Opioid Analgesia and Opioid-Induced Adverse Effects: A Review
Abstract
1. Introduction
1.1. Pain
1.2. Opioid Receptors
1.3. Endogenous Opioid Ligands
1.4. Exogenous Opioid Ligands
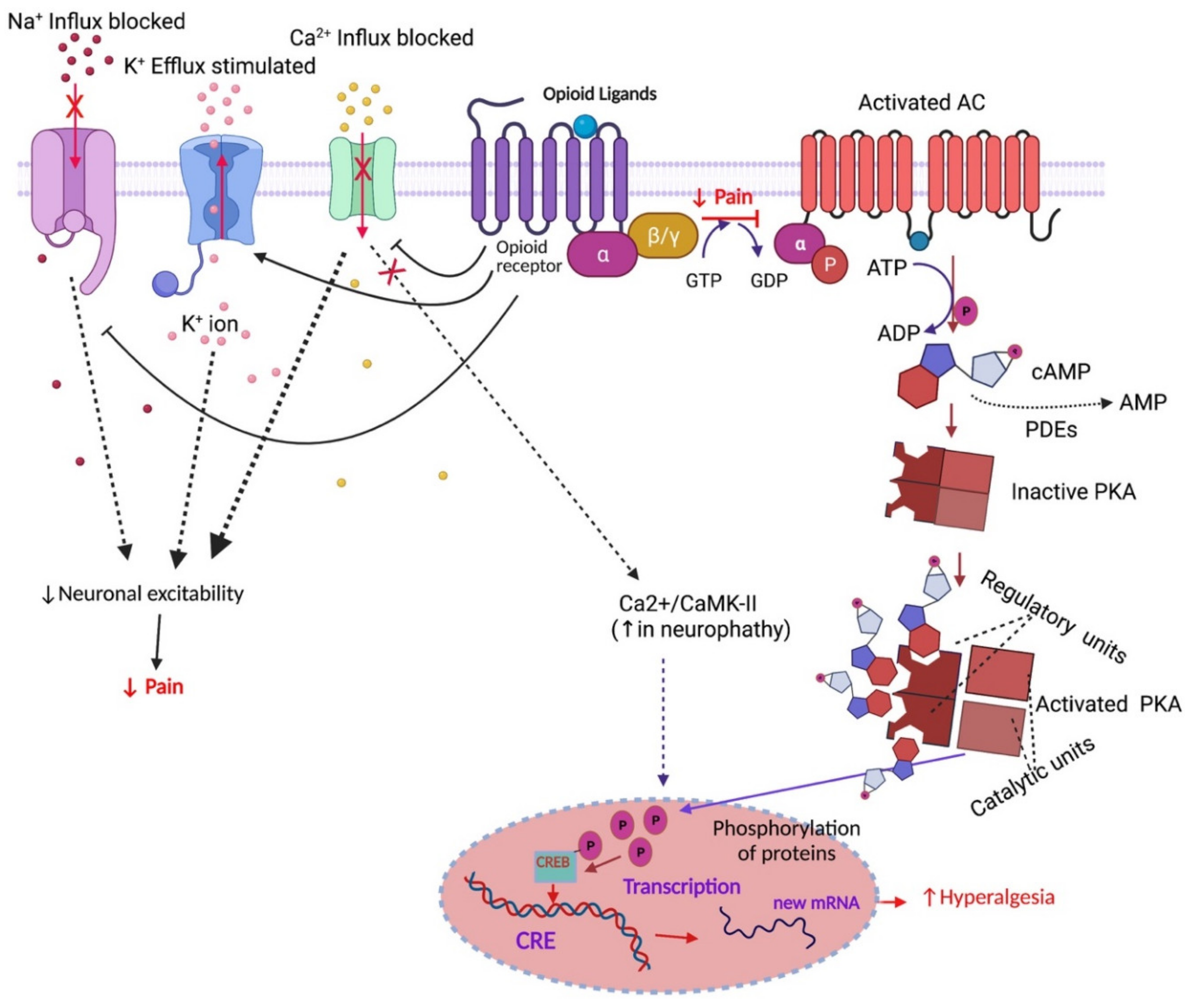
