocellular carcinoma), and HRT-18 (human colorectal adenocarcinoma) | IC50 41.76 to 76.1 μg/mL | Yes a | [17] | ||
| Tetragonula biroi (Friese 1898) | Lagunas, Philippines | Ethanolic extract | Gastric cancer cell lines (AGS, MKN-45, NUGC-4, and MKN-74) | Regression of macroscopic and histological lesions | In vitro and in vivo | Yes a | [29] | |
| Scaptotrigona aff. postica (Latreille 1807) | Maranhão, Brazil | Propolis | Hydroethanolic extract | Ehrlich solid tumor | Inhibition of tumor progression | In vivo | Partially a | [28] |
| Scaptotrigona bipunctata (Lepeletier 1836) | Paraná, Brazil | Ethanolic extract | MDA-MB-231 (triple-negative human breast adenocarcinoma), MCF-7 (human breast adenocarcinoma), HeLa (human cervical adenocarcinoma), HepG2 (human hepatocellular carcinoma), and HRT-18 (human colorectal adenocarcinoma) | Cytotoxicity IC50 54.89 to 112.23 μg/mL | In vitro | Yes a | [17] | |
| Scaptotrigona bipunctata (Lepeletier 1836) | Santa Catarina, Brazil | Ethanolic extract | Human melanoma (SK-MEL-28) | Decreased migration and invasion of melanoma cells | Yes a | [32] | ||
| Scaptotrigona depilis (Moure 1942) | Mato Grosso do Sul, Brazil | Ethanolic extract | Human erythroleukemia cell line (K562) | Decrease in cell growth to 32.6 ± 3.2% at 500 μg/mL | Yes a | [31] | ||
| Scaptotrigona sp. | Maranhão, Brazil | Ethanolic extract | Human glioblastoma (U251 and U343) | Anti-proliferative activity | No | [22] | ||
| Tetrigona apicalis (Smith 1857) | Perak, Malaysia | Ethanolic extract | Human breast adenocarcinoma (MCF-7) | Proliferation inhibition IC50 32.70 μg/mL | Yes a | [44] |
2.1. Cytotoxicity Tests
2.1.1. Ehrlich Tumor
2.1.2. Glioblastoma
2.1.3. Erythroleukemia
2.1.4. Melanoma
2.1.5. Osteosarcoma Cells
2.1.6. Laryngeal Carcinoma
2.1.7. Ovarian Adenocarcinoma
2.1.8. Colorectal Adenocarcinoma
2.1.9. Carcinoma of the Pharynx
2.1.10. Gastric Adenocarcinoma
2.2. Chemical Identification of Antitumor Extracts from Propolis and Geopropolis
| Bee Species | Place of Origin | Product | Class of Compounds | Chemical Compounds | Method | Ref. |
|---|---|---|---|---|---|---|
| Scaptotrigona bipunctata (Latreille 1807) | Paraná/Santa Catarina, Brazil | Propolis | Alkaloids | Lelobanonoline, 2-[6-(2-hydroxy-propyl)-1-methyl-[2]piperidyl]-1-phenylethanone, norlobelanidine, norlobeline, lobeline, and lobelanidine | HPLC/MS | [17,32] |
| Terpenes | α-Amyrin/β-amyrin and 4R,5R,9R,10R-13-hydroxypodocarp-8(14)-en-19-oic acid | |||||
| Phenolic compounds (phenolic acids, flavonoids, coumarin, stilbenes, phenylpropanoids, and tannins) | Vicenin, liquiritigenin, formononetin, drupanin, p-coumaric acid, acid ferulic, biochanin A, kaempferol methyl ether, dihydrokaempferide, retusin 8-methyl ether, betuletol, artepillin C, 4-hydroxy-3(E)-(4-hydroxy-3- methyl-2-butenyl)-5-prenylcinnamic acid, 3-hydroxy-2,2-dimethyl-8-prenyl-2H-1-benzopyran-6-propenoic acid, artepillin C derivative, anacardic acid, dicaffeoylquinic, and (E)-3-{4-hydroxy-3-[(E)-4-(2,3-dihydrocinnamoyloxy)-3-methyl-2-butenyl]-5-prenylphenyl}-2-propenoic acid | |||||
| Fatty acids | Palmitic acid, oleic acid, stearic acid, and eicosapentaenoic acid | |||||
| Melipona fasciculata (Smith 1854) | Maranhão, Brazil | Geopropolis | Phenolic compounds | Anacardic acid, heptedecenyl salicylic acid, nonadecenyl salicylic acid, pentadecenyl salicylic acid, heptadecadecylresorcinol, nonadecadecylresorcinol, pentadecadecadienylresorcinol, heptedecadienylresorcinol, taxifolin 7-O-rhamnoside, isoschaftoside, typhaneoside, dihydroquercetin-C-glycoside, narigenin-C-glycoside, vitexin-O-gallate, glycosylated pinobanksin, dihydroquercetin-3-O-rhamnoside, and gallocatechin-xylose | HPLC/MS | [8,30] |
