New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. General
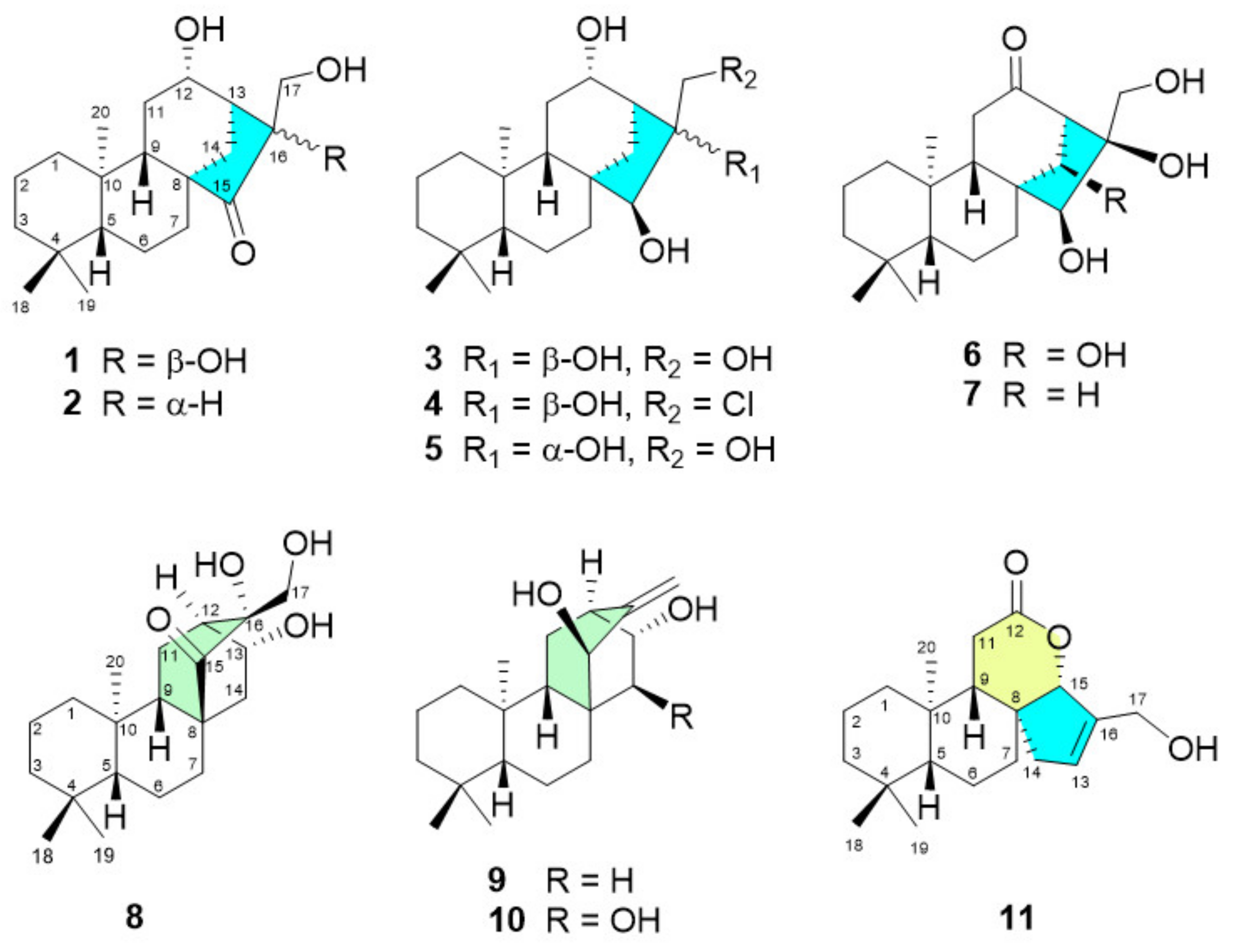
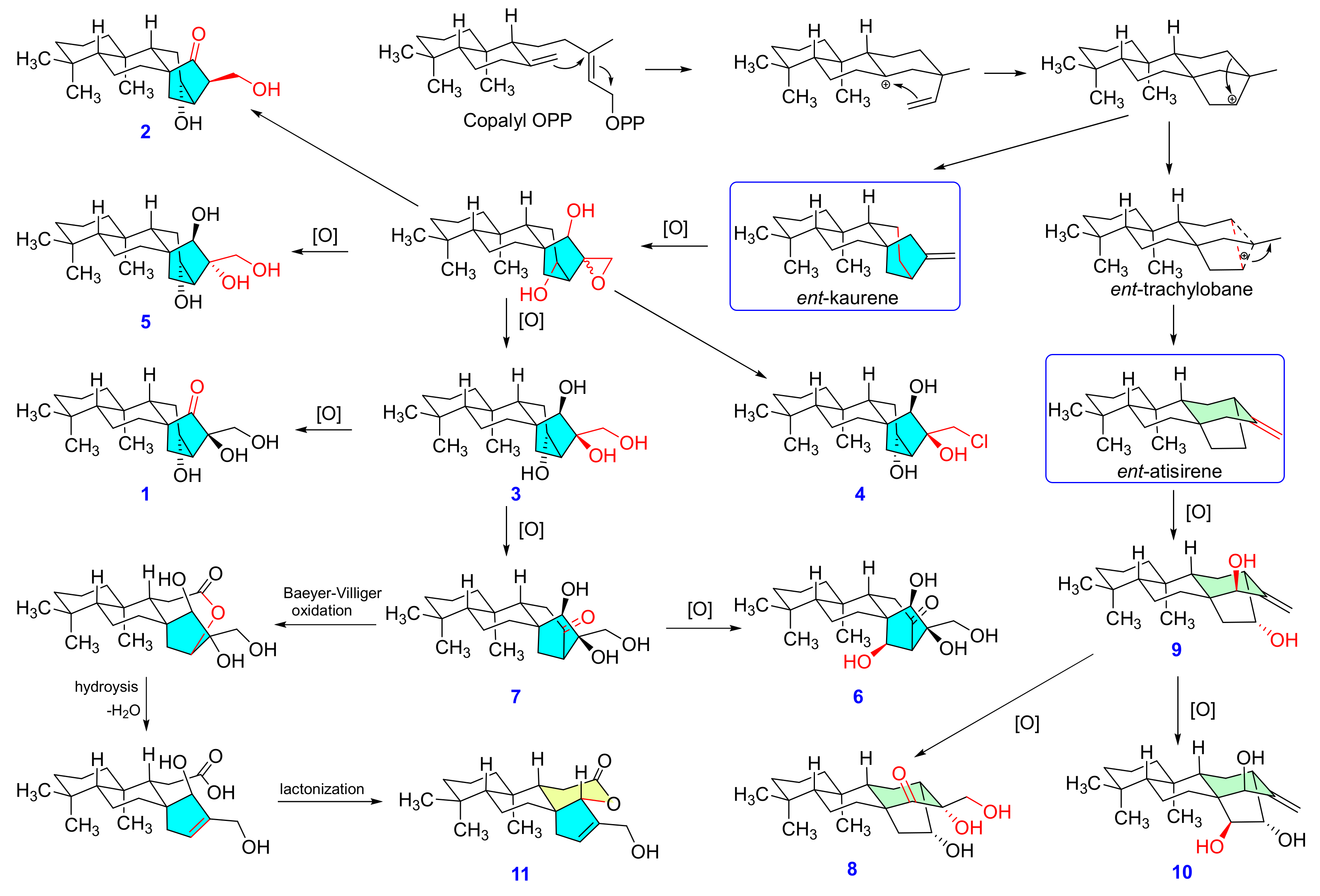
2.2. Structure Elucidation of the Isolated Diterpenoids
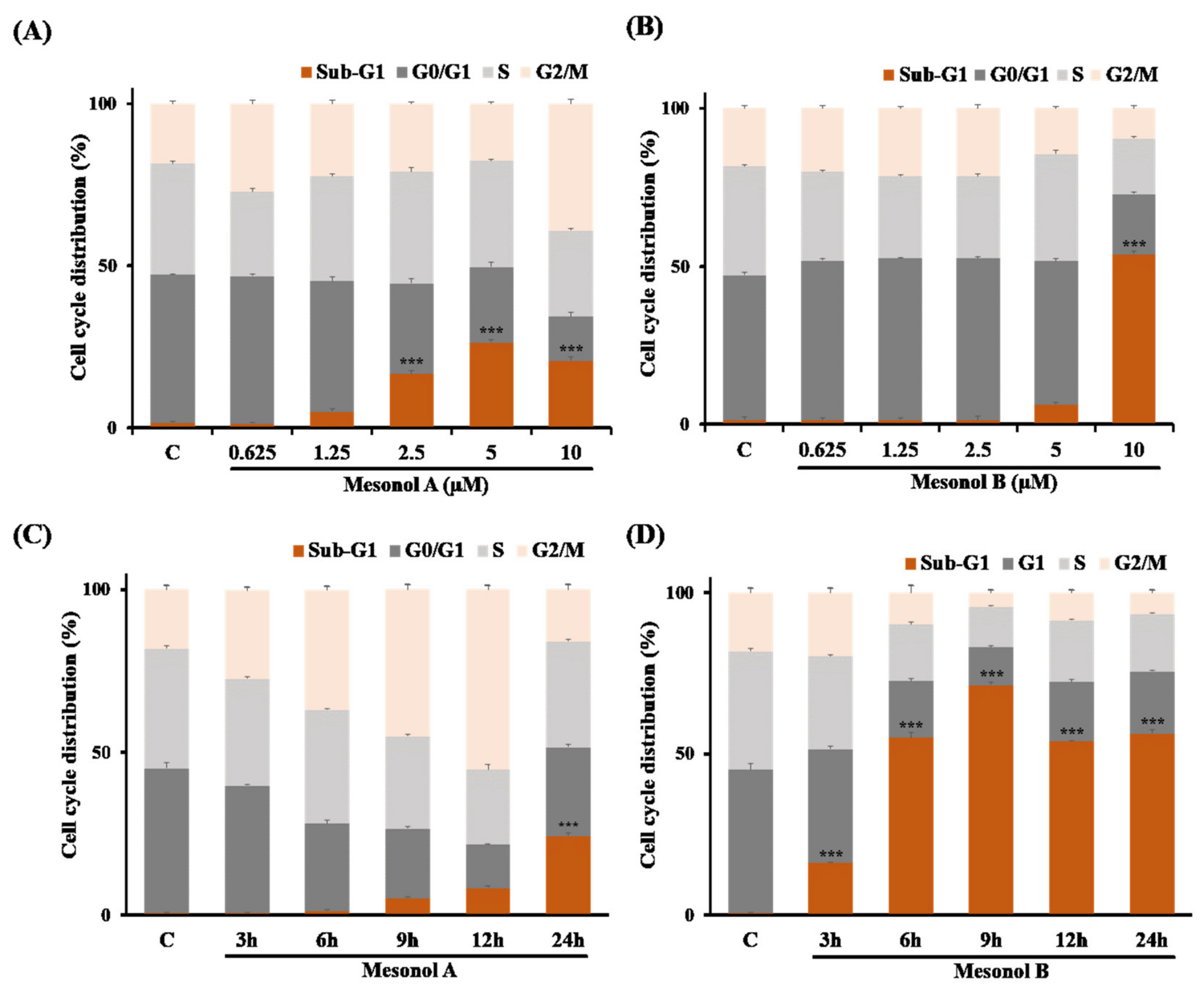
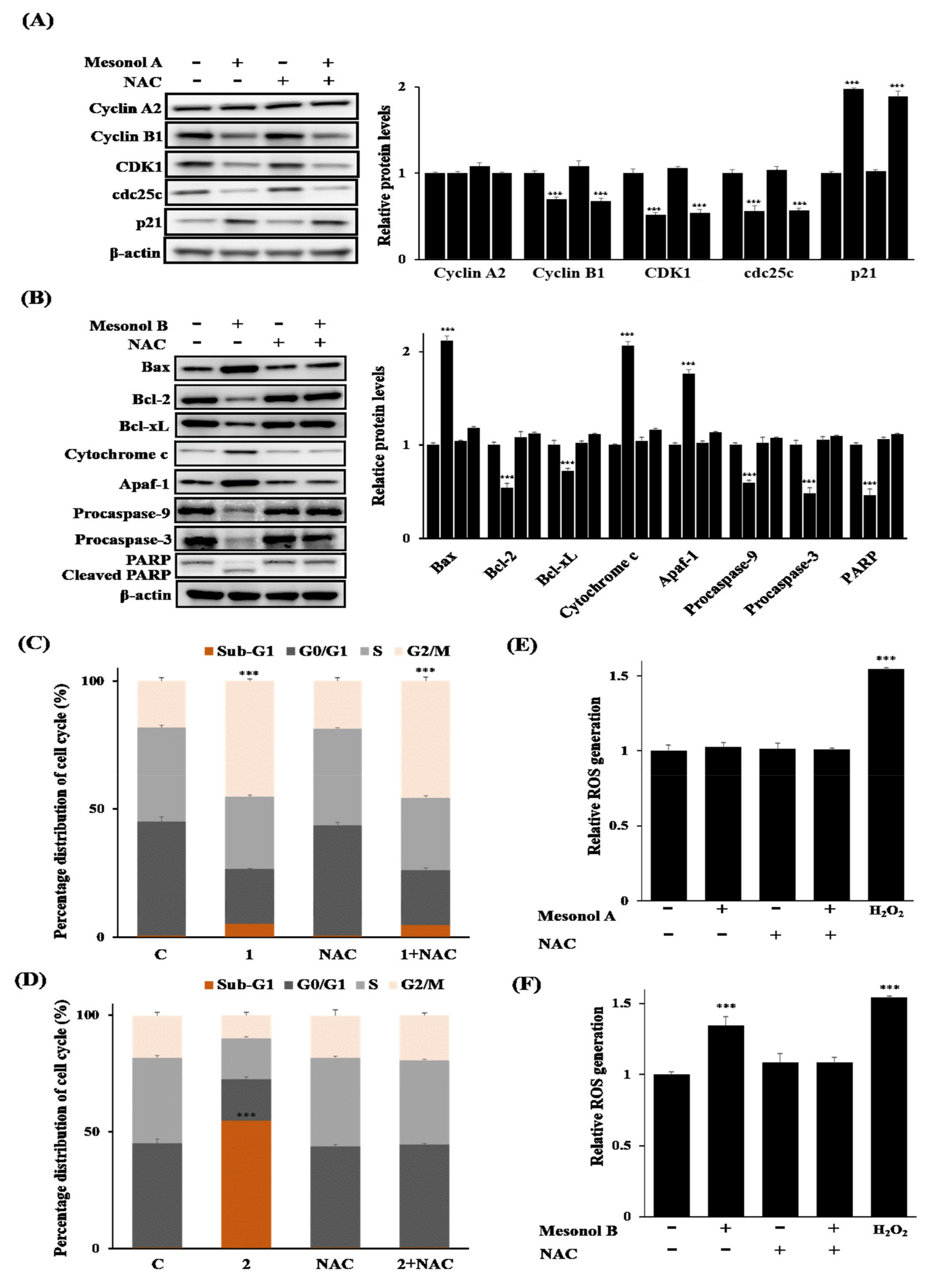
2.3. Biological Studies
3. Discussion
4. Materials and Methods
4.1. General
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Spectral Measurements
4.5. X-ray Crystallographic Data for 1–3, and 7
4.5.1. Mesonol A (1)
4.5.2. Mesonol B (2)
4.5.3. Mesonol C (3)
4.5.4. Mesonol G (7)
4.6. Cytotoxicity Assay
4.7. Western Blot Analysis
4.8. Cell Cycle Analysis
4.9. Measurement of ROS Generation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Bon, R.S.; Waldmann, H. Bioactivity-guided navigation of chemical space. Acc. Chem. Res. 2010, 43, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Case, B.C.; Hauck, M.L.; Yeager, R.L.; Simkins, A.H.; de Serres, M.; Schmith, V.D.; Dillberger, J.E.; Page, R.L. The pharmacokinetics and pharmacodynamics of GW395058, a peptide agonist of the thrombopoietin receptor, in the dog, a large-animal model of chemotherapy-induced thrombocytopenia. Stem Cells 2000, 18, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Kma, L. Synergistic effect of resveratrol and radiotherapy in control of cancers. Asian Pac. J. Cancer Prev. 2013, 14, 6197–6208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Chahar, M.K.; Sharma, N.; Dobhal, M.P.; Joshi, Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011, 5, 2346–2352. [Google Scholar] [CrossRef] [Green Version]
- Isah, T. Anticancer alkaloids from trees: Development into drugs. Pharmacogn. Rev. 2016, 10, 90–99. [Google Scholar] [CrossRef] [Green Version]
- Spatafora, C.; Tringali, C. Natural-derived polyphenols as potential anticancer agents. Anti Cancer Agents Med. Chem. 2012, 12, 902–918. [Google Scholar] [CrossRef]