1.5. Opioid Receptor-Mediated Signalling Pathways
2. Opioid-Induced Adverse Effects
2.1. Analgesic Tolerance
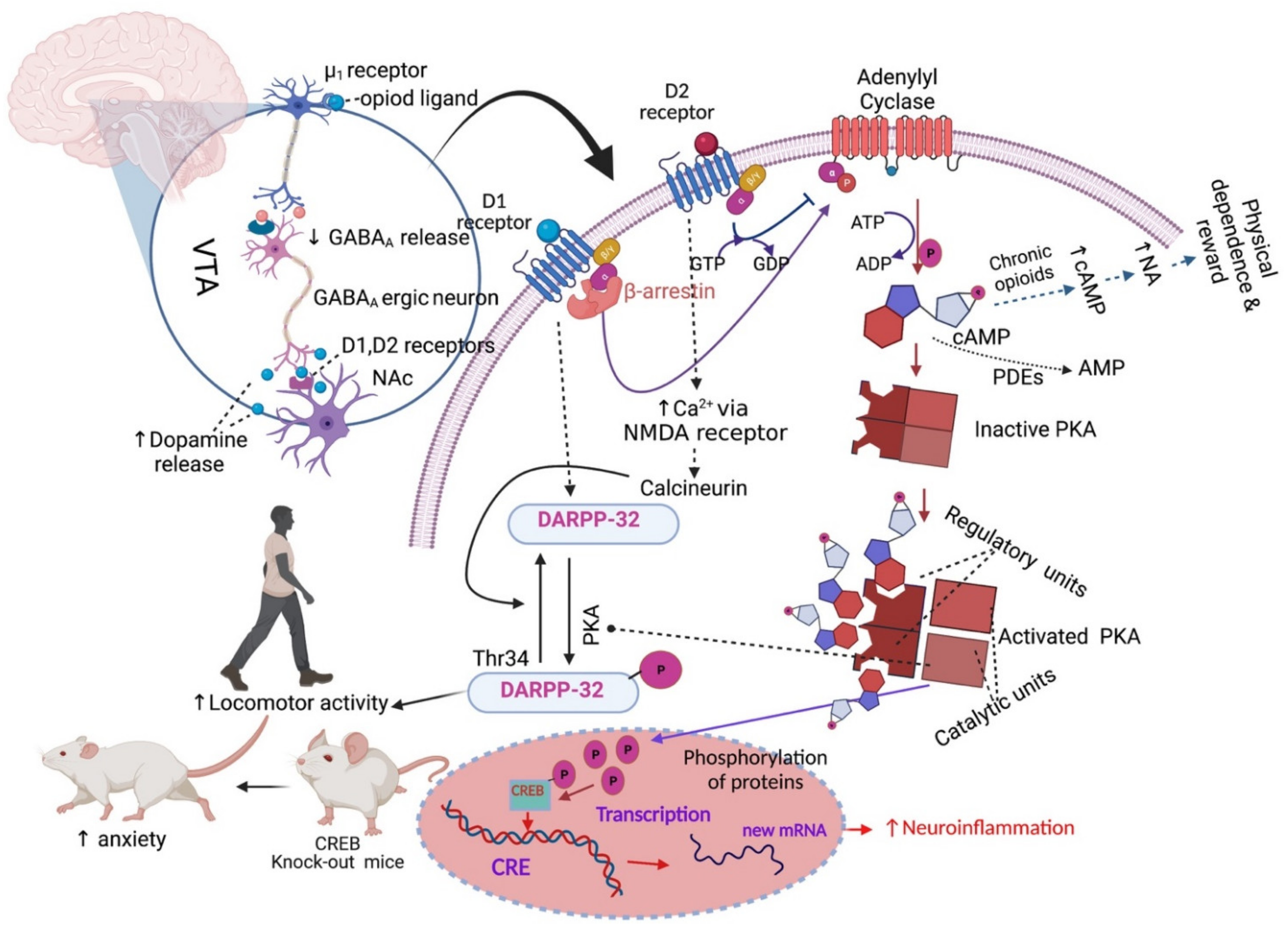
2.2. Addiction and Physical Dependence