| Terpenes | Lupeol, α-amyrin, β-amyrin, α-amyrenone, β-amyrenone, triterpene ketone, taraxerone, dipterocarpol, marsformosanone, and 3-[Xyl]-28-Glc-phytophthalacagenin | |||||
| Anthraquinone | Xantholaccaic acid A | |||||
| Organic acids | Glycuronic acid, methylmalonic acid, and gluconic acid | |||||
| Melipona scutellaris (Latreille 1811) | Bahia, Brazil | Geopropolis | Benzofenones | Propensaeure 3-phenyl-trimethylsilylester and 1,2-benzenedicarboxylic acid | GC/MS | [24] |
| Melipona quadrifasciata quadrifasciata (Lepetetier 1836)) | Paraná, Brazil | Propolis | Phenolic compounds | p-Coumaric acid, ferulic acid, ellagic acid, gallic acid, naringenin, aromadendrin, isosakuranetin, dihydrokaempferide, aromadendrin methyl ether, cinnamoyl-galloyl-hexoside, anacardic acid, cinnamoyl-coumaroyl-hexoside, dicoumaroyl-hexoside, digalloyl-cinnamoyl-hexoside, digalloyl-coumaroyl-hexoside, cinnamoyl-coumaroyl-galloyl hexoside, and dicoumaroyl-galloyl-hexoside | HPLC/MS | [17] |
| Terpenes | Sugiol, pimaric acid, isocupressic acid, cupressic acid, junicedric acid, mangiferonic acid, and isomangiferolic acid | |||||
| Melipona quadrifasciata anthidioides (Lepeletier 1836) | Mato Grosso do Sul, Brazil | Propolis | Phenolic compounds | p-Coumaric acid, vanilic acid, caffeic acid, vanillin, ferulic acid, benzoic acid, quercetin, luteolin, cinnamic acid, and apigenin | HPLC/MS; GC/MS | [31] |
| Terpenes | Stigmasterol, β-sitosterol, β-amyrin, taraxasterol, α-amyrin, β-amyrin acetate, and pinusenocarp | |||||
| Melipona quadrifasciata anthidioides (Lepeletier 1836) | Santa Catarina, Brazil | Propolis | Phenolic compounds | 7-O-methyl aromadendrin, 5-hydroxy-4′,7-dimethoxy flavone, 2′-hydroxynaringenin, narigenin, and p-coumaric | HPLC/MS | [32] |
| Phenylpropanoids | 4-O-(6″-O-p-coumaroyl-β-D-glucopyranosyl)- and 6-O-cinnamoyl-1-O-p-coumaroyl-β-D-glucopyranoside | |||||
| Terpenes | Abieta-8,11,13,15-tetraen-18-oic acid, abietic acid, 7-hydroxydehydroabietic acid, and inumakiol | |||||
| Melipona orbignyi (Guérin-Méneville 1844) | Mato Grosso do Sul, Brazil | Propolis | Phenolic compounds | Dihydrocinnamic acids, cinnamic acids, benzoic acids, coumarin C-prenylated acids, and long-chain caffeates | GC/MS | [33] |
| Melipona mondury (Smith 1863) | Bahia, Brazil | Geopropolis | Phenolic compounds | Gallic acid | HPLC/MS | [15] |
| Terpenes | Not specified | |||||
| Trigona spp. | Maharashtra, India | Propolis | Unidentified | Unidentified | - | [26] |
| Indonesia | Propolis | Alkaloids, flavonoids, saponins, tannins, steroids, and triterpenes | Unidentified | Chemical approach | [34] | |
| Scaptotrigonaaff. postica (Latreille 1807) | Maranhão, Brazil | Propolis | Terpenes and coumarins | Unidentified | Phytochemical approach | [28] |
| Scaptotrigona depilis (Moure 1942) | Mato Grosso do Sul, Brazil | Propolis | Terpenes | β-Sitosterol, β-amyrin, α-amyrin, and β-amyrin acetate | GC/MS; HPLC/MS | [31] |
| Phenolic compounds | Vanillin, p-coumaric acid, ferulic acid, benzoic acid, and cinnamic acid | |||||
| Tetragonula biroi (Friese 1898) | Lagunas, Philippines | Propolis | Carbohydrates, steroids, alkaloids, anthraquinones, and phenols | Unidentified | Phytochemical approach | [29] |
| Tetragonisca fiebrigi (Schwartz 1938) | Mato Grosso do Sul, Brazil | Propolis | Phenolic acids | Benzoic acid, cinnamic acid, p-coumaric acid, 3-phenyl-p-coumaric acid, and benzyl caffeate | GC/MS | [20] |
