Initial Experience of Clinical Use of [99mTc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Subject Population
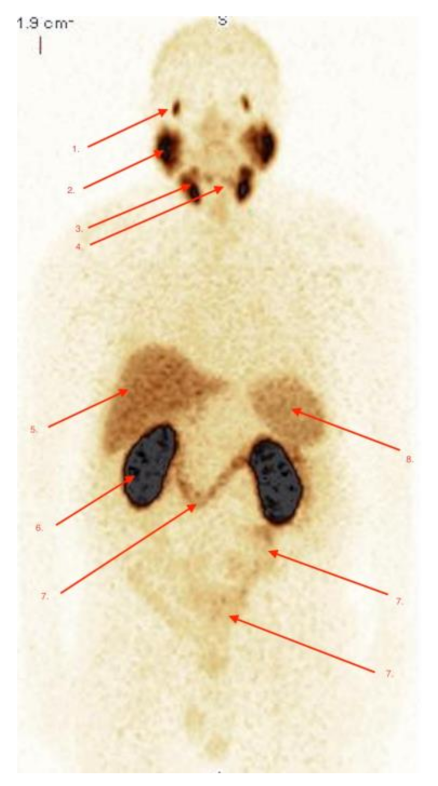
2.2. Biodistribution of Radiopharmaceutical
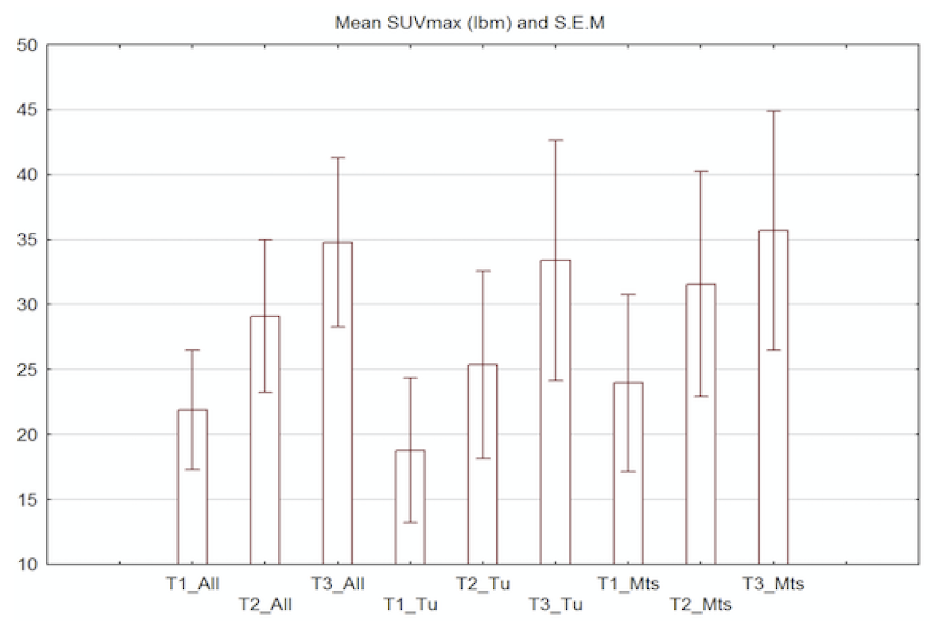
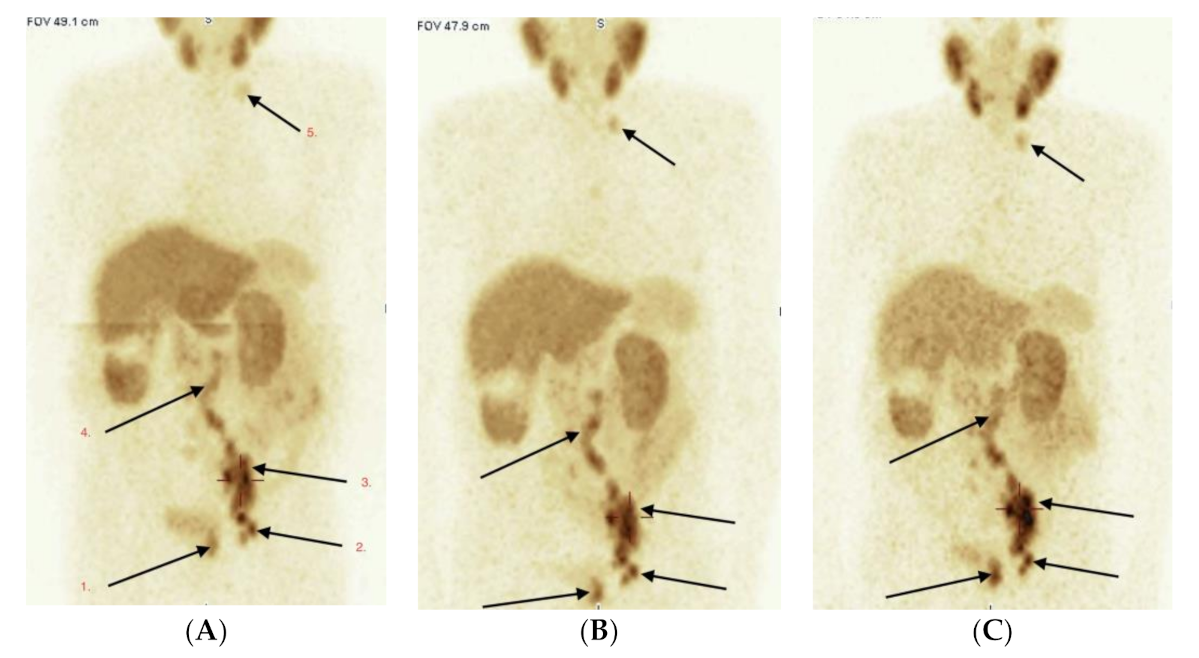
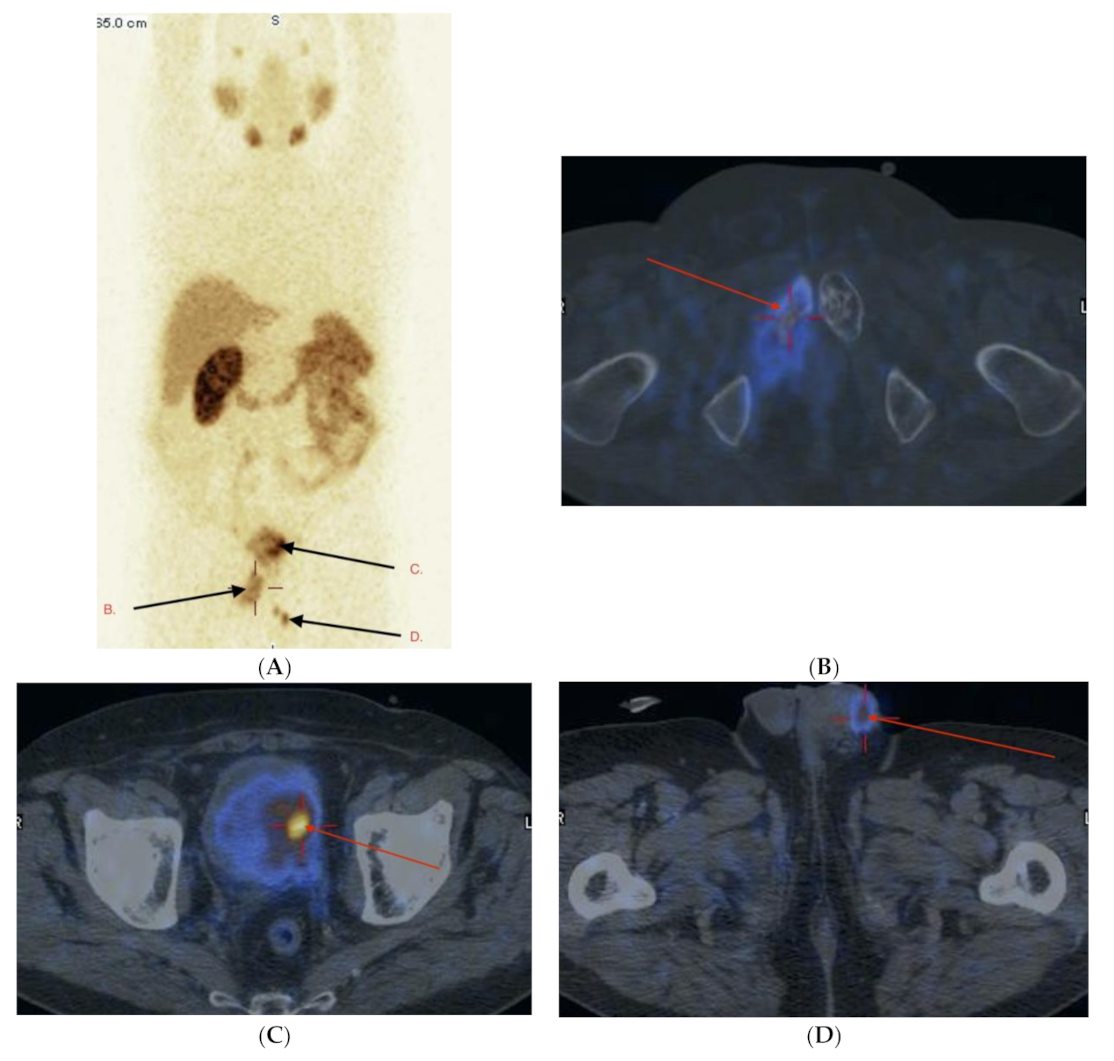
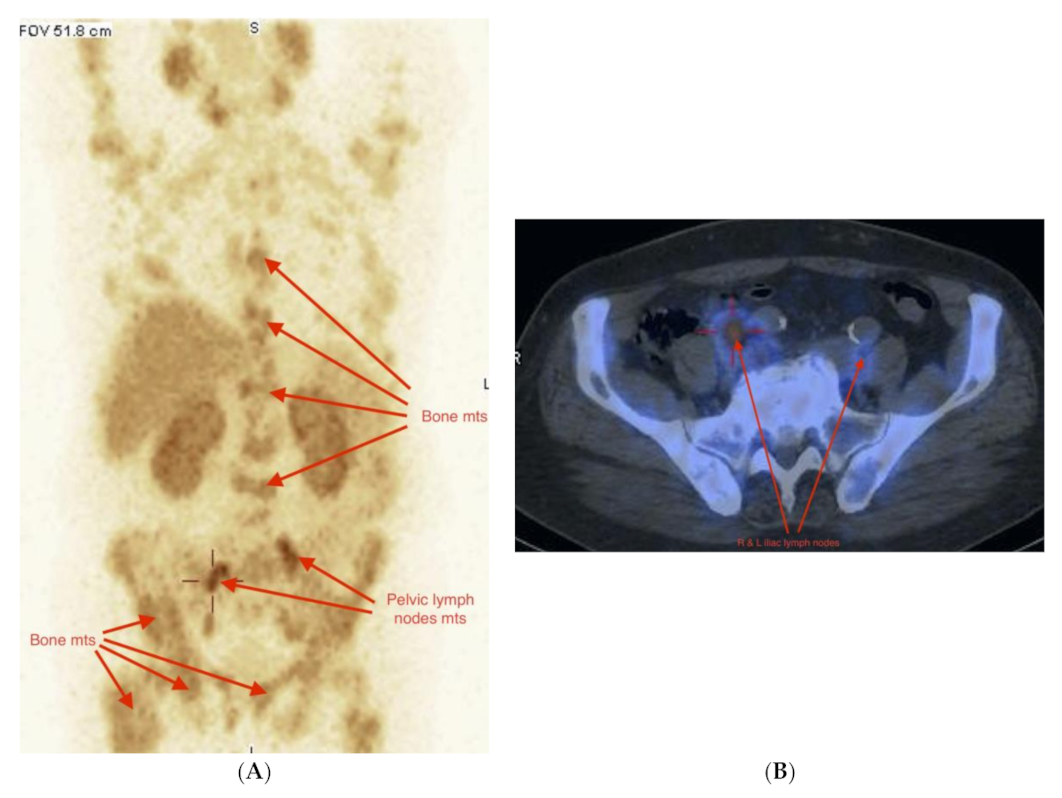
2.3. Imaging Studies
2.4. Detection of Active Pathology in Patients
2.5. Adverse Events (Safety)
3. Discussion
4. Materials and Methods
4.1. Study Drug
4.1.1. PSMA-T4 Freeze-Dried Kit for Radiopharmaceutical Preparation
4.1.2. Preparation of [99mTc]Tc-PSMA-T4 and Evaluation of Radiochemical Purity
4.2. Image Acquisition
4.3. Image Analysis
4.4. Adverse Events (Safety)
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Pt No | Age | Indication | GlS ** | R/P # | Primary Diagnosis | TNM $ | PSA before Scan (ng/mL) | Doubling Time PSA (Months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 53 | Stage | 4 + 5 | P | 21.01.2014 | cTxN1M1 | 4.38 | n.d. |
| 2 | 65 | Rec | 5 + 3 | R | 01.02.2014 | pT3bN0Mx | 0.1 | >12.0 |
| 3 | 66 | Stage | 3 + 4 | P | 01.06.2014 | cTxN1M1 | 35.2 | |
| 4 | 65 | Rec | 4 + 3 | R | 12.04.2018 | pT3bN0Mx | 10.4 | 1.2 |
| 5 | 67 | Rec | 4 + 3 | R | 02.03.2018 | pT3aN0M0 | 1.41 | n.d. |
| 6 | 66 | Rec | 5 + 4 | R | 11.05.2016 | pT3bN0Mx | 1.73 | 4.7 |
| 7 | 78 | Stage | 5 + 5 | P | 27.03.2019 | cTxNxM1 | 10.2 | |
| 8 | 72 | Rec | 3 + 4 | R | 22.12.2015 | pT2aN0M0 | 1.54 | 2.0 |
| 9 | 56 | Stage * | 4 + 4 | R | 30.04.2019 | pT3bN1Mx | 25.9 | |
| 10 | 69 | Stage | 4 + 3 | P | 13.04.2018 | cT4N1M1 | 54.8 | n.d. |
| 11 | 72 | Rec | 4 + 3 | R | 30.11.2018 | pT2cN0Mx | 0.48 | n.d. |
| 12 | 76 | Rec | 4 + 5 | R(Rtx) | 23.05.2013 | cT2cNxM0 | 0.28 | >12.0 |
| 13 | 66 | Stage | 4 + 5 | P | 02.02.2012 | cTxNxM1 | 0.35 | n.d. |