- Bras, M.; Queenan, B.; Susin, S.A. Programmed cell death via mitochondria: Different modes of dying. Biochemistry 2005, 70, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G. Mitochondrial control of apoptosis: An introduction. Biochem. Biophys. Res. Commun. 2003, 304, 433–435. [Google Scholar] [CrossRef]
- Matsuura, K.; Canfield, K.; Feng, W.; Kurokawa, M. Metabolic regulation of apoptosis in cancer. Int. Rev. Cell Mol. Biol. 2016, 327, 43–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.J.; Liao, J.C.; Chiu, C.S.; Huang, S.S.; Lin, T.H.; Deng, J.S. Anti-inflammatory activities of aqueous extract of Mesona procumbens in experimental mice. J. Sci. Food Agric. 2012, 92, 1186–1193. [Google Scholar] [CrossRef]
- Yeh, C.T.; Huang, W.H.; Yen, G.C. Antihypertensive effects of Hsian-tsao and its active compound in spontaneously hypertensive rats. J. Nutr. Biochem. 2009, 20, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Hung, Y.L.; Hsieh, C.L. Protective effect of extracts of Mesona procumbens Hemsl. on DNA damage in human lymphocytes exposed to hydrogen peroxide and UV irradiation. Food Chem. Toxicol. 2000, 38, 747–754. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Hung, Y.L. Contributions of major components to the antimutagenic effect of Hsian-Tsao (Mesona procumbens Hemsl.). J. Agric. Food. Chem. 2001, 49, 5000–5004. [Google Scholar] [CrossRef] [PubMed]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Hsian-Tsao (Mesona procumbens Heml.) prevents against rat liver fibrosis induced by CCl(4) via inhibition of hepatic stellate cells activation. Food Chem. Toxicol. 2008, 46, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xu, Z.P.; Xu, C.J.; Meng, J.; Ding, G.Q.; Zhang, X.M.; Weng, Y. Renal protective activity of hsian-tsao extracts in diabetic rats. Biomed. Environ. Sci. 2008, 21, 222–227. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.X.; Liang, Z.M.; Tsang, S.W.; Zhang, H.J. Mechanistic pathways and molecular targets of plant-derived anticancer ent-kaurane diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Lin, Z.; Shen, X.; Yoshio, T.; Tetsuro, F. ent-kaurene diterpenoids from Rabdosia loxothyrsa. Phytochemistry 1991, 30, 603–606. [Google Scholar] [CrossRef]
- Zhan, R.; Li, X.-N.; Du, X.; Wang, W.-G.; Dong, K.; Su, J.; Li, Y.; Pu, J.-X.; Sun, H.-D. ent-Atisane and ent-kaurane diterpenoids from Isodon rosthornii. Fitoterapia 2013, 88, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrek, J.S. Neutral diterpenoids of Helianthus annuus. J. Nat. Prod. 1984, 47, 822–827. [Google Scholar] [CrossRef]
- Shi, H.M.; Williams, I.D.; Sung, H.H.Y.; Zhu, H.X.; Ip, N.Y.; Min, Z.D. Cytotoxic diterpenoids from the roots of Euphorbia ebracteolata. Planta Med. 2005, 71, 349–354. [Google Scholar] [CrossRef]
- De Heluani, C.S.; Catalán, C.A.; Hernández, L.R.; Burgueño-Tapia, E.; Joseph-Nathan, P. Three new diterpenoids based on the novel sarcopetalane skeleton from Croton sarcopetalus. J. Nat. Prod. 2000, 63, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, A.; Lee, W.S.; Yun, J.W.; Lu, J.N.; Chang, S.H.; Jeong, J.H.; Kim, G.S.; Jung, J.M.; Hong, S.C. Tetraarsenic hexoxide induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt suppression and p38 MAPK activation in SW620 human colon cancer cells. PLoS ONE 2017, 12, e0174591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash Bipin, C.; El-Deiry Wafik, S. Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol. Cell. Biol. 2005, 25, 3364–3387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtin, J.F.; Donovan, M.; Cotter, T.G. Regulation and measurement of oxidative stress in apoptosis. J. Immunol. Methods 2002, 265, 49–72. [Google Scholar] [CrossRef] [Green Version]