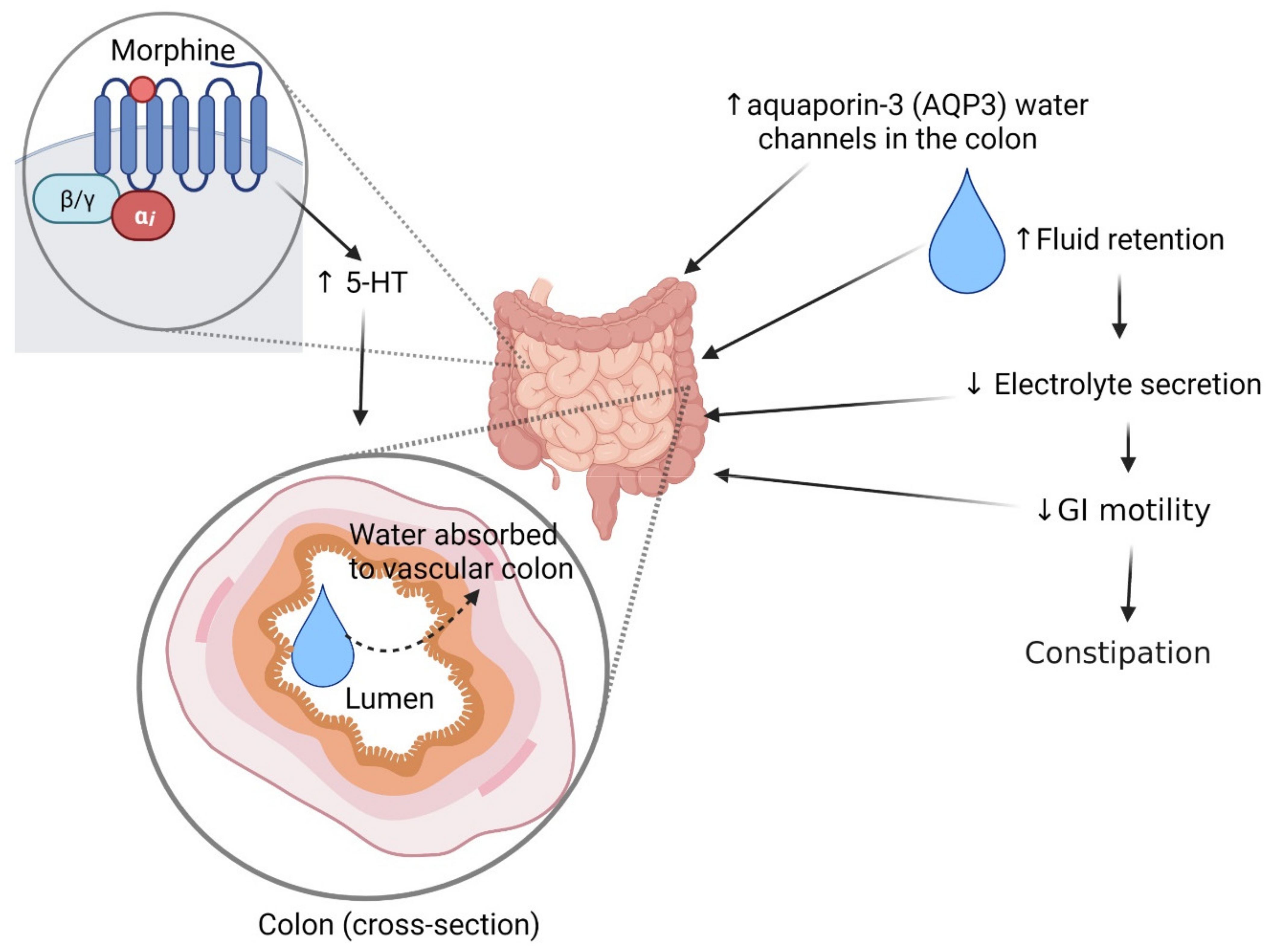
2.3. Constipation
2.4. Nausea and Vomiting
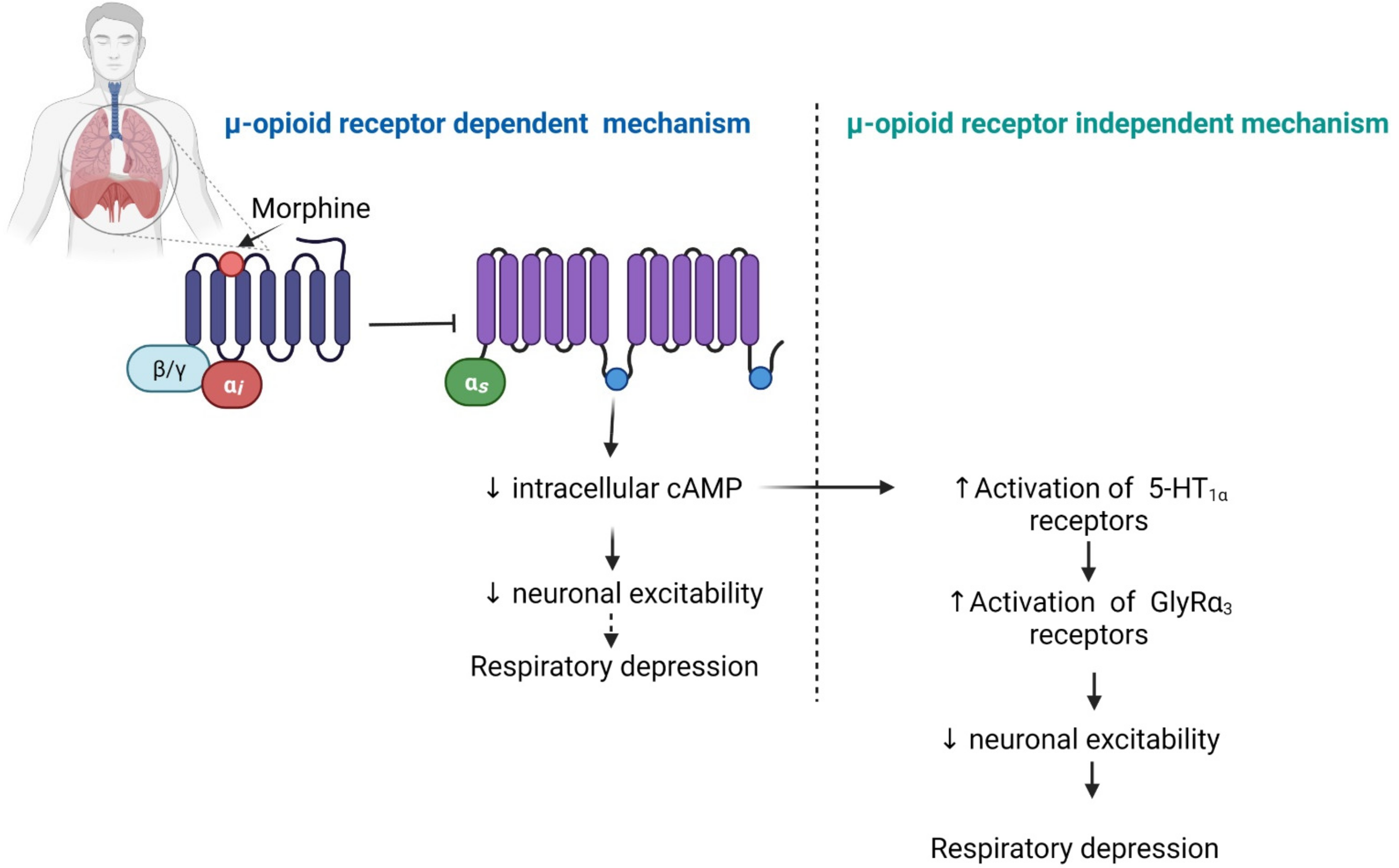
2.5. Respiratory Depression
2.6. Other Adverse Effects
3. Discussion and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IASP. Pain, IASP Terminology. International Association for the Study of Pain (IASP). Available online: https://www.iasp-pain.org/resources/terminology/#pain (accessed on 26 October 2021).