| 14 | 59 | Rec | 3 + 3 | R(Rtx) | 04.06.2008 | cT2cNxM0 | 0.84 | >12.0 |
| 15 | 69 | Rec | 3 + 4 | R | 30.07.2019 | pT3bN1Mx | 9.39 | 4.1 |
| 16 | 68 | Rec | 3 + 4 | R | 17.10.2018 | pT3bN1Mx, | 0.1 | |
| 17 | 63 | Rec | 3 + 5 | R | 26.02.2013 | pT3cN1Mx | 5.99 | n.d. |
| 18 | 69 | Rec | 3 + 5 | R | 01.09.2017 | pT3bN0M0 | 5.68 | 10.0 |
| 19 | 68 | Rec | 4 + 3 | R | 08.04.2019 | pT2bN0Mx | 0.23 | |
| 20 | 81 | Stage | 3 + 3 | P | 18.04.2018 | cTxNxM1 | 29.6 | >12.0 |
| 21 | 67 | Rec | 4 + 3 | R | 25.05.2018 | pT3bN1Mx | 12.4 | n.d. |
| 22 | 71 | Stage | 4 + 5 | P | 29.03.2019 | cTxN1M1 | 7.2 | |
| 23 | 74 | Rec | 4 + 3 | R | 20.11.2018 | pT3bN0M0 | 0.2 | n.d. |
| 24 | 66 | Stage * | 4 + 3 | P | 29.05.2019 | cT3bN1Mx | 6.94 | >12.0 |
| 25 | 66 | Rec | 3 + 4 | R | 10.03.2015 | pT2aN0M0 | 1.6 | n.d. |
| 26 | 62 | Rec | 3 + 4 | R | 02.04.2014 | pT2CN0M0 | 0.12 | 9.0 |
| 27 | 87 | Stage | 4 + 4 | P | 18.03.2019 | cTxNxM1 | 228.0 | >12.0 |
| 28 | 59 | Rec | 3 + 3 | R | 03.08.2005 | pT2cN0Mx | 3.3 | 3.0 |
| 29 | 69 | Rec | 5 + 5 | R | 20.12.2018 | pT3bN1Mx | 0.14 | |
| 30 | 66 | Rec | 3 + 4 | R | 10.06.2016 | pT2cN0Mx | 0.02 | 5.0 |
| 31 | 76 | Stage * | 4 + 3 | R | 01.02.2019 | pT3bN0Mx | 6.63 | >12.0 |
| Therapy | Number of Subjects n (%) |
|---|---|
| Radical prostatectomy | 22 (71) |
| Palliative treatment including different combination of radiotherapy, chemotherapy, hormonotherapy | 9 (29) |
| Radical surgery (Intention to Treat–ITT) | 20 (65) |
| R0 | 10 (50) |
| R1/R2 | 10/0 (50/0) |
| Radical Radiotherapy (Rtx) | 2 (10) |
| Gleason Score (GlS) n = 31 | Number of subjects n (%) |
| GLS = 6 (3 + 3) | 3 (10) |
| GLS = 7 (3 + 4 or 4 + 3) | 16 (52) |
| GLS = 8 (3 + 5; 4 + 4 or 5 + 3) | 5 (16) |
| GlS = 9 (4 + 5 or 5 + 4) | 5 (16) |
| GlS = 10 (5 + 5) | 2 (6) |
| Risk group | Number of subjects n (%) |
| Intermediate | 19 (62) |
| High | 12 (32) |
| Indication to Scanning n (%) | Number of subjects n (%) |
| Evaluation of disease extent—palliative settings | 8 (25) |
| Evaluation of disease extent—before radical therapy | 3 (10) |
| Evaluation of Recurrence after radical therapy | 20 (65) |
| PSA at imaging (mean, range) | Mean (range) |
| Radical therapy | 3.34 (0.02–12.4) |
| Palliative | 52.3 (7.2–228) |
| Target are of Interpretation of Results [99mTc]Tc-PSMA-T4 Scan | True Positive n: Sensitivity (%) | True Negative n; Specificity (%) | False Negative n Negative Predictive Value (%) | False Positive n; Positive Predictive Value (%) |
|---|---|---|---|---|
| Prostate (n = 15) | 11 (92) | 3 (100) | 1 (75) | 0 (100) |
| Pelvic lymph nodes (per patient) | 10 (83) | 19 (100) | 2 (91) | 0 (100) |
| Abdominal/thoracic/other lymph nodes or soft tissue involvement (per patient) | 9 (100) | 21 (95) | 0 (100) | 1 (90) |
| Bone mts (per patient) | 11 (100) | 20 (100) | 0 (100) | 0 (100) |
References
- Shariat, S.F.; Scardino, P.T.; Lilja, H. Screening for prostate cancer: An update. Can. J. Urol. 2008, 15, 4363–4374. [Google Scholar]
- Hricak, H.; Choyke, P.L.; Eberhardt, S.C.; Leibel, S.A.; Scardino, P.T. Imaging prostatę cancer: A multidisciplinary perspective. Radiology 2007, 243, 28–53. [Google Scholar] [CrossRef]
- Hou, A.H.; Swanson, D.; Barqawi, A.B. Modalities for Imaging of Prostate Cancer. Adv. Urol. 2009, 2009, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Toegel, S.; Hoffmann, O.; Wadsak, W.; Ettlinger, D.; Mien, L.-K.; Wiesner, K.; Nguemo, J.; Viernstein, H.; Kletter, K.; Dudczak, R.; et al. Uptake of bone-seekers is solely associated with mineralization. A study with 99mTc-MDP, 153Sm-EDTMP and 18F-fluoride on osteoblasts. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef]
- Israeli, R.S.; Powell, C.T.; Fair, W.R.; Heston, W.D. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993, 53, 227–230. [Google Scholar]
- Horoszewicz, J.S.; Kawinski, E.; Murphy, G.P. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer. Res. 1987, 7, 927–935. [Google Scholar] [PubMed]
- Gomes, C.; Oliveira, J.M.; Faria, D.B.; Vieira, T.S.; Silva, F.A.; Vale, J.; Pimentel, F.L. 68Ga-prostate-specific membrane antigen positron emission tomography/computed tomography for prostate cancer imaging: A narrative literature review. World J. Nucl. Med. 2017, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schafer, M.A.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel Preclinical and Radiopharmaceutical Aspects of [68Ga]Ga-PSMA-HBED-CC: A New PET Tracer for Imaging of Prostate Cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenboeck, S.M.; Rauscher, I.; Bluemel, C.; Fendler, W.P.; Rowe, S.P.; Pomper, M.G.; Asfhar-Oromieh, A.; Herrmann, K.; Eiber, M. PSMA Ligands for PET Imaging of Prostate Cancer. J. Nucl. Med. 2017, 58, 1545–1552. [Google Scholar] [CrossRef] [Green Version]
- Pinto, J.T.; Suffoletto, B.P.; Berzin, T.; Qiao, C.H.; Lin, S.; Tong, W.P.; May, F.; Mukherjee, B.; Heston, W.D. Prostate-specific membrane antigen: A novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 1996, 2, 1445–1451. [Google Scholar] [PubMed]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.; Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Perner, S.; Hofer, M.D.; Kim, R.; Shah, R.B.; Li, H.; Möller, P.; Hautmann, R.E.; Gschwend, J.E.; Kuefer, R.; Rubin, M.A. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum. Pathol. 2007, 38, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.L., Jr.; Grob, B.M.; Haley, C.; Grossman, K.; Newhall, K.; Petrylak, D.; Troyer, J.; Konchuba, A.; Schellhammer, P.F.; Moriarty, R. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology 1996, 48, 326–334. [Google Scholar] [CrossRef]
- Ross, J.S.; Sheehan, C.E.; Fisher, H.A.G.; Kaufamn, R.P., Jr.; Kaur, P.; Grey, K.; Webb, I.; Gray, G.S.; Mosher, R.; Kallakuri, B.V.S. Correlation of primary tumor prostatę specific mem-brane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 6357–6362. [Google Scholar]
- Vallabhajosula, S.; Kuji, I.; Hamacher, K.A.; Konishi, S.; Kostakoglu, L.; Kothari, P.A.; Milowski, M.I.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: Prediction of 90Y-J591 radiation dosim-etry based on 111In or 177Lu? J. Nucl. Med. 2005, 46, 634–641. [Google Scholar]
- Kabasakal, L.; Demirci, E.; Ocak, M.; Akyel, R.; Nematyazar, J.; Aygun, A.; Halac, M.; Talat, Z.; Araman, A. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 2015, 36, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; Gage, K.L.; Mease, R.C.; Senthamizhchelvan, S.; Holt, D.P.; Jeffrey-Kwanisai, A.; Endres, C.J.; Dannals, R.F.; Sgouros, G.; Lodge, M.; et al. Biodistribution, tumor detection, and radiation dosimetry of 18F DCFBC, a low-molecular-weight inhibitor of prostatespecific membrane antigen, in patients. J. Nucl. Med. 2012, 53, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Afshar-Oromieh, A.; Zechmann, C.M.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Holland-Letz, T.; Hadaschik, B.A.; Giesel, F.L.; Debus, J.; et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Hillier, S.M.; Maresca, K.P.; Femia, F.J.; Marquis, J.C.; Foss, C.A.; Nguyen, N.; Zimmerman, C.N.; Barrett, J.A.; Eckelman, W.C.; Pomper, M.G.; et al. Preclinical evaluation of novel glutamateurea- lysine analogs that target prostate specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009, 69, 6932–6940. [Google Scholar] [CrossRef] [Green Version]
- Maresca, K.P.; Hillier, S.M.; Lu, G.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. Small molecule inhibitors of PSMA incorporating technetium-99m for imaging prostate cancer: Effects of chelate design on pharmacokinetics. Inorg. Chim. Acta 2012, 389, 168–175. [Google Scholar] [CrossRef]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen for molecular imaging of prostate cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.R.; Foss, C.A.; Castanares, M.; Meanse, R.C.; Byun, Y.; Fox, J.J.; Hilton, J.; Lupoid, S.E.; Kazikowski, A.P.; Pomper, M.G. Synthesis and evaluation of technetium-99m- and rheni-um-labeled inhibitors of the prostate-specific membrane antigen (PSMA). J. Med. Chem. 2008, 51, 4504–4517. [Google Scholar] [CrossRef] [Green Version]
- Kularatne, S.A.; Zhou, Z.; Yang, J.; Post, C.B.; Low, P.S. Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted 99mTc-radioimaging agents. Mol. Pharm. 2009, 6, 790–800. [Google Scholar] [CrossRef]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.J. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [PubMed] [Green Version]
- Xiaoping, X.; Zhang, J.; Hu, S.; He, S.; Bao, X.; Ma, X.; Luo, J.; Cheng, J.; Zhang, Y. 99mTc-labeling and evaluation of a HYNIC modified small-molecular inhibitor of prostate-specific membrane antigen. Nucl. Med. Biol. 2017, 48, 69–75. [Google Scholar]
- Ferro-Flores, G.; Luna-Gutierreez, M.; Ocampo-Garcia, B.; Saantos-Cuevas, C.; Azorin-Vega, E.; Jimenez-Mancilla, N.; Orocio-Rodrigues, E.; Davanzo, J.; Garcia-Perez, F.O. Clinical transla-tion of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, F.O.; Davanzo, J.; López-Buenrostro, S.; Santos-Cuevas, C.; Ferro-Flores, G.; Jímenez-Ríos, M.A.; Scavuzzo, A.; Santana-Ríos, Z.; Medina-Ornelas, S. Head to head comparison performance of 99mTc-EDDA/HYNIC-iPSMA SPECT/CT and 68Ga-PSMA-11 PET/CT a prospective stud y in biochemical recurrence prostate cancer patients. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 332–340. [Google Scholar]
- Vallabhajosula, S.; Nikolopoulou, A.; Babich, J.W.; Osborne, J.R.; Tagawa, S.T.; Lipaa, I.; Solnes, L.; Maresca, K.P.; Armor, T.; Joyal, J.L.; et al. 99mTc-Labeled Small-Molecule Inhibitors of Prostate-Specific Membrane Antigen: Pharmacokinetics and Biodistribution Studies in Healthy Subjects and Patients with Metastatic Prostate Cancer. J. Nucl. Med. 2014, 55, 1791–1798. [Google Scholar] [CrossRef] [Green Version]
- Maurer, T.; Graefen, M.; van der Poel, H.; Hamdy, F.; Briganti, A.; Eiber, M.; Wester, J.-J.; Leeuwen, A.B.J. Prostate Specific Membrane Antigen—Guided Surgery. JNM 2020, 61, 6–12. [Google Scholar] [CrossRef]
- Sikora, A.E.; Maurin, M.; Jaron, A.W.; Pijarowska-Kruszyna, J.; Orzelowska, M.; Janota, B.; Radzik, M.; Garnuszek, P. PSMA Inhibitor Derivatives for Labelling with 99mTc via HYNIC, a Radiopharmaceutical Kit, Radiopharmaceutical Preparations and Their Use in Prostate Cancer Diagnostics. European Patent Bulletin Number EP3721907A1, 14 October 2020. [Google Scholar]
- Sergieva, S.; Mangaldjiev, R.; Dimcheva, M.; Bozhil, R. SPECT-Computed Tomography with New 99mTc Prostate-Specific Membrane Antigen-T4 Tracer in Patients with Recurrent Prostate Cancer. World J. Nucl. Med. 2019, 18, 317–323. [Google Scholar]
- Sergieva, S.; Mangaldgiev, R.; Dimcheva, M.; Nedev, K.; Zahariev, Z.; Robev, B. SPECT-CT Imaging with 99mTc-PSMA-T4 in patients with Reccurrent Prostate Cancer. Nucl. Med. Rev. 2021, 24, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Benešová, M.; Bauder-Wüst, U.; Schäfer, M.; Klika, K.D.; Mier, W.; Haberkorn, U.; Kopka, K.; Eder, M. Linker Modification Strategies to Control the Pros-tate-Specific Membrane Antigen (PSMA)-Targeting and Pharmacokinetic Properties of DOTA-Conjugated PSMA Inhibitors. J. Med. Chem. 2016, 59, 1761–1775. [Google Scholar] [CrossRef]
- Hovels, M.; Heesakkers, A.; Adang, M.; Jager, G.J.; Hoogeveen, Y.L.; Severenes, J.L.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Maurer, T.; Gschwend, J.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.-J.; Heck, M.; Kubler, H.; Beer, A.J.; et al. Diagnostic efficacy of 68gallium-PSMA PET compared to conventional imaging in lymph node staging of 130 consecutive patients with intermediate to high-risk prostate cancer. J. Urol. 2015, 195, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćwikła, J.B.; Roslan, M.; Skoneczna, I.; Kempińska-Wróbel, M.; Maurin, M.; Rogowski, W.; Janota, B.; Szarowicz, A.; Garnuszek, P. Initial Experience of Clinical Use of [99mTc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study. Pharmaceuticals 2021, 14, 1107. https://doi.org/10.3390/ph14111107
Ćwikła JB, Roslan M, Skoneczna I, Kempińska-Wróbel M, Maurin M, Rogowski W, Janota B, Szarowicz A, Garnuszek P. Initial Experience of Clinical Use of [99mTc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study. Pharmaceuticals. 2021; 14(11):1107. https://doi.org/10.3390/ph14111107
Chicago/Turabian StyleĆwikła, Jarosław B., Marek Roslan, Iwona Skoneczna, Monika Kempińska-Wróbel, Michał Maurin, Wojciech Rogowski, Barbara Janota, Anna Szarowicz, and Piotr Garnuszek. 2021. "Initial Experience of Clinical Use of [99mTc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study" Pharmaceuticals 14, no. 11: 1107. https://doi.org/10.3390/ph14111107
APA StyleĆwikła, J. B., Roslan, M., Skoneczna, I., Kempińska-Wróbel, M., Maurin, M., Rogowski, W., Janota, B., Szarowicz, A., & Garnuszek, P. (2021). Initial Experience of Clinical Use of [99mTc]Tc-PSMA-T4 in Patients with Prostate Cancer. A Pilot Study. Pharmaceuticals, 14(11), 1107. https://doi.org/10.3390/ph14111107