- Morales, A.; Pérez, P.; Mendoza, R.; Compagnone, R.; Suarez, A.I.; Arvelo, F.; Ramírez, J.L.; Galindo-Castro, I. Cytotoxic and proapoptotic activity of ent-16beta-17alpha-dihydroxykaurane on human mammary carcinoma cell line MCF-7. Cancer Lett. 2005, 218, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.W.; Zhang, J.; Yang, W.H.; Wang, B.; Wang, J.W. Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells through mitochondria-mediated death pathway. Toxicol. In Vitro 2011, 25, 51–63. [Google Scholar] [CrossRef]
- Yao, R.; Chen, Z.; Zhou, C.C.; Luo, M.; Shi, X.; Li, J.; Gao, Y.; Zhou, F.; Pu, J.; Sun, H.; et al. Xerophilusin B induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma cells and does not cause toxicity in nude mice. J. Nat. Prod. 2015, 78, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Ma, C.Y.; Wang, M.L.; Lu, J.H.; Hu, P.; Chen, J.W.; Li, X.; Chen, Y. Five new ent-kaurane diterpenes from Annona squamosa L. pericarps. Nat. Prod. Res. 2020, 34, 2243–2247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, L.X.; Feng, Q.M.; Wu, H.; Liu, Y.L.; Wang, D.Q.; Dai, L.P.; Wang, Z.M. A new ent-kaurane diterpene from Isodon henryi. Nat. Prod. Res. 2021, 35, 2346–2352. [Google Scholar] [CrossRef] [PubMed]

| No | 1 a | 2 b | 3 b | 4 b | 5 b | 6 b | 7 a | 8 a | 9 b | 10 b | 11 a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.71 td (3.6, 13.2) 1.74 m | 0.74 td (3.5, 13.0) 1.75 m | 0.82 m 1.83 m | 0.82 m 1.83 m | 0.82 m 1.81 m | 0.88 m 1.76 brd (12.5) | 0.83 m 1.76 m | 0.82 m 1.65 m | 0.88 m 1.61 m | 0.90 m 1.56 m | 0.98 td (4.2, 13.2) 1.62 m |
| 2 | 1.37 m 1.60 m | 1.42 m 1.70 m | 1.40 m 1.68 m | 1.40 m 1.68 m | 1.40 m 1.69 m | 1.45 m 1.66 m | 1.42 m 1.65 m | 1.37 m 1.64 m | 1.38 m 1.64 m | 1.38 m 1.64 m | 1.46 m 1.64 m |
| 3 | 1.16 m 1.38 m | 1.18 m 1.41 m | 1.17 td (4.5, 14.0) 1.39 m | 1.18 td (4.5, 14.0) 1.40 m | 1.17 td (4.5, 13.0) 1.40 m | 1.21 td (4.5, 13.5) 1.42 m | 1.21 td (4.2, 12.0) 1.39 m | 1.15 m 1.38 m | 1.19 m 1.40 m | 1.19 td (4.0, 13.0) 1.41 m | 1.22 td (3.6, 13.2) 1.42 m |
| 5 | 0.88 m | 0.90 m | 0.83 m | 0.82 m | 0.83 dd (2.0, 12.5) | 0.95 dd (2.0, 11.5) | 0.85 dd (2.4, 11.4) | 0.72 dd (1.8, 12.0) | 0.82 dd (2.0, 12.0) | 0.89 m | 1.06 dd (2.4, 10.5) |
| 6 | 1.37 m 1.66 m | 1.40 m 1.65 m | 1.39 m 1.57 m | 1.41 m 1.58 m | 1.40 m 1.57 m | 1.42 m 1.63 m | 1.36 m 1.64 m | 1.35 m 1.58 m | 1.40 m 1.55 m | 1.41 m 1.56 m | 1.50 m 1.69 m |
| 7 | 1.34 m 1.79 td (6.0, 14.4) | 1.37 m 1.83 td (6.0, 14.5) | 1.40 m 1.71 m | 1.43 m 1.70 m | 1.49 m 1.67 m | 1.44 m 2.27 m | 1.55 td (6.6, 11.4) 1.77 m | 1.23 m 1.85 td (4.8, 11.2) | 1.11 m 1.85 td (5.0, 13.5) | 1.61 m 1.88 td (3.0, 13.5) | 1.37 m 1.77 m |
| 9 | 1.19 m | 1.17 d (9.5) | 1.54 brd (10.0) | 1.54 brd (9.5) | 1.51 m | 1.94 d (11.0) | 1.88 brd (10.2) | 1.35 m | 1.51 m | 1.57 m | 1.74 dd (3.6, 13.2) |
| 11 | 1.70 m (2H) | 1.48 m 1.78 m | 1.58 m 1.85 m | 1.58 m 1.84 m | 1.63 m 1.78 m | 2.23 brd (18.0) 2.71 dd (11.5, 18.0) | 2.22 brd (17.4) 2.63 dd (10.2, 17.4) | 1.80 m 1.96 ddd (2.4, 7.2, 13.8) | 1.36 m 1.97 ddd (2.0, 7.5, 13.0) | 1.35 m 1.83 ddd (2.0, 8.0, 13.0) | 2.32 dd (13.8, 17.4) 2.47 dd (3.6, 17.4) |
| 12 | 4.12 m | 4.05 m | 4.07 m | 4.07 m | 3.96 m | - | - | 2.27 brq (3.0) | 2.30 brs | 2.36 m | - |
| 13 | 2.48 brt (3.6) | 2.67 m | 2.04 brt (4.5) | 2.11 brt (4.0) | 2.10 brs | 2.77 brs | 2.60 brd (4.8) | 4.31 brd (9.0) | 3.89 ddt (1.5, 3.5, 10.0) | 3.60 m | 5.91 brs |
| 14 | 1.34 m 2.73 brd (13.2) | 1.24 dd (4.0, 12.5) 2.86 brd (12.5) | 0.71 dd (4.5, 12.5) 2.32 brd (12.5) | 0.77 dd (4.5, 13.0) 2.32 brd (13.0) | 1.27 dd (4.5, 12.0) 2.32 d (12.0) | 4.24 brs | 1.34 m 2.28 brd (13.2) | 1.52 ddd (1.8, 10.2, 15.0) 2.14 brdd (3.0, 15.0) | 1.31 m 1.98 dd (3.5, 15.0) | 3.99 m | 2.16 brd (17.4) 2.66 brd (17.4) |
| 15 | - | - | 3.10 brs | 3.18 brs | 3.43 brs | 3.65 brs | 3.31 brs | - | 3.47 brt (2.0) | 3.98 m | 4.77 d (1.8) |
| 16 | - | 2.63 m | - | - | - | - | - | - | - | - | |
| 17 | 3.40 d (11.4) 3.55 d (11.4) | 3.60 dd (8.0, 11.0) 3.92 dd (5.0, 11.0) | 3.29 d (11.0) 3.45 d (11.0) | 3.48 d (11.0) 3.62 d (11.0) | 3.67 d (11.0) 3.72 d (11.0) | 3.55 d (11.0) 3.60 d (11.0) | 3.33 d (11.4) 3.42 d (11.4) | 3.55 d (12.0) 3.62 d (12.0) | 5.08 t (1.5) 5.17 t (1.5) | 5.06 t (2.5) 5.13 t (2.5) | 4.14 d (14.4) 4.18 d (14.4) |
| 18 | 0.83 s | 0.87 s | 0.86 s | 0.86 s | 0.86 s | 0.85 s | 0.81 s | 0.85 s | 0.87 s | 0.87 s | 0.87 s |
| 19 | 0.87 s | 0.91 s | 0.89 s | 0.87 s | 0.88 s | 0.92 s | 0.88 s | 0.86 s | 0.90 s | 0.91 s | 0.89 s |
| 20 | 1.29 s | 1.31 s | 1.25 s | 1.25 s | 1.27 s | 0.84 s | 0.88 s | 1.21 s | 1.13 s | 1.05 s | 0.87 s |
| No | 1 a | 2 b | 3 b | 4 b | 5 b | 6 b | 7 a | 8 a | 9 b | 10 b | 11 a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39.2, CH2 | 39.0, CH2 | 40.1, CH2 | 40.1, CH2 | 40.0, CH2 | 39.7, CH2 | 39.5, CH2 | 39.4, CH2 | 39.6, CH2 | 39.6, CH2 | 37.9, CH2 |
| 2 | 18.0, CH2 | 17.8, CH2 | 18.2, CH2 | 18.1, CH2 | 18.1, CH2 | 18.0, CH2 | 17.9, CH2 | 17.7, CH2 | 17.9, CH2 | 17.9, CH2 | 17.9, CH2 |
| 3 | 41.7, CH2 | 41.7, CH2 | 41.9, CH2 | 41.9, CH2 | 41.9, CH2 | 41.5, CH2 | 41.6, CH2 | 41.7, CH2 | 41.9, CH2 | 41.8, CH2 | 41.6, CH2 |
| 4 | 32.7, qC | 32.7, qC | 32.8, qC | 32.8, qC | 32.7, qC | 32.8, qC | 32.7, qC | 32.5, qC | 32.5, qC | 32.5, qC | 32.7, qC |
| 5 | 55.2, CH | 55.4, CH | 55.1, CH | 55.0, CH | 55.2, CH | 55.4, CH | 55.1, CH | 55.2, CH | 55.2, CH | 55.4, CH | 55.8, CH |
| 6 | 17.8, CH2 | 18.3, CH2 | 19.7, CH2 | 19.7, CH2 | 19.9, CH2 | 18.9, CH2 | 19.4, CH2 | 17.6, CH2 | 18.2, CH2 | 17.7, CH2 | 18.9, CH2 |
| 7 | 33.1, CH2 | 33.5, CH2 | 39.8, CH2 | 39.7, CH2 | 39.8, CH2 | 77.1, CH | 37.8, CH2 | 29.8, CH2 | 39.6, CH2 | 37.4, CH2 | 39.2, CH2 |
| 8 | 51.5, qC | 52.6, qC | 45.2, qC | 45.7, qC | 46.9, qC | 51.9, qC | 46.3, qC | 45.4, qC | 38.9, qC | 44.9, qC | 43.1, qC |
| 9 | 55.4, CH | 54.1, CH | 47.5, CH | 47.4, CH | 47.6, CH | 45.1, CH | 47.1, CH | 47.7, CH | 42.7, CH | 43.0, CH | 47.9, CH |
| 10 | 38.3, qC | 38.1, qC | 37.6, qC | 37.5, qC | 37.5, qC | 38.8, qC | 38.7, qC | 38.8, qC | 37.4, qC | 37.4, qC | 36.6, qC |
| 11 | 26.4, CH2 | 26.2, CH2 | 26.4, CH2 | 26.2, CH2 | 26.4, CH2 | 38.4, CH2 | 37.9, CH2 | 14.5, CH2 | 20.9, CH2 | 21.4, CH | 26.8, CH2 |
| 12 | 65.6, CH | 64.8, CH | 66.3, CH | 66.1, CH | 65.7, CH | 212.5, qC | 214.3, qC | 41.0, CH2 | 44.3, CH | 44.8, CH | 173.9, qC |
| 13 | 43.9, CH | 39.5, CH | 46.6, CH | 48.1, CH | 51.1, CH | 65.6, CH | 57.4, CH | 63.2, CH | 67.4, CH | 77.2, CH | 131.8, CH |
| 14 | 27.0, CH2 | 30.9, CH2 | 28.8, CH2 | 28.7, CH2 | 28.7, CH2 | 30.5, CH2 | 33.4, CH2 | 34.1, CH2 | 37.8, CH2 | 73.2, CH2 | 37.0, CH2 |
| 15 | 223.0, qC | 222.7, qC | 80.3, CH | 81.3, CH | 89.2, CH | 70.9, CH | 79.0, CH | 216.0, qC | 74.8, CH | 67.9, CH | 95.3, CH |
| 16 | 81.2, qC | 55.2, CH | 75.8, qC | 75.2, qC | 81.1, qC | 77.5, qC | 76.9, qC | 75.2, qC | 155.1, qC | 153.1, qC | 142.2, qC |
| 17 | 64.0, CH2 | 58.1, CH2 | 69.1, CH2 | 53.9, CH2 | 63.2, CH2 | 69.4, CH2 | 68.1, CH2 | 64.0, CH2 | 110.0, CH2 | 109.0, CH2 | 58.2, CH2 |
| 18 | 20.4, CH3 | 20.4, CH3 | 20.7, CH3 | 20.6, CH3 | 20.6, CH3 | 20.5, CH3 | 20.5, CH3 | 20.5, CH3 | 20.9, CH3 | 20.8, CH3 | 20.5, CH3 |
| 19 | 32.6, CH3 | 32.6, CH3 | 32.7, CH3 | 32.7, CH3 | 32.7, CH3 | 32.5, CH3 | 32.4, CH3 | 32.4, CH3 | 32.6, CH3 | 32.5, CH3 | 32.4, CH3 |
| 20 | 15.2, CH3 | 15.2, CH3 | 15.4, CH3 | 15.4, CH3 | 15.5, CH3 | 15.5, CH3 | 15.4, CH3 | 15.4, CH3 | 15.0, CH3 | 15.0, CH3 | 13.7, CH3 |
| Compounds | IC50 Values * (µM) | |||||
|---|---|---|---|---|---|---|
| A549 | Hep-3B | PC-3 | HT29 | U937 | RAW 264.7 | |
| 1 | 17.36 ± 0.28 | 12.08 ± 0.55 | 12.47 ± 0.24 | 9.10 ± 0.38 | 2.66 ± 0.14 | >50 |
| 2 | 7.39 ± 0.42 | 7.06 ± 0.38 | 4.19 ± 0.31 | 2.78 ± 0.03 | 1.97 ± 0.09 | >50 |
| 3 | 17.12 ± 0.36 | 17.08 ± 0.73 | 16.49 ± 0.55 | 14.18 ± 0.31 | 6.73 ± 0.36 | >50 |
| 4 | 19.39 ± 0.38 | 19.86 ± 0.73 | 15.96 ± 0.21 | 12.64 ± 0.55 | 8.25 ± 0.24 | >50 |
| 5 | >20 | >20 | >20 | >20 | >20 | >50 |
| 6 | >20 | >20 | >20 | >20 | >20 | >50 |
| 7 | >20 | >20 | >20 | >20 | >20 | >50 |
| 8 | (-) a | (-) a | (-) a | (-) a | (-) a | (-) a |
| 9 | >20 | >20 | >20 | >20 | >20 | >50 |
| 10 | >20 | >20 | >20 | >20 | >20 | >50 |
| 11 | >20 | >20 | >20 | >20 | >20 | >50 |
| CPT-11 | 15.26 ± 0.42 | 23.21 ± 0.38 | 31.03 ± 0.28 | 15.11 ± 0.48 | 4.95 ± 0.43 | (-) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-T.; Liaw, C.-C.; Lin, Y.-C.; Liao, G.-Y.; Chao, C.-H.; Chiou, C.-T.; Kuo, Y.-H.; Lee, K.-T. New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells. Pharmaceuticals 2021, 14, 1108. https://doi.org/10.3390/ph14111108
Huang H-T, Liaw C-C, Lin Y-C, Liao G-Y, Chao C-H, Chiou C-T, Kuo Y-H, Lee K-T. New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells. Pharmaceuticals. 2021; 14(11):1108. https://doi.org/10.3390/ph14111108
Chicago/Turabian StyleHuang, Hung-Tse, Chia-Ching Liaw, Yu-Chi Lin, Geng-You Liao, Chih-Hua Chao, Chun-Tang Chiou, Yao-Haur Kuo, and Kung-Ta Lee. 2021. "New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells" Pharmaceuticals 14, no. 11: 1108. https://doi.org/10.3390/ph14111108
APA StyleHuang, H.-T., Liaw, C.-C., Lin, Y.-C., Liao, G.-Y., Chao, C.-H., Chiou, C.-T., Kuo, Y.-H., & Lee, K.-T. (2021). New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells. Pharmaceuticals, 14(11), 1108. https://doi.org/10.3390/ph14111108