- Woolf, C.J.; Bennett, G.J.; Doherty, M.; Dubner, R.; Kidd, B.; Koltzenburg, M.; Lipton, R.; Loeser, J.D.; Payne, R.; Torebjork, E. Towards a mechanism-based classification of pain? Pain 1998, 77, 227–229. [Google Scholar] [CrossRef]
- Woolf, C.J.; Mannion, R.J. Neuropathic pain: Aetiology, symptoms, mechanisms, and management. Lancet 1999, 353, 1959–1964. [Google Scholar] [CrossRef]
- Fitzcharles, M.-A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef]
- Nijs, J.; Lahousse, A.; Kapreli, E.; Bilika, P.; Saraçoğlu, I.; Malfliet, A.; Coppieters, I.; De Baets, L.; Leysen, L.; Roose, E.; et al. Nociplastic pain criteria or recognition of central sensitization? Pain phenotyping in the past, present and future. J. Clin. Med. 2021, 10, 3203. [Google Scholar] [CrossRef]
- IASP. Central Sensitization, IASP Terminology. Available online: https://www.iasp-pain.org/resources/terminology/?navItemNumber=576#Centralsensitization (accessed on 25 August 2021).
- Cai, Z.; Ratka, A. Opioid system and Alzheimer’s disease. Neuromol. Med. 2012, 14, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82, S121–S126. [Google Scholar] [CrossRef]
- Holden, J.E.; Jeong, Y.; Forrest, J.M. The endogenous opioid system and clinical pain management. AACN Clin. Issues Adv. Pr. Acute Crit. Care 2005, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Beckett, A.H.; Casy, A.F. Synthetic analgesics: Stereochemical considerations. J. Pharm. Pharmacol. 2011, 6, 986–1001. [Google Scholar] [CrossRef]
- Martin, W.R.; Eades, C.G.; Thompson, J.A.; Huppler, R.E.; Gilbert, P.E. The effects of morphine-and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Borsodi, A.; Bruchas, M.; Caló, G.; Chavkin, C.; Christie, M.J.; Civelli, O.; Connor, M.; Cox, B.M.; Devi, L.A.; Evans, C.; et al. Opioid Receptors, Introduction. IUPHAR/BPS Guide to PHARMACOLOGY. Available online: http://www.guidetoimmunopharmacology.org/GRAC/FamilyIntroductionForward?familyId=50 (accessed on 26 October 2021).
- Alexander, S.P.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.; Spedding, M.; Peters, J.A.; Harmar, A.J.; Collaborators, C. The concise guide to PHARMACOLOGY 2013/14: G Protein-Coupled receptors. Br. J. Pharmacol. 2013, 170, 1459–1581. [Google Scholar] [CrossRef] [PubMed]
- Waldhoer, M.; Bartlett, S.E.; Whistler, J.L. Opioid receptors. Annu. Rev. Biochem. 2004, 73, 953–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mestek, A.; Liu, J.; Yu, L. Molecular cloning of a rat kappa opioid receptor reveals sequence similarities to the mu and delta opioid receptors. Biochem. J. 1993, 295, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Mollereau, C.; Parmentier, M.; Mailleux, P.; Butour, J.-L.; Moisand, C.; Chalon, P.; Caput, D.; Vassart, G.; Meunier, J.-C. ORL1, a novel member of the opioid receptor family. FEBS Lett. 1994, 341, 33–38. [Google Scholar] [CrossRef]
- Granier, S.; Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Weis, W.; Kobilka, B.K. Structure of the δ-opioid receptor bound to naltrindole. Nat. Cell Biol. 2012, 485, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Filizola, M.; Devi, L.A. How opioid drugs bind to receptors. Nat. Cell Biol. 2012, 485, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Manglik, A.; Kruse, A.C.; Kobilka, T.S.; Thian, F.S.; Mathiesen, J.M.; Sunahara, R.K.; Pardo, L.; Weis, W.; Kobilka, B.K.; Granier, S. Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nat. Cell Biol. 2012, 485, 321–326. [Google Scholar] [CrossRef]
- Thompson, A.A.; Liu, W.; Chun, E.; Katritch, V.; Wu, H.; Vardy, E.; Huang, X.-P.; Trapella, C.; Guerrini, R.; Calo, G.; et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nat. Cell Biol. 2012, 485, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wacker, D.; Mileni, M.; Katritch, V.; Han, G.W.; Vardy, E.; Liu, W.; Thompson, A.A.; Huang, X.-P.; Carroll, F.I.; et al. Structure of the human κ-opioid receptor in complex with JDTic. Nat. Cell Biol. 2012, 485, 327–332. [Google Scholar] [CrossRef]
- Matthes, H.W.D.; Maldonado, R.; Simonin, F.; Valverde, O.; Slowe, S.; Kitchen, I.; Befort, K.; Dierich, A.; Le Meur, M.; Dollé, P.; et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nat. Cell Biol. 1996, 383, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, B.L. Opioids: First lessons from knockout mice. Trends Pharmacol. Sci. 1999, 20, 19–26. [Google Scholar] [CrossRef]
- Sora, I.; Takahashi, N.; Funada, M.; Ujike, H.; Revay, R.S.; Donovan, D.M.; Miner, L.L.; Uhl, G.R. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc. Natl. Acad. Sci. USA 1997, 94, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.D.; Henderson, G.; McKnight, A.T.; Paterson, S.J. 75 years of opioid research: The exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006, 147, S153–S162. [Google Scholar] [CrossRef]
- Williams, J.T.; Ingram, S.L.; Henderson, G.; Chavkin, C.; Von Zastrow, M.; Schulz, S.; Koch, T.; Evans, C.J.; Christie, M.J. Regulation of µ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 2013, 65, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Machelska, H.; Celik, M.Ö. Advances in achieving opioid analgesia without side effects. Front. Pharmacol. 2018, 9, 1388. [Google Scholar] [CrossRef]
- Darcq, E.; Kieffer, B.L. Opioid receptors: Drivers to addiction? Nat. Rev. Neurosci. 2018, 19, 499–514. [Google Scholar] [CrossRef]
- Romberg, R.; Sarton, E.; Teppema, L.; Matthes, H.W.; Kieffer, B.L.; Dahan, A. Comparison of morphine-6-glucuronide and morphine on respiratory depressant and antinociceptive responses in wild type and mu-opioid receptor deficient mice. Br. J. Anaesth. 2003, 91, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Shibasaki, Y.; Matsumoto, K.; Shibasaki, M.; Hasegawa, M.; Wang, E.; Masukawa, D.; Yoshizawa, K.; Horie, S.; Suzuki, T. Mechanisms that underlie μ-opioid receptor agonist–induced constipation: Differential involvement of μ-opioid receptor sites and responsible regions. J. Pharmacol. Exp. Ther. 2013, 347, 91–99. [Google Scholar] [CrossRef]
- Daniels, D.J.; Lenard, N.R.; Etienne, C.L.; Law, P.-Y.; Roerig, S.C.; Portoghese, P.S. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA 2005, 102, 19208–19213. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.A.A.; Walwyn, W.; Nozaki, C.; Filliol, D.; Erbs, E.; Matifas, A.; Evans, C.; Kieffer, B.L. Ligand-directed trafficking of the-opioid receptor in vivo: Two paths toward analgesic tolerance. J. Neurosci. 2010, 30, 16459–16468. [Google Scholar] [CrossRef]
- Fraser, G.L.; Gaudreau, G.-A.; Clarke, P.B.S.; Ménard, D.P.; Perkins, M.N. Antihyperalgesic effects of δ opioid agonists in a rat model of chronic inflammation. Br. J. Pharmacol. 2000, 129, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Cahill, C.M.; Morinville, A.; Hoffert, C.; O’Donnell, D.; Beaudet, A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: Implications for pain control. Pain 2003, 101, 199–208. [Google Scholar] [CrossRef]
- Gaveriaux-Ruff, C.; Nozaki, C.; Nadal, X.; Hever, X.C.; Weibel, R.; Matifas, A.; Reiss, D.; Filliol, D.; Nassar, M.A.; Wood, J.N.; et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain 2011, 152, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Kabli, N.; Cahill, C.M. Anti-Allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain 2007, 127, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Brainin-Mattos, J.; Smith, N.D.; Malkmus, S.; Rew, Y.; Goodman, M.; Taulane, J.; Yaksh, T.L. Cancer-related bone pain is attenuated by a systemically available δ-opioid receptor agonist. Pain 2006, 122, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Vanderah, T.W. Delta and kappa opioid receptors as suitable drug targets for pain. Clin. J. Pain 2010, 26, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Gendron, L.; Mittal, N.; Beaudry, H.; Walwyn, W. Recent advances on the δ opioid receptor: From trafficking to function. Br. J. Pharmacol. 2015, 172, 403–419. [Google Scholar] [CrossRef]
- Dykstra, L.A.; Schoenbaum, G.M.; Yarbrough, J.; McNutt, R.; Chang, K.J. A novel delta opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J. Pharmacol. Exp. Ther. 1993, 267, 875–882. [Google Scholar] [PubMed]
- Negus, S.S.; Butelman, E.R.; Chang, K.J.; DeCosta, B.; Winger, G.; Woods, J.H. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J. Pharmacol. Exp. Ther. 1994, 270, 1025–1034. [Google Scholar]
- Negus, S.S.; Gatch, M.; Mello, N.K.; Zhang, X.; Rice, K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J. Pharmacol. Exp. Ther. 1998, 286, 362–375. [Google Scholar]
- Nozaki, C.; Le Bourdonnec, B.; Reiss, D.; Windh, R.T.; Little, P.J.; Dolle, R.E.; Kieffer, B.L.; Gaveriaux-Ruff, C. δ-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: Analgesia, locomotion, and receptor internalization. J. Pharmacol. Exp. Ther. 2012, 342, 799–807. [Google Scholar] [CrossRef]
- Broom, D.C.; Nitsche, J.F.; Pintar, J.E.; Rice, K.C.; Woods, J.H.; Traynor, J.R. Comparison of receptor mechanisms and efficacy requirements for δ-agonist-induced convulsive activity and antinociception in mice. J. Pharmacol. Exp. Ther. 2002, 303, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Eisenach, J.C.; Carpenter, R.; Curry, R. Analgesia from a peripherally active κ-opioid receptor agonist in patients with chronic pancreatitis. Pain 2003, 101, 89–95. [Google Scholar] [CrossRef]
- Riviere, P. Peripheral kappa-opioid agonists for visceral pain. Br. J. Pharmacol. 2004, 141, 1331–1334. [Google Scholar] [CrossRef]
- Moon, S.W.; Park, E.H.; Suh, H.R.; Ko, D.H.; Kim, Y.I.; Han, H.C. The contribution of activated peripheral kappa opioid receptors (kORs) in the inflamed knee joint to anti-nociception. Brain Res. 2016, 1648, 11–18. [Google Scholar] [CrossRef]
- Bileviciute-Ljungar, I.; Spetea, M. Contralateral but not systemic administration of the κ-opioid agonist U-50,488H induces anti-nociception in acute hindpaw inflammation in rats. Br. J. Pharmacol. 2001, 132, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Keita, H.; Kayser, V.; Guilbaud, G. Antinociceptive effect of a kappa-opioid receptor agonist that minimally crosses the blood-brain barrier (ICI 204448) in a rat model of mononeuropathy. Eur. J. Pharmacