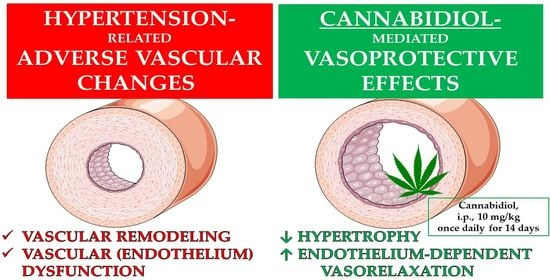
Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension
Abstract
1. Introduction
2. Results
2.1. General
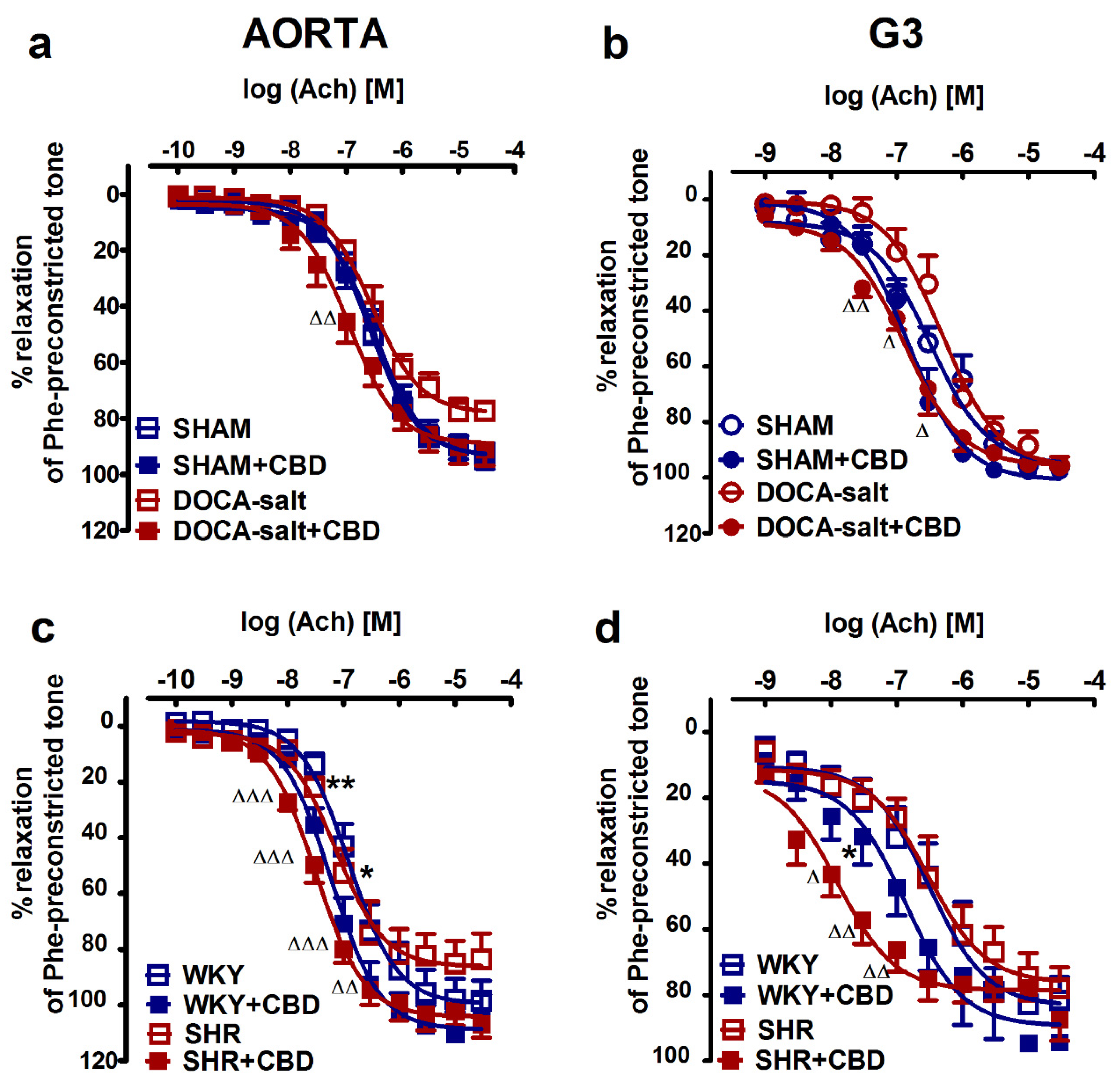
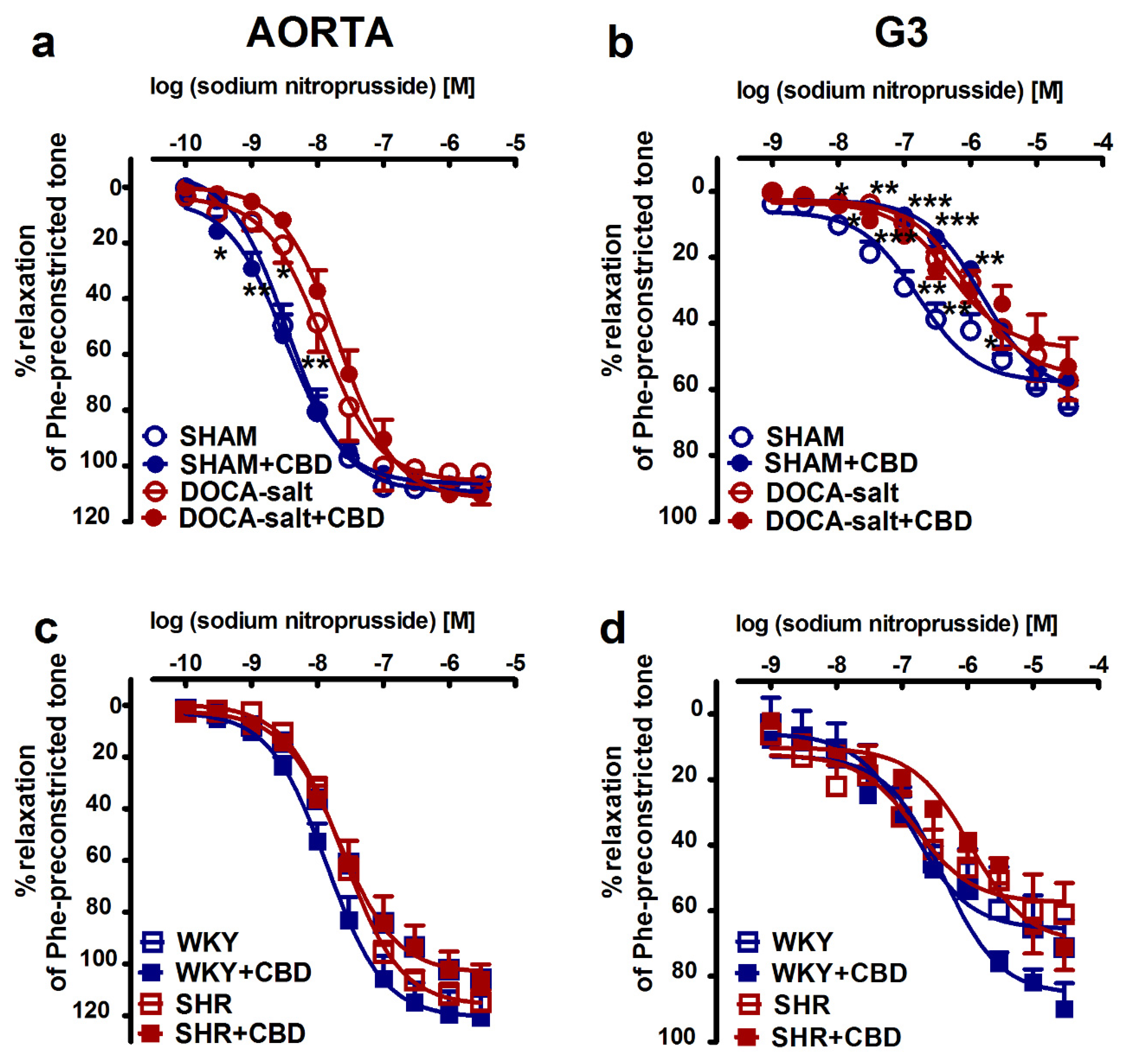
2.2. Influences of Hypertension and Chronic Administration of CBD on Vasodilatory Effects of Acetylcholine and Sodium Nitroprusside in Aortas and Mesenteric G3 Arteries
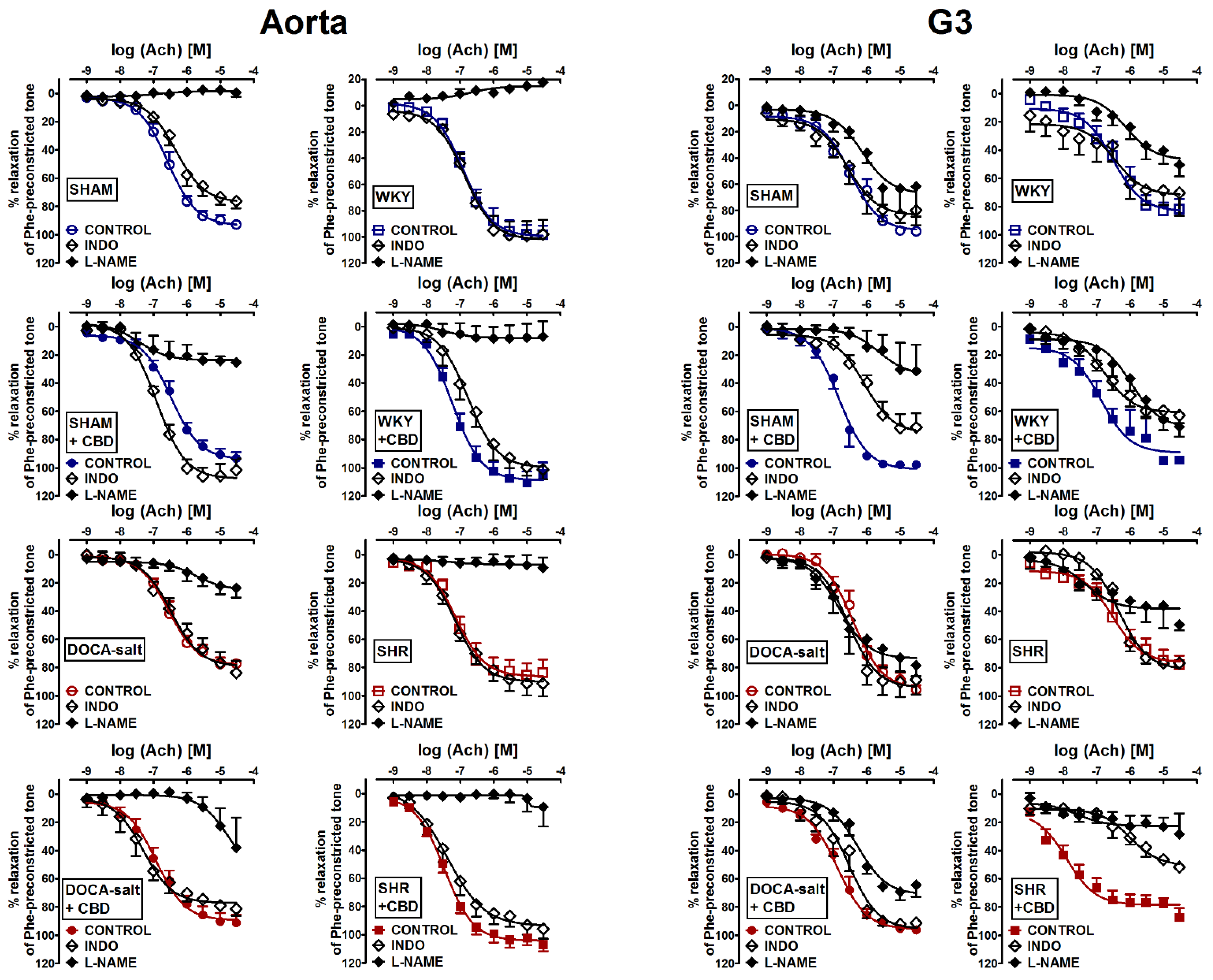
2.3. Evaluation of Endothelial Mediators Involved in Vascular Effects of Acetylcholine-Induced Relaxation in Aortas and Mesenteric G3 Arteries
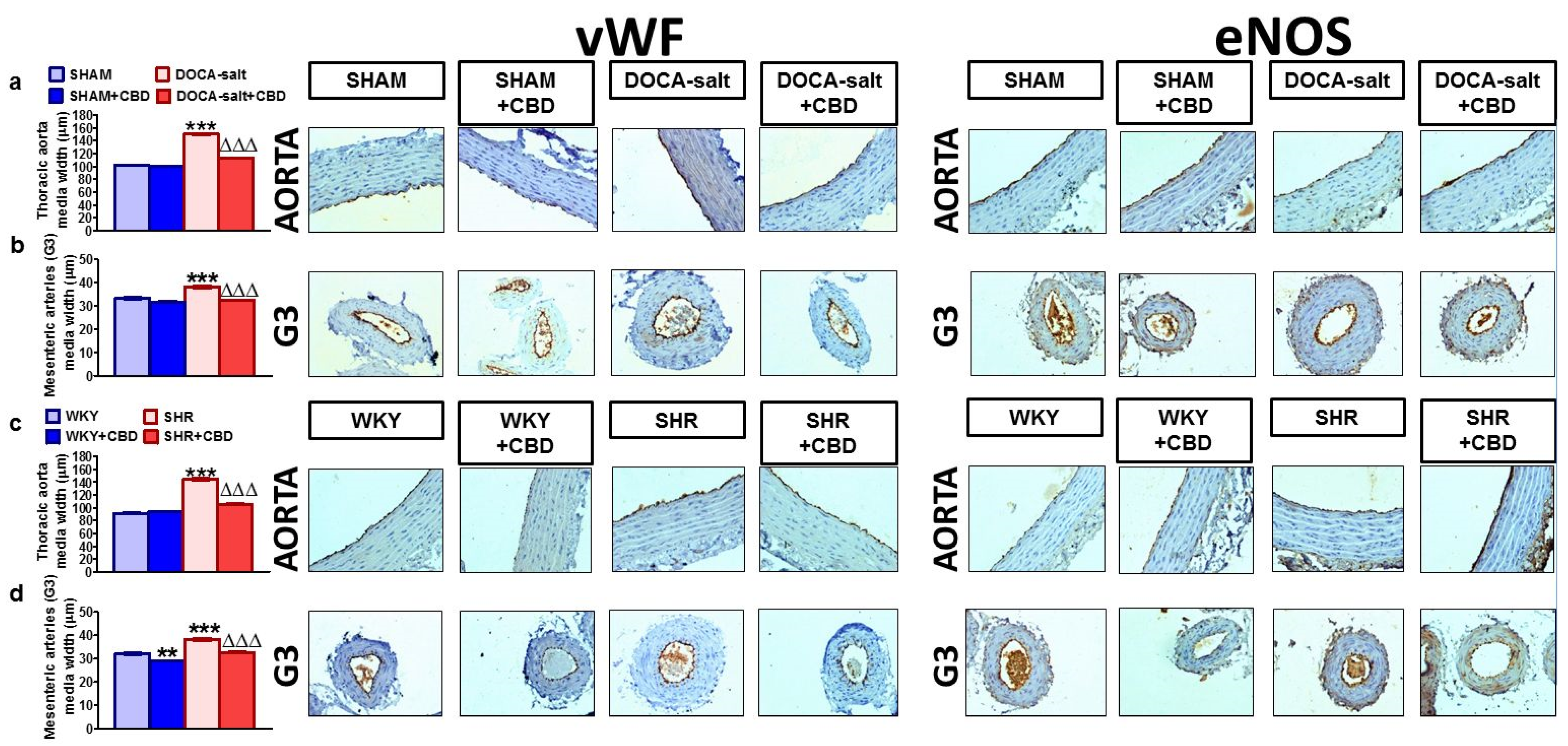
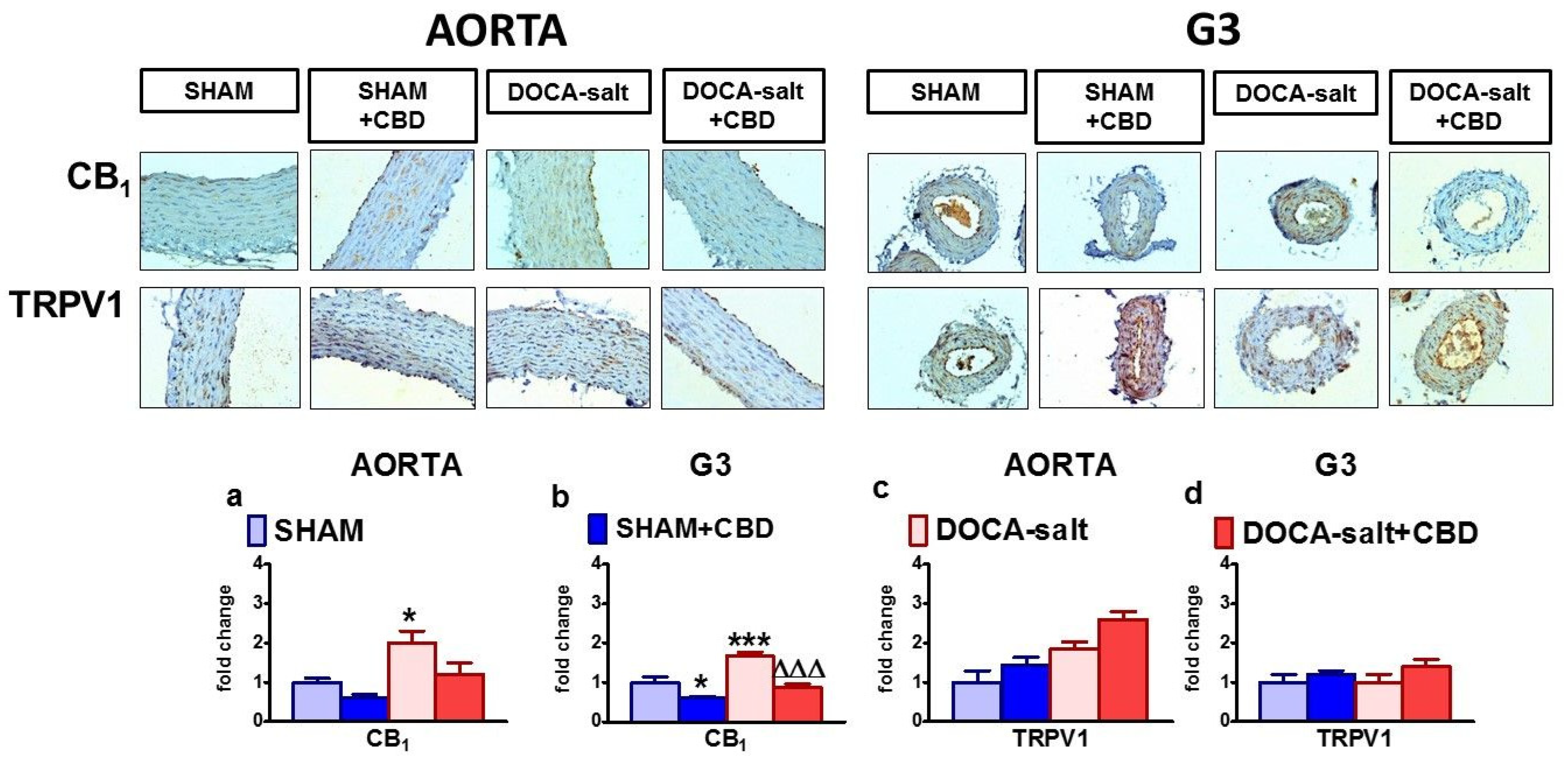
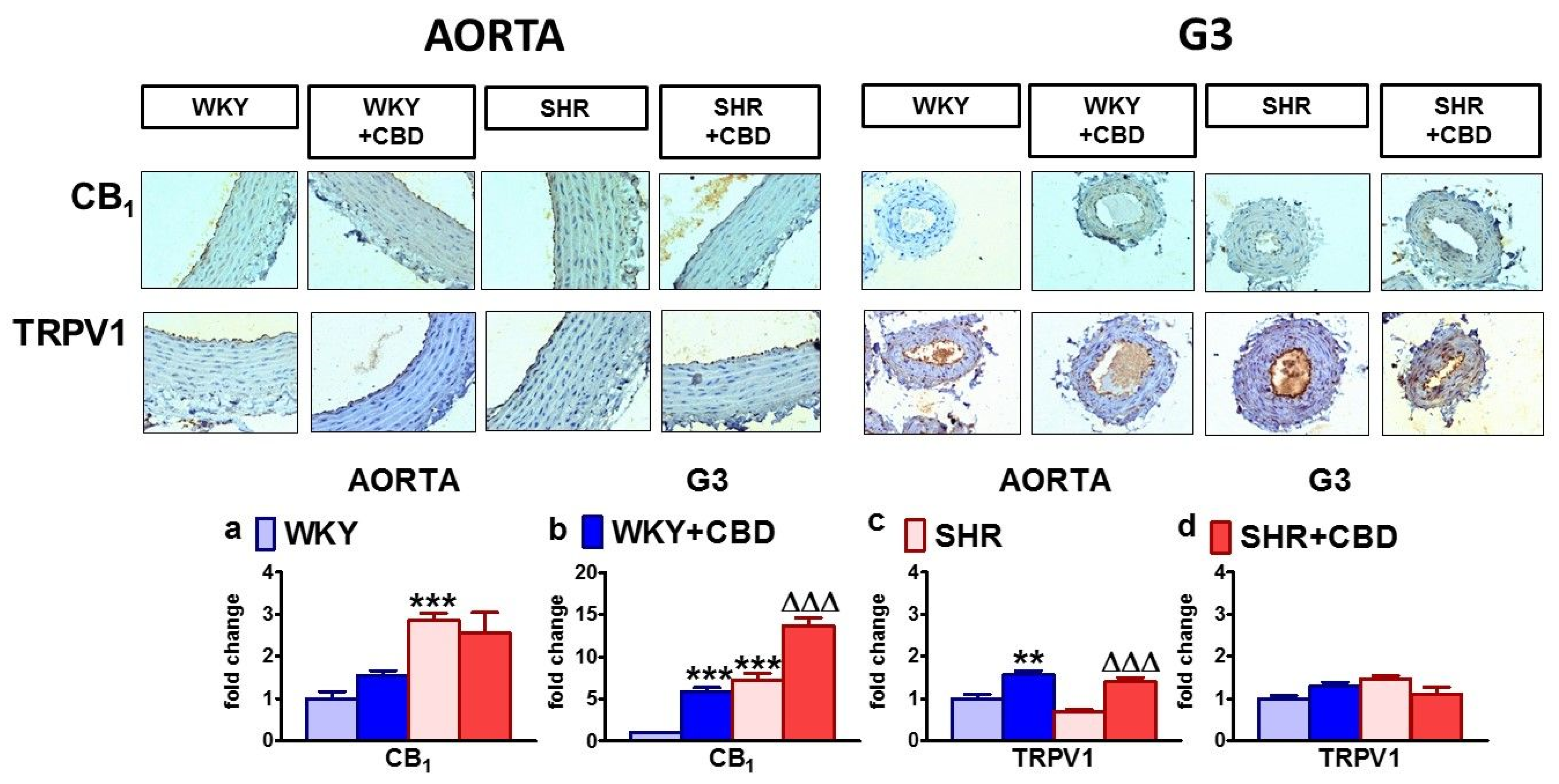
2.4. Influences of Hypertension and Chronic Administration of CBD on Vascular Remodeling and Immunohistochemical Staining of von Willebrand Factor; eNOS, CB1 and TRPV1 Receptors in Isolated Aortas and Mesenteric G3 Arteries
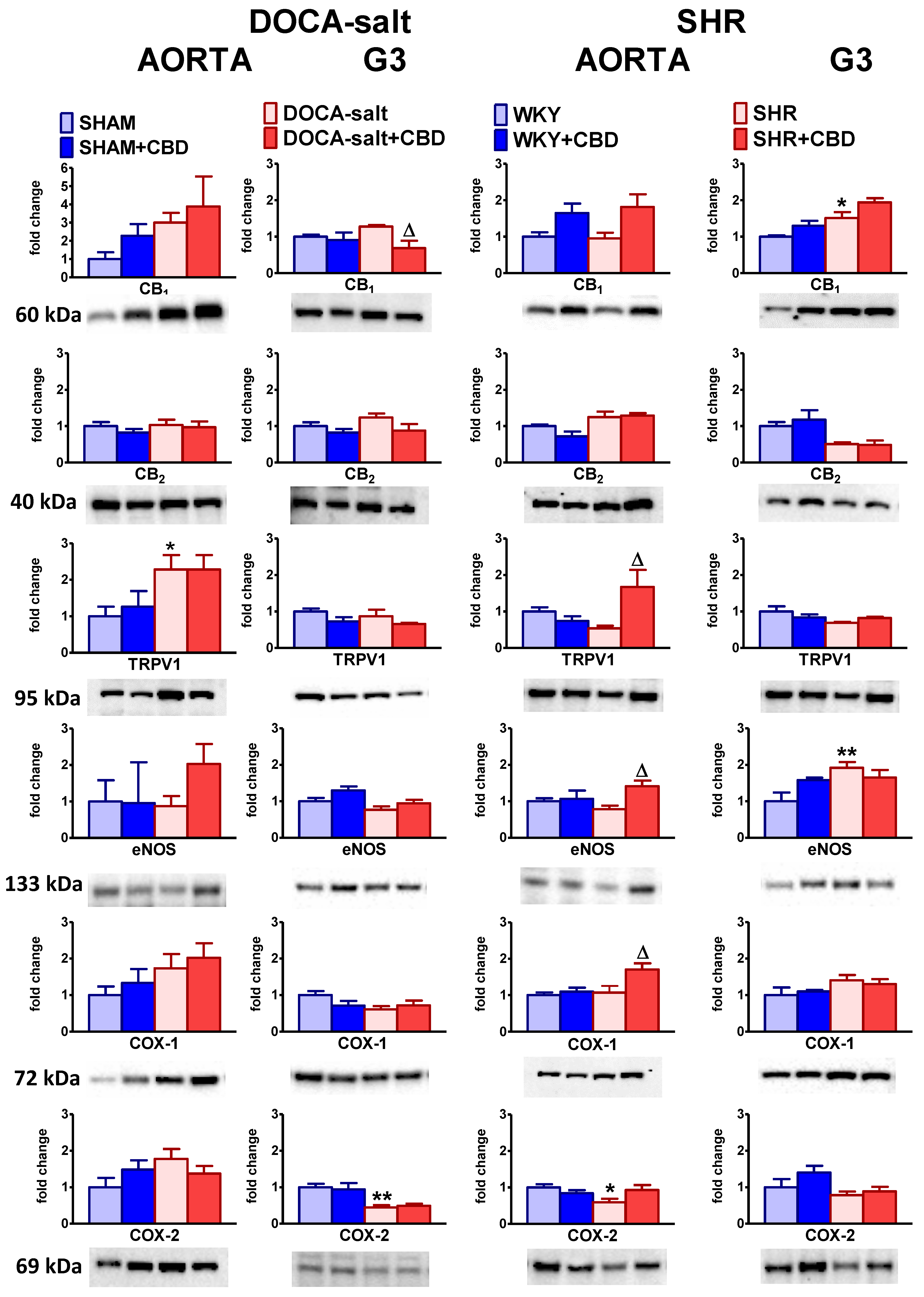
2.5. Influences of Hypertension and Chronic Administration of CBD on Vascular Expression of CB1, CB2 and TRPV1 Receptors; COX-1; COX-2; and eNOS in Isolated Aortas and Mesenteric G3 Arteries
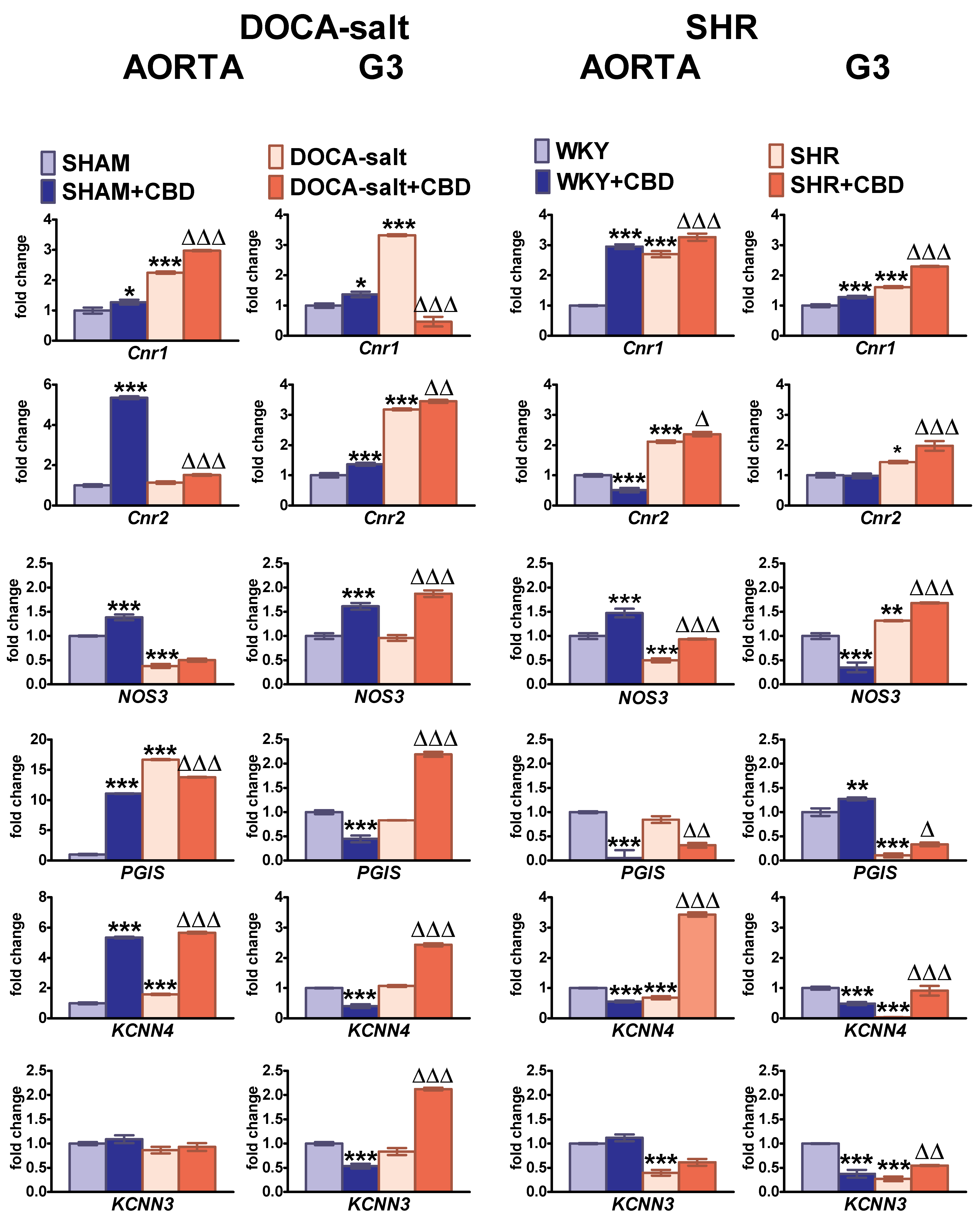
2.6. Influences of Hypertension and Chronic Administration of CBD on Cnr1, Cnr2, eNOS, PGIS, KCa3.1 and KCa2.3 Gene Expression in Isolated Aortas and Mesenteric G3 Arteries
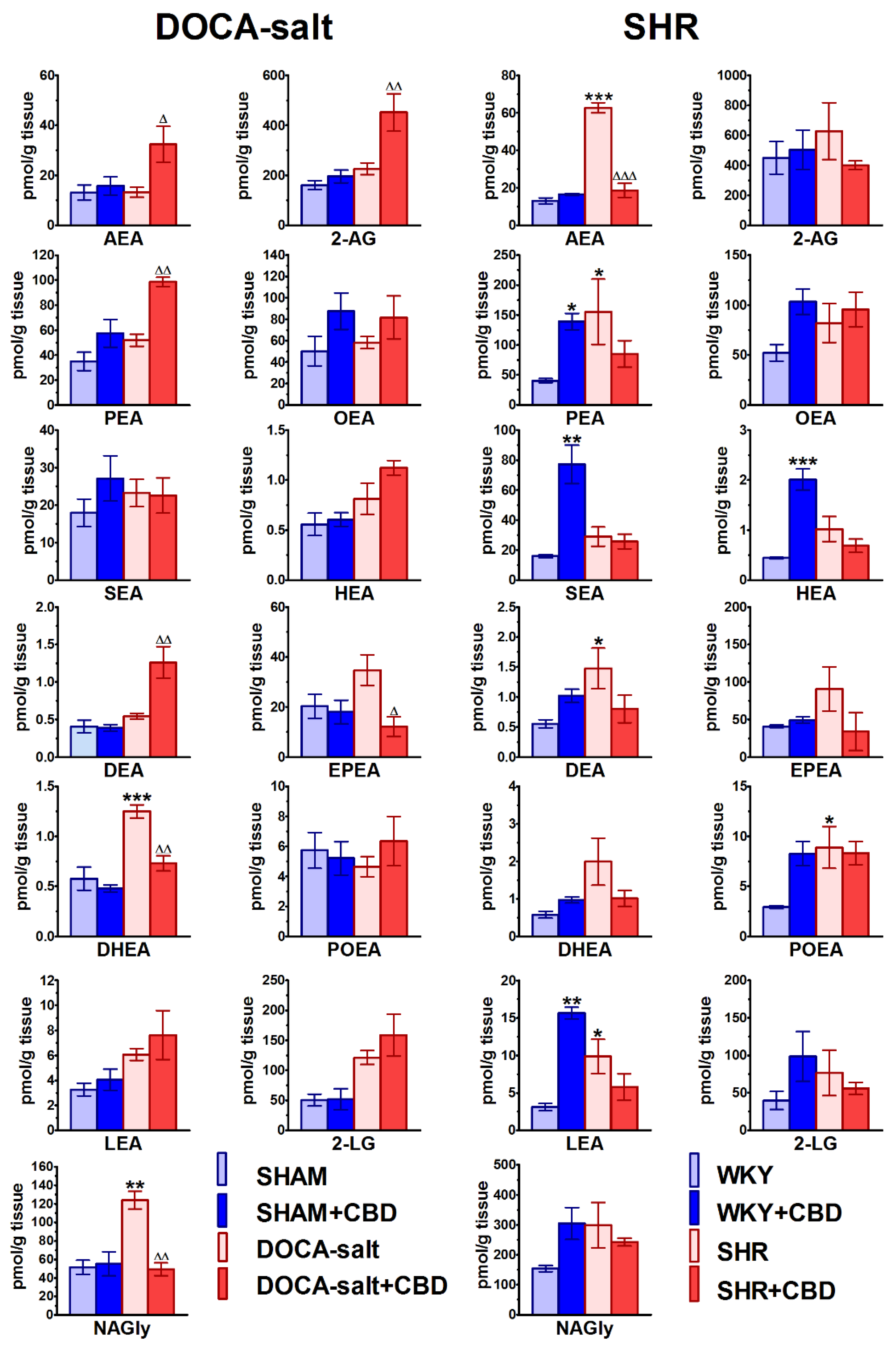
2.7. Influences of Hypertension and Chronic Administration of CBD on Endocannabinoid Levels in Isolated Aortas
3. Discussion
3.1. Vascular Changes Related to Hypertension
3.2. Vascular Changes Induced by CBD in Hypertension and Normotension
3.3. Limitations
4. Materials and Methods
4.1. Animals
4.2. Chronic Treatment with Cannabidiol
4.3. Vessel Preparation
4.4. Concentration-Response Curves
4.5. Thickness of Media in Aortas and Mesenteric G3 Arteries
4.6. Immunohistochemistry
4.7. Western Blots
4.8. RT-qPCR
4.9. Determination of Endocannabinoids
4.10. Drugs
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| 2-AG | 2-Arachidonoylglycerol |
| AEA | Anandamide |
| ANOVA | Analysis of variance |
| BP | Blood pressure |
| Cnr1 | Cannabinoid receptor 1 protein coding gene |
| Cnr2 | Cannabinoid receptor 2 protein coding gene |
| COX | Cyclooxygenase |
| CRCs | Concentration response curves |
| CBD | Cannabidiol |
| DMF | N,N-Dimethylformamide |
| DEA | Docosatetraenoyl ethanolamide |
| DHEA | Docosahexaenoyl ethanolamide |
| eNOS | Endothelial nitric oxide synthase |
| EPEA | Eicosapentaenoyl ethanolamide |
| FAAH | Fatty acid amide hydrolase |
| HEA | Homo-γ-linolenyl ethanolamide |
| HR | Heart rate |
| LEA | Linolenoyl ethanolamide |
| 2-LG | Linoleoylglycerol |
| i.p. | Intraperitoneally |
| KCa3.1 | Intermediate potassium calcium-activated channel |
| KCa2.3 | Small potassium calcium-activated channel |
| KCNN3 | Potassium calcium-activated channel subfamily N member 3 protein coding gene |
| KCNN4 | Potassium calcium-activated channel subfamily N member 4 protein coding gene |
| kDA | Kilodaltons |
| LC-MS | Ultrahigh performance liquid chromatography-tandem mass spectrometry |
| L-NAME | NG-Nitro-L-arginine methyl ester |
| MRM | Multiple reaction monitoring |
| NAGly | N-Arachidonoyl glycine |
| N.D. | Not determined |
| NO | Nitric oxide |
| OEA | Oleoyl ethanolamide |
| PEA | Palmitoyl ethanolamide |
| POEA | Palmitoleoyl ethanolamide |
| PGIS | Prostaglandin I2 synthase protein coding gene |
| Phe | Phenylephrine |
| PPAR | Peroxisome proliferator-activated receptors |
| RIPA | Radioimmunoprecipitation assay |
| RT-PCR | Real time reverse transcription-polymerase chain reaction |
| SBP | Systolic blood pressure |
| SHAM | Sham operated Wistar rats |
| SHR | Spontaneously hypertensive rats |
| SNP | Sodium nitroprusside |
| SOD | Superoxide dismutase |
| SEA | Stearoyl ethanolamide |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| vWF | Von Willebrand factor |
| WKY | Wistar Kyoto Rats |
| ZDF | Zucker Diabetic Fatty rats |
References
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.C.; Lang, N.N.; Touyz, R.M. Drug Treatment of Hypertension: Focus on Vascular Health. Drugs 2016, 76, 1529–1550. [Google Scholar] [CrossRef]
- Brandes, R.P. Endothelial dysfunction and hypertension. Hypertension 2014, 64, 924–928. [Google Scholar] [CrossRef]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597. Int. J. Mol. Sci. 2021, 22, 4833. [Google Scholar] [CrossRef]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef]
- Page, R.L., 2rd; Allen, L.A.; Kloner, R.A.; Carriker, C.R.; Martel, C.; Morris, A.A.; Piano, M.R.; Rana, J.S.; Saucedo, J.F.; American Heart Association Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; et al. Medical Marijuana, Recreational Cannabis, and Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e131–e152. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Karpińska, O.; Toczek, M.; Harasim, E.; Kasacka, I.; Malinowska, B. Protective role of cannabinoid CB1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA-salt hypertensive rats. Life Sci. 2016, 151, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef]
- Malinowska, B.; Toczek, M.; Pędzińska-Betiuk, A.; Schlicker, E. Cannabinoids in arterial, pulmonary and portal hypertension - mechanisms of action and potential therapeutic significance. Br. J. Pharmacol. 2019, 176, 1395–1411. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol ameliorates monocrotaline-induced pulmonary hypertension in rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef] [PubMed]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, K.A.; Tan, G.D.; O’Sullivan, S.E. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2017, 2, e93760. [Google Scholar] [CrossRef]
- Sultan, S.R.; O’Sullivan, S.E.; England, T.J. The effects of acute and sustained cannabidiol dosing for seven days on the haemodynamics in healthy men: A randomised controlled trial. Br. J. Clin. Pharmacol. 2020, 86, 1125–1138. [Google Scholar] [CrossRef]
- Offertáler, L.; Mo, F.M.; Bátkai, S.; Liu, J.; Begg, M.; Razdan, R.K.; Martin, B.R.; Bukoski, R.D.; Kunos, G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003, 63, 699–705. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.P.; Wheal, A.J.; Randall, M.D.; O’Sullivan, S.E. Cannabinoids alter endothelial function in the Zucker rat model of type 2 diabetes. Eur. J. Pharmacol. 2013, 720, 376–382. [Google Scholar] [CrossRef]
- Stanley, C.P.; Hind, W.H.; Tufarelli, C.; O’Sullivan, S.E. Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc. Res. 2015, 107, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol improves vasorelaxation in Zucker diabetic fatty rats through cyclooxygenase activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In vivo cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of Zucker Diabetic Fatty rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Mazeh, A.C.; Angus, J.A.; Wright, C.E. Cannabidiol selectively inhibits the contraction of rat small resistance arteries: Possible role for CGRP and voltage-gated calcium channels. Eur. J. Pharmacol. 2021, 891, 173767. [Google Scholar] [CrossRef]
- Schwartz, M.; Böckmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features by cannabidiol in vascular smooth muscle cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Irie, K.; Fujioka, M.; Orito, K.; Abe, K.; Hasebe, N.; Egashira, N.; et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J. Neurochem. 2007, 102, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Hayakawa, K.; Abe, K.; Ikeda, T.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine 1A receptor-dependent mechanism. Stroke 2005, 36, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Valdepeñas, L.; Martínez-Orgado, J.A.; Benito, C.; Millán, A.; Tolón, R.M.; Romero, J. Cannabidiol reduces lipopolysaccharide-induced vascular changes and inflammation in the mouse brain: An intravital microscopy study. J. Neuroinflamm. 2011, 8, 5. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Drel, V.R.; Obrosova, I.G.; Pacher, P. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H610–H619. [Google Scholar] [CrossRef]
- Pędzińska-Betiuk, A.; Weresa, J.; Schlicker, E.; Harasim-Symbor, E.; Toczek, M.; Kasacka, I.; Gajo, B.; Malinowska, B. Chronic cannabidiol treatment reduces the carbachol-induced coronary constriction and left ventricular cardiomyocyte width of the isolated hypertensive rat heart. Toxicol. Appl. Pharmacol. 2021, 411, 115368. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Blann, A. von Willebrand factor: A marker of endothelial dysfunction in vascular disorders? Cardiovasc. Res. 1997, 34, 255–265. [Google Scholar] [CrossRef]
- Mancini, I.; Baronciani, L.; Artoni, A.; Colpani, P.; Biganzoli, M.; Cozzi, G.; Novembrino, C.; Boscolo Anzoletti, M.; De Zan, V.; Pagliari, M.T.; et al. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J. Thromb. Haemost. 2021, 19, 513–521. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal models of hypertension: A scientific statement from the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef]
- Resstel, L.B.N.; Tavares, R.F.; Lisboa, S.F.S.; Joca, S.R.L.; Corrêa, F.M.A.; Guimarães, F.S. 5-HT1A receptors are involved in the cannabidiol induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009, 156, 181–188. [Google Scholar] [CrossRef]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Wenceslau, C.F.; McCarthy, C.G.; Earley, S.; England, S.K.; Filosa, J.A.; Goulopoulou, S.; Gutterman, D.D.; Isakson, B.E.; Kanagy, N.L.; Martinez-Lemus, L.A.; et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. Am. J. Physiol. Heart Circ. Physiol. 2021, 321, H77–H111. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]
- Jiang, J.; Zheng, J.P.; Li, Y.; Gan, Z.; Jiang, Y.; Huang, D.; Li, H.; Liu, Z.; Ke, Y. Differential contribution of endothelium-derived relaxing factors to vascular reactivity in conduit and resistance arteries from normotensive and hypertensive rats. Clin. Exp. Hypertens. 2016, 38, 393–398. [Google Scholar] [CrossRef]
- Bernatova, I. Endothelial dysfunction in experimental models of arterial hypertension: Cause or consequence? Biomed. Res. Int. 2014, 2014, 598271. [Google Scholar] [CrossRef] [PubMed]
- Kloza, M.; Baranowska-Kuczko, M.; Toczek, M.; Kusaczuk, M.; Sadowska, O.; Kasacka, I.; Kozłowska, H. Modulation of Cardiovascular Function in Primary Hypertension in Rat by SKA-31, an Activator of KCa2.x and KCa3.1 Channels. Int. J. Mol. Sci. 2019, 20, 4118. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.W.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization: Age, gender and blood pressure, do they matter? Acta Physiol. (Oxf.) 2017, 219, 108–123. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.M.; Peng, J.; Zhang, Y.S.; Li, X.H.; Yuan, Q.; Hu, C.P.; Deng, H.W.; Li, Y.J. Role of anandamide transporter in regulating calcitonin gene-related peptide production and blood pressure in hypertension. J. Hypertens. 2009, 27, 1224–1232. [Google Scholar] [CrossRef]
- Al Suleimani, Y.M.; Al Mahruqi, A.S. The endogenous lipid N-arachidonoyl glycine is hypotensive and nitric oxide-cGMP-dependent vasorelaxant. Eur. J. Pharmacol. 2017, 794, 209–215. [Google Scholar] [CrossRef]
- Bondarenko, A.I. Cannabinoids and Cardiovascular System. Adv. Exp. Med. Biol. 2019, 1162, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Williams, G.; Doherty, P. 2-Linoleoylglycerol Is a Partial Agonist of the Human Cannabinoid Type 1 Receptor that Can Suppress 2-Arachidonolyglycerol and Anandamide Activity. Cannabis Cannabinoid Res. 2019, 4, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S.; Randall, M.D. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br. J. Pharmacol. 2007, 150, 641–651. [Google Scholar] [CrossRef]
- Ho, W.S.; Barrett, D.A.; Randall, M.D. Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br. J. Pharmacol. 2008, 155, 837–846. [Google Scholar] [CrossRef]
- AlSuleimani, Y.M.; Hiley, C.R. Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. Eur. J. Pharmacol. 2013, 702, 1–11. [Google Scholar] [CrossRef]
- Martín Giménez, V.M.; Noriega, S.E.; Kassuha, D.E.; Fuentes, L.B.; Manucha, W. Anandamide and endocannabinoid system: An attractive therapeutic approach for cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2018, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, C.; Ortisi, E.; Pulvirenti, L.; Reibaldi, M.; Scollo, D.; Amato, R.; Avitabile, T.; Longo, A. Ocular hypotensive effect of oral palmitoyl-ethanolamide: A clinical trial. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6096–6100. [Google Scholar] [CrossRef]
- Marichal-Cancino, B.A.; González-Hernández, A.; MaassenVanDenBrink, A.; Ramírez-San Juan, E.; Villalón, C.M. Potential Mechanisms Involved in Palmitoylethanolamide-Induced Vasodepressor Effects in Rats. J. Vasc. Res. 2020, 57, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Fusco, R.; Biundo, F.; D’Amico, R.; Benedetto, F.; Di Paola, R.; Cuzzocrea, S. Palmitoylethanolamide and Polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharm. Res. 2017, 123, 83–92. [Google Scholar] [CrossRef]
- Buzinari, T.C.; Oishi, J.C.; De Moraes, T.F.; Vatanabe, I.P.; Selistre-de-Araújo, H.S.; Pestana, C.R.; Rodrigues, G.J. Treatment with sodium nitroprusside improves the endothelial function in aortic rings with endothelial dysfunction. Eur. J. Pharm. Sci. 2017, 105, 144–149. [Google Scholar] [CrossRef]
- Tang, E.H.; Feletou, M.; Huang, Y.; Man, R.Y.; Vanhoutte, P.M. Acetylcholine and sodium nitroprusside cause long-term inhibition of EDCF-mediated contractions. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2434–H2440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, D.B.; Xu, H.W.; Yang, G.J.; Yang, J.M.; Fang, H.; Tang, J.Y. Effects of rosuvastatin correlated with the down-regulation of CYP4A1 in spontaneously hypertensive rats. Microvasc. Res. 2015, 98, 88–93. [Google Scholar] [CrossRef]
- Park, J.K.; Mervaala, E.M.; Muller, D.N.; Menne, J.; Fiebeler, A.; Luft, F.C.; Haller, H. Rosuvastatin protects against angiotensin II-induced renal injury in a dose-dependent fashion. J. Hypertens. 2009, 27, 599–605. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, Y.; Li, Y.; Zhao, L.; Gong, Y.; Wang, M.; Wang, M.; Fu, G.; Zhang, W. Rosuvastatin Reverses Hypertension-Induced Changes in the Aorta Structure and Endothelium-Dependent Relaxation in Rats Through Suppression of Apoptosis and Inflammation. J. Cardiovasc. Pharmacol. 2020, 75, 584–595. [Google Scholar] [CrossRef]
- Rickard, A.J.; Morgan, J.; Chrissobolis, S.; Miller, A.A.; Sobey, C.G.; Young, M.J. Endothelial cell mineralocorticoid receptors regulate deoxycorticosterone/salt-mediated cardiac remodeling and vascular reactivity but not blood pressure. Hypertension 2014, 63, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Goodman, A.G.; Wiley, J.L.; Pondelick, A.M.; Craft, R.M. Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain. J. Pharm. Exp. Ther. 2020, 373, 416–428. [Google Scholar] [CrossRef]
- Wanner, N.M.; Colwell, M.; Drown, C.; Faulk, C. Developmental cannabidiol exposure increases anxiety and modifies genome-wide brain DNA methylation in adult female mice. Clin. Epigenet. 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.S. Modulation by 17β-estradiol of anandamide vasorelaxation in normotensive and hypertensive rats: A role for TRPV1 but not fatty acid amide hydrolase. Eur. J. Pharmacol. 2013, 701, 49–56. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104, Erratum in: Eur. Heart J. 2019, 40, 475. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 7, e13–e115, Erratum in: Hypertension 2018, 71, e140–e144. [Google Scholar] [CrossRef]
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989. [Google Scholar] [CrossRef]
- Silmore, L.H.; Willmer, A.R.; Capparelli, E.V.; Rosania, G.R. Food effects on the formulation, dosing, and administration of cannabidiol (CBD) in humans: A systematic review of clinical studies. Pharmacotherapy 2021, 41, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Frost, M.; Keable, A.; Baseley, D.; Sealy, A.; Andreea Zbarcea, D.; Gatherer, M.; Yuen, H.M.; Sharp, M.M.; Weller, R.O.; Attems, J.; et al. Vascular α1A Adrenergic Receptors as a Potential Therapeutic Target for IPAD in Alzheimer’s Disease. Pharmaceuticals 2020, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Luque-Córdoba, D.; Calderón-Santiago, M.; Luque de Castro, M.D.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC-MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef]
| Group AORTA | SHAM | SHAM + CBD | DOCA-Salt | DOCA-Salt + CBD | WKY | WKY + CBD | SHR | SHR + CBD |
|---|---|---|---|---|---|---|---|---|
| Ach | (8) | (8) | (6) | (7) | (8) | (8) | (8) | (8) |
| pEC50 | 6.6 ± 0.1 | 6.5 ± 0.1 | 6.5 ± 0.1 | 6.9 ± 0.1 ∆ | 6.9 ± 0.1 | 7.2 ± 0.1 | 7.1 ± 0.1 | 7.5 ± 0.1 ∆ |
| Rmax (%) | 92.9 ± 2.8 | 93.4 ± 4.6 | 77.3 ± 2.4 | 91.2 ± 5.8 | 98.5 ± 7.2 | 104.0 ± 7.9 | 83.7 ± 9.4 | 106.8 ± 5.0 |
| Ach + L-NAME | (4) | (4) | (4) | (4) | (5) | (5) | (6) | (6) |
| pEC50 | - | - | - | - | - | - | - | - |
| Rmax (%) | 0.5 ± 3.1 ### | 25.5 ± 2.1 ### | 23.7 ± 6.7 ### | 37.9 ± 21.1 ## | 17.8 ± 1.5 ### | 7.0 ± 10.8 ### | 9.3 ± 7.2 ### | 9.5 ± 13.7 ### |
| Ach + INDO | (6) | (4) | (4) | (4) | (5) | (5) | (6) | (6) |
| pEC50 | 6.3 ± 0.1 | 6.9 ± 0.1 #,a | 6.5 ± 0.1 | 7.3 ± 0.1 #,a | 6.9 ± 0.1 | 6.8 ± 0.1 #,a | 7.1 ± 0.1 | 7.4 ± 0.1 |
| Rmax (%) | 76.3 ± 4.9 ## | 101.6 ± 8.1 | 83.8 ± 8.4 | 81.3 ± 5.0 | 97.9 ± 11.1 | 101.2 ± 6.9 | 91.3 ± 9.1 | 95.9 ± 7.0 |
| SNP | (7) | (7) | (7) | (7) | (8) | (6) | (7) | (8) |
| pEC50 | 8.4 ± 0.1 | 8.5 ± 0.1 | 7.9 ± 0.1 ** | 7.7 ± 0.1 | 7.6 ± 0.1 | 7.9 ± 0.1 | 7.6 ± 0.1 | 7.6 ± 0.1 |
| Rmax (%) | 107.3 ± 1.8 | 107.2 ± 1.2 | 102.6 ± 1.1 | 110.3 ± 3.6 | 106.3 ± 6.0 | 121.5 ± 9.1 | 115.7 ± 6.3 | 106.3 ± 6.0 |
| Group G3 | SHAM | SHAM + CBD | DOCA-Salt | DOCA-Salt + CBD | WKY | WKY + CBD | SHR | SHR + CBD |
|---|---|---|---|---|---|---|---|---|
| Ach | (7) | (5) | (6) | (6) | (8) | (8) | (7) | (8) |
| pEC 50 | 6.5 ± 0.1 | 6.8 ± 0.1 | 6.4 ± 0.1 | 6.9 ± 0.1 ∆∆ | 6.5 ± 0.1 | 6.9 ± 0.1 * | 6.5 ± 0.1 | 7.9 ± 0.1 ∆∆∆ |
| Rmax (%) | 95.9 ± 2.7 | 97.7 ± 2.6 | 95.7 ± 3.2 | 96.3 ± 2.1 | 81.6 ± 7.3 | 94.3 ± 2.0 | 78.0 ± 6.5 | 87.3 ± 6.6 |
| Ach + L-NAME | (4) | (4) | (4) | (6) | (6) | (6) | (5) | (5) |
| pEC50 | 6.1 ± 0.1 # | - | 6.8 ± 0.1 # | 6.3 ± 0.1 ## | 6.1 ± 0.1 # | 6.0 ± 0.1 ### | - | - |
| Rmax (%) | 61.7 ± 20.9 # | 31.3 ± 18.5 ## | 78.5 ± 7.4 | 64.4 ± 8.4 ## | 50.3 ± 8.2 | 71.0 ± 6.9 ## | 49.6 ± 3.9 ## | 28.4 ± 14.0 ### |
| Ach + INDO | (5) | (5) | (5) | (6) | (5) | (6) | (5) | (5) |
| pEC50 | 6.6 ± 0.1 | 6.1 ± 0.1 ##,a | 6.6 ± 0.1 | 6.5 ± 0.1 # | 6.5 ± 0.1 | 6.8 ± 0.1 | 6.3 ± 0.1 | 6.0 ± 0.1 ###,a |
| Rmax (%) | 79.9 ± 4.8 | 71.3 ± 10.0 | 88.7 ± 10.4 | 91.4 ± 1.6 | 70.3 ± 16.3 | 62.9 ± 5.6 ### | 76.6 ± 1.6 | 51.8 ± 1.0 # |
| SNP | (7) | (6) | (5) | (5) | (5) | (5) | (6) | (7) |
| pEC 50 | 6.8 ± 0.2 | 5.8 ± 0.1 *** | 6.0 ± 0.1 ** | 6.3 ± 0.1 | 6.8 ± 0.1 | 6.4 ± 0.1 | 6.8 ± 0.1 | 6.0 ± 0.1 ∆∆∆ |
| Rmax (%) | 65.1 ± 6.4 | 57.7 ± 8.0 | 57.2 ± 6.1 | 53.0 ± 8.5 | 71.2 ± 8.4 | 90.0 ± 7.8 | 61.0 ± 9.5 | 71.1 ± 7.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Kusaczuk, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals 2021, 14, 1120. https://doi.org/10.3390/ph14111120
Baranowska-Kuczko M, Kozłowska H, Kloza M, Kusaczuk M, Harasim-Symbor E, Biernacki M, Kasacka I, Malinowska B. Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals. 2021; 14(11):1120. https://doi.org/10.3390/ph14111120
Chicago/Turabian StyleBaranowska-Kuczko, Marta, Hanna Kozłowska, Monika Kloza, Magdalena Kusaczuk, Ewa Harasim-Symbor, Michał Biernacki, Irena Kasacka, and Barbara Malinowska. 2021. "Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension" Pharmaceuticals 14, no. 11: 1120. https://doi.org/10.3390/ph14111120
APA StyleBaranowska-Kuczko, M., Kozłowska, H., Kloza, M., Kusaczuk, M., Harasim-Symbor, E., Biernacki, M., Kasacka, I., & Malinowska, B. (2021). Vasoprotective Endothelial Effects of Chronic Cannabidiol Treatment and Its Influence on the Endocannabinoid System in Rats with Primary and Secondary Hypertension. Pharmaceuticals, 14(11), 1120. https://doi.org/10.3390/ph14111120