| Phenylpropanoids | Cinnamyl caffeate, hydrocinnamic acid, and hydrocinnamic acid ethyl ester | |||||
| Terpene | Kaurenoic acid | |||||
| Sugars | Fructose and glucose | |||||
| Lipids | Tocopherol, cholesterol, and retinol | |||||
| Tetrigona apicalis (Smith 1857) | Perak, Malaysia | Propolis | Hydrocarbon | Undecane | GC/MS | [44] |
| Phenolic compound | Myristicin | |||||
| Terpenes | β-Elemene, α-cubebene, copaene, cyperene, α-gurjunene, caryophyllene, α-caryophyllene, γ -cadinene, germacrene D, bicyclogermacrene, δ-amorphene, β-selinene, aromadendr-1-ene, spathulenol, caryophyllene oxide, 1, 2-dimethyl-3, 5-bis(1-methylethenyl)-, humulene epoxide II, α-cadinol, aristolene epoxide, taraxerone, β-amyrin, and α-amyrin | |||||
| Plebeia remota (Holmberg 1903) | Paraná, Brazil | Propolis | Fatty acid | Arachidonic acid | HPLC/MS | [17] |
| Terpenes | Sugiol, totarol, communic acid, agathic acid, isocupressic acid, cupressic acid, dihydroagathic acid, and 15-acetoxy-cupressic acid |
2.3. Isolation of Compounds


3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Camargo, R.C.R.; de Oliveira, K.L.; Berto, M.I. Mel de abelhas sem ferrão: Proposta de regulamentação. Brazilian J. Food Technol. 2017, 20, 1–7. [Google Scholar] [CrossRef]
- Villas-Bôas, J. Manual Tecnológico: Mel de abelhas sem ferrão; Instituto Sociedade, População e Natureza (ISPN): Brasília, Brasil, 2012; ISBN 9788563288080. [Google Scholar]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.D.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; da Rocha, C.Q.; Lima, S.T.D.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Garcia, J.B.S.; Pinheiro, M.S.D.; Pereira, F.A.N.; Camelo, D.D.S.; de Morais, S.V.; Freitas, J.R.B.; da Rocha, C.Q.; Ribeiro, M.N.D.S.; et al. Anti-Inflammatory and Antioxidant Activity of Pollen Extract Collected by Scaptotrigona affinis postica: In silico, in vitro, and in vivo Studies. Antioxidants 2020, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Hrncir, M.; Jarau, S.; Barth, F.G. Stingless bees (Meliponini): Senses and behavior. J. Comp. Physiol. A 2016, 202, 597–601. [Google Scholar] [CrossRef]
- Lavinas, F.C.; Macedo, E.H.B.C.; Sá, G.B.L.; Amaral, A.C.F.; Silva, J.R.A.; Azevedo, M.M.B.; Vieira, B.A.; Domingos, T.F.S.; Vermelho, A.B.; Carneiro, C.S.; et al. Brazilian stingless bee propolis and geopropolis: Promising sources of biologically active compounds. Rev. Bras. Farmacogn. 2019, 29, 389–399. [Google Scholar] [CrossRef]
- de Carvalho, R.M.; Martins, C.; da Mourão, J. Meliponiculture in Quilombola communities of Ipiranga and Gurugi, Paraíba state, Brazil: An ethnoecological approach. J. Ethnobiol. Ethnomed. 2014, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Barboza, J.R.; Pereira, F.A.N.; Fernandes, R.A.; Vasconcelos, C.C.; Cartágenes, M.D.S.D.S.; Oliveira Lopes, A.J.; de Melo, A.C.; Guimarães, I.D.S.; da Rocha, C.Q.; Ribeiro, M.N.D.S. Cytotoxicity and Pro-Apoptotic, Antioxidant and Anti-Inflammatory Activities of Geopropolis Produced by the Stingless Bee Melipona fasciculata Smith. Biology 2020, 9, 292. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Shehu, A.; Ismail, S.; Rohin, M.; Harun, A.; Aziz, A.; Haque, M. Antifungal Properties of Malaysian Tualang Honey and Stingless Bee Propolis against Candida Albicans and Cryptococcus Neoformans. J. Appl. Pharm. Sci. 2016, 044–050. [Google Scholar] [CrossRef]
- Villas-Bôas, J. Manual Tecnológico de Aproveitamento Integral dos Produtos das Abelhas Nativas Sem Ferrão, 2nd ed.; Instituto Sociedade, População e Natureza (ISPN): Asa Norte, Brasília, 2018. [Google Scholar]
- de Souza, S.A.; da Silva, T.M.G.; da Silva, E.M.S.; Camara, C.A.; Silva, T.M.S. Characterisation of phenolic compounds by UPLC-QTOF-MS/MS of geopropolis from the stingless bee Melipona subnitida (jandaíra). Phytochem. Anal. 2018, 29, 549–558. [Google Scholar] [CrossRef]
- Dutra, R.P.; Abreu, B.V.D.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef]
- Batista, M.C.A.; Abreu, B.V.B.; Dutra, R.P.; Cunha, M.S.; Amaral, F.M.M.; Torres, L.M.B.; Ribeiro, M.N.S. Chemical composition and antioxidant activity of geopropolis produced by Melipona fasciculata (Meliponinae) in flooded fields and cerrado areas of Maranhão State, northeastern Brazil. Acta Amaz. 2016, 46, 315–322. [Google Scholar] [CrossRef]
- Dos Santos, T.L.A.; Queiroz, R.F.; Sawaya, A.C.H.F.; Lopez, B.G.-C.; Soares, M.B.P.; Bezerra, D.P.; Rodrigues, A.C.B.C.; de Paula, V.F.; Waldschmidt, A.M. Melipona mondury produces a geopropolis with antioxidant, antibacterial and antiproliferative activities. An. Acad. Bras. Cienc. 2017, 89, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.; Fernandes-Silva, C.C.; Salatino, A.; Message, D.; Negri, G. Antioxidant Activity of a Geopropolis from Northeast Brazil: Chemical Characterization and Likely Botanical Origin. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Surek, M.; Fachi, M.M.; de Fátima Cobre, A.; de Oliveira, F.F.; Pontarolo, R.; Crisma, A.R.; de Souza, W.M.; Felipe, K.B. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 2021, 269, 113662. [Google Scholar] [CrossRef]
- Dutra, R.P.; Bezerra, J.L.; da Silva, M.C.P.; Batista, M.C.A.; Patrício, F.J.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farmacogn. 2019, 29, 287–293. [Google Scholar] [CrossRef]
- Yosri, N.; Abd El-Wahed, A.A.; Ghonaim, R.; Khattab, O.M.; Sabry, A.; Ibrahim, M.A.A.; Moustafa, M.F.; Guo, Z.; Zou, X.; Algethami, A.F.M.; et al. Anti-Viral and Immunomodulatory Properties of Propolis: Chemical Diversity, Pharmacological Properties, Preclinical and Clinical Applications, and In Silico Potential against SARS-CoV-2. Foods 2021, 10, 1776. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; dos Santos, U.P.; da Rocha, P.D.S.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; dos Santos, E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca fiebrigi (Jataí). Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- de Sousa-Fontoura, D.M.N.; Olinda, R.G.; Viana, G.A.; de FM Costa, K.M.; Batista, J.S.; Serrano, R.M.O.T.; Silva, O.M.D.; Camara, C.A.; Silva, T.M.S. Wound healing activity and chemical composition of geopropolis from Melipona subnitida. Rev. Bras. Farmacogn. 2020, 30, 367–373. [Google Scholar] [CrossRef]
- Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Soares, A.E.E.; Tone, L.G. Antiproliferative effects of Tubi-bee propolis in glioblastoma cell lines. Genet. Mol. Biol. 2011, 34, 310–314. [Google Scholar] [CrossRef]
- Cinegaglia, N.C.; Bersano, P.R.O.; Araújo, M.J.A.M.; Búfalo, M.C.; Sforcin, J.M. Anticancer Effects of Geopropolis Produced by Stingless Bees on Canine Osteosarcoma Cells In Vitro. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- da Cunha, M.G.; Franchin, M.; Galvão, L.; de Ruiz, A.; de Carvalho, J.E.; Ikegaki, M.; de Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial and antiproliferative activities of stingless bee Melipona scutellaris geopropolis. BMC Complement. Altern. Med. 2013, 13, 23. [Google Scholar] [CrossRef]
- Bartolomeu, A.R.; Frión-Herrera, Y.; da Silva, L.M.; Romagnoli, G.G.; de Oliveira, D.E.; Sforcin, J.M. Combinatorial effects of geopropolis produced by Melipona fasciculata Smith with anticancer drugs against human laryngeal epidermoid carcinoma (HEp-2) cells. Biomed. Pharmacother. 2016, 81, 48–55. [Google Scholar] [CrossRef]
- Choudhari, M.K.; Haghniaz, R.; Rajwade, J.M.; Paknikar, K.M. Anticancer Activity of Indian Stingless Bee Propolis: An In Vitro Study. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Vongsak, B.; Chonanant, C.; Machana, S. In Vitro Cytotoxicity of Thai Stingless Bee Propolis from Chanthaburi Orchard. Walailak J. Sci. Technol. 2016, 14, 741–747. [Google Scholar]
- Araújo, M.J.A.M.; Dutra, R.P.; Costa, G.C.; Reis, A.S.; Assunção, A.K.M.; Libério, S.A.; Maciel, M.C.G.; Silva, L.A.; Guerra, R.N.M.; Ribeiro, M.N.S.; et al. Efeito do tratamento com própolis de Scaptotrigona aff. postica sobre o desenvolvimento do tumor de Ehrlich em camundongos. Rev. Bras. Farmacogn. 2010, 20, 580–587. [Google Scholar] [CrossRef][Green Version]
- Desamero, M.J.; Kakuta, S.; Tang, Y.; Chambers, J.K.; Uchida, K.; Estacio, M.A.; Cervancia, C.; Kominami, Y.; Ushio, H.; Nakayama, J.; et al. Tumor-suppressing potential of stingless bee propolis in in vitro and in vivo models of differentiated-type gastric adenocarcinoma. Sci. Rep. 2019, 9, 19635. [Google Scholar] [CrossRef]
- Araujo, M.; Bufalo, M.; Conti, B.; Fernandes, A.R.Y., Jr.; Trusheva, B.; Bankova, V.; Sforcin, J. The chemical composition and pharmacological activities of geopropolis produced by Melipona fasciculata Smith in northeast Brazil. J. Mol. Pathophysiol. 2015, 4, 12. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; de Picoli Souza, K.; dos Santos, E.L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cisilotto, J.; Sandjo, L.P.; Faqueti, L.G.; Fernandes, H.; Joppi, D.; Biavatti, M.W.; Creczynski-Pasa, T.B. Cytotoxicity mechanisms in melanoma cells and UPLC-QTOF/MS2 chemical characterization of two Brazilian stingless bee propolis: Uncommon presence of piperidinic alkaloids. J. Pharm. Biomed. Anal. 2018, 149, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.F.; dos Santos, U.P.; Macorini, L.F.B.; de Melo, A.M.M.F.; Balestieri, J.B.P.; Paredes-Gamero, E.J.; Cardoso, C.A.L.; de Picoli Souza, K.; dos Santos, E.L. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae). Food Chem. Toxicol. 2014, 65, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Mangunwidjaja, D.; Sunarti, T.; Suparno, O.; Setiyono, A. Investigating the antioxidant and anticytotoxic activities of propolis collected from five regions of Indonesia and their abilities to induce apoptosis. Emirates J. Food Agric. 2014, 26, 390. [Google Scholar] [CrossRef]
- Amalia, E.; Diantini, A.; Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Chitkul, B.; Koontongkaew, S. Cytotoxic Activity of Propolis Extracts from the Stingless Bee Trigona Sirindhornae Against Primary and Metastatic Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2017, 18, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Sukemi; Yadi; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Puthong, S.; Arung, E.T.; Chanchao, C. In vitro cytotoxicity of Indonesian stingless bee products against human cancer cell lines. Asian Pac. J. Trop. Biomed. 2014, 4, 549–556. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Phuwapraisirisan, P.; Puthong, S.; Palaga, T.; Arung, E.T.; Chanchao, C. Propolis from the Stingless Bee Trigona incisa from East Kalimantan, Indonesia, Induces In Vitro Cytotoxicity and Apoptosis in Cancer Cell lines. Asian Pacific J. Cancer Prev. 2015, 16, 6581–6589. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Lirdprapamongkol, K.; Palaga, T.; Puthong, S.; Phuwapraisirisan, P.; Svasti, J.; Chanchao, C. Molecular mechanism of cardol, isolated from Trigona incisa stingless bee propolis, induced apoptosis in the SW620 human colorectal cancer cell line. BMC Pharmacol. Toxicol. 2017, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Umthong, S.; Puthong, S.; Chanchao, C. Trigona laeviceps Propolis from Thailand: Antimicrobial, Antiproliferative and Cytotoxic Activities. Am. J. Chin. Med. 2009, 37, 855–865. [Google Scholar] [CrossRef]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ismail, W.I.W.; Hussin, N.N.; Mazlan, S.N.F.; Hussin, N.H.; Radzi, M.N.F.M. Physicochemical Analysis, Antioxidant and Anti Proliferation Activities of Honey, Propolis and Beebread Harvested from Stingless Bee. IOP Conf. Ser. Mater. Sci. Eng. 2018, 440, 012048. [Google Scholar] [CrossRef]
- Mohamed, W.A.S.; Ismail, N.Z.; Omar, E.A.; Abdul Samad, N.; Adam, S.K.; Mohamad, S. GC-MS Evaluation, Antioxidant Content, and Cytotoxic Activity of Propolis Extract from Peninsular Malaysian Stingless Bees, Tetrigona Apicalis. Evidence-Based Complement. Altern. Med. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- da Silva, P.I.C.; Freitas, J.J.D.S.; Domingues, R.J.D.S. Manual Básico de ONCOLOGIA EXPERIMENTAL Tumor de Ehrlich; Universidade do Estado do Pará: Belém, Brazil, 2017. [Google Scholar]
- Georgiu, C.; MihuŢ, E.; Raus, I.; Mirescu, Ş.C.; Szabo, L.; Şovrea, A.S. Pediatric glioblastoma with giant cells and “supratentorial” primitive neuroectodermal component-case report and review of the literature. Rom. J. Morphol. Embryol. 2015, 56, 1165–1171. [Google Scholar] [PubMed]
- Boddu, P.; Benton, C.B.; Wang, W.; Borthakur, G.; Khoury, J.D.; Pemmaraju, N. Erythroleukemia-historical perspectives and recent advances in diagnosis and management. Blood Rev. 2018, 32, 96–105. [Google Scholar] [CrossRef]
- Pluta, R.M. Melanoma. JAMA 2011, 305, 2368. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine Osteosarcoma: A Naturally Occurring Disease to Inform Pediatric Oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Thun, M.J. Cancer Statistics, 2009. CA. Cancer J. Clin. 2009, 59, 225–249. [Google Scholar] [CrossRef]
- Santos, M.D.O. Estimativa 2018: Incidência de Câncer no Brasil. Rev. Bras. Cancerol. 2018, 64, 119–120. [Google Scholar] [CrossRef]
- Haggar, F.; Boushey, R. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [CrossRef]
- Ghoncheh, M.; Mohammadian, M.; Mohammadian-Hafshejani, A.; Salehiniya, H. The Incidence and Mortality of Colorectal Cancer and Its Relationship With the Human Development Index in Asia. Ann. Glob. Heal. 2017, 82, 726. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Gu, D.; Zeng, H. Antitumor effects of beta-amyrin in Hep-G2 liver carcinoma cells are mediated via apoptosis induction, cell cycle disruption and activation of JNK and P38 signalling pathways. J. BUON. 2018, 23, 965–970. [Google Scholar] [PubMed]
- Qi, G.; Chen, J.; Shi, C.; Wang, Y.; Mi, S.; Shao, W.; Yu, X.; Ma, Y.; Ling, J.; Huang, J. Cinnamic Acid (CINN) Induces Apoptosis and Proliferation in Human Nasopharyngeal Carcinoma Cells. Cell. Physiol. Biochem. 2016, 40, 589–596. [Google Scholar] [CrossRef]
- Ma, X.-C.; Dong, S.; Zhang, S.-Y.; Jia, N.; Ou, S.-L. Taraxerone triterpene inhibits cancer cell growth by inducing apoptosis in non-small cell lung cancer cells. Bangladesh J. Pharmacol. 2016, 11, 342. [Google Scholar] [CrossRef]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Antiproliferative effect of p-Coumaric acid targets UPR activation by downregulating Grp78 in colon cancer. Chem. Biol. Interact. 2018, 291, 16–28. [Google Scholar] [CrossRef]
- Pang, S.; Yee, M.; Saba, Y.; Chino, T. Artepillin C as a targeting survivin molecule in oral squamous cell carcinoma cells in vitro: A preliminary study. J. Oral Pathol. Med. 2018, 47, 48–52. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Ko, E.-B.; Choi, K.-C. Gallic acid, a phenolic acid, hinders the progression of prostate cancer by inhibition of histone deacetylase 1 and 2 expression. J. Nutr. Biochem. 2020, 84, 108444. [Google Scholar] [CrossRef]
- Qi, Y.; Ding, Z.; Yao, Y.; Ren, F.; Yin, M.; Yang, S.; Chen, A. Apigenin induces apoptosis and counteracts cisplatin-induced chemoresistance via Mcl-1 in ovarian cancer cells. Exp. Ther. Med. 2020, 20, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, M.; Rosalen, P.; Franchin, M.; de Alencar, S.; Ikegaki, M.; Ransom, T.; Beutler, J. Antiproliferative Constituents of Geopropolis from the Bee Melipona scutellaris. Planta Med. 2015, 82, 190–194. [Google Scholar] [CrossRef]
- Oanh, V.T.K.; Thoa, H.T.; Hang, N.T.M.; Phuong, D.T.L.; Lien, N.T.P.; Popova, M.; Trusheva, B.; Bankova, V.; Le, T.N. New dihydrochromene and xanthone derivatives from Lisotrigona furva propolis. Fitoterapia 2021, 149, 104821. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, M.T.T.; Nguyen, N.T.; Awale, S. Chemical Constituents of Propolis from Vietnamese Trigona minor and Their Antiausterity Activity against the PANC-1 Human Pancreatic Cancer Cell Line. J. Nat. Prod. 2017, 80, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Van Do, T.N.; Nguyen, M.T.T.; Dang, P.H.; Tho, L.H.; Awale, S.; Nguyen, N.T. A New alkenylphenol from the propolis of stingless bee trigona minor. Nat. Prod. Commun. 2018, 13, 69–70. [Google Scholar] [CrossRef]
- Thanh, L.N.; Thoa, H.T.; Oanh, N.T.T.; Giap, T.H.; Quyen, V.T.; Ha, N.T.T.; Phuong, D.T.L.; Lien, N.T.P.; Hang, N.T.M. Cycloartane triterpenoids and biological activities from the propolis of the stingless bee Lisotrigona furva. Vietnam J. Chem. 2021, 59, 426–430. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, F.A.N.; Barboza, J.R.; Vasconcelos, C.C.; Lopes, A.J.O.; Ribeiro, M.N.d.S. Use of Stingless Bee Propolis and Geopropolis against Cancer—A Literature Review of Preclinical Studies. Pharmaceuticals 2021, 14, 1161. https://doi.org/10.3390/ph14111161
Pereira FAN, Barboza JR, Vasconcelos CC, Lopes AJO, Ribeiro MNdS. Use of Stingless Bee Propolis and Geopropolis against Cancer—A Literature Review of Preclinical Studies. Pharmaceuticals. 2021; 14(11):1161. https://doi.org/10.3390/ph14111161
Chicago/Turabian StylePereira, Francisco Assis Nascimento, Josianne Rocha Barboza, Cleydlenne Costa Vasconcelos, Alberto Jorge Oliveira Lopes, and Maria Nilce de Sousa Ribeiro. 2021. "Use of Stingless Bee Propolis and Geopropolis against Cancer—A Literature Review of Preclinical Studies" Pharmaceuticals 14, no. 11: 1161. https://doi.org/10.3390/ph14111161
APA StylePereira, F. A. N., Barboza, J. R., Vasconcelos, C. C., Lopes, A. J. O., & Ribeiro, M. N. d. S. (2021). Use of Stingless Bee Propolis and Geopropolis against Cancer—A Literature Review of Preclinical Studies. Pharmaceuticals, 14(11), 1161. https://doi.org/10.3390/ph14111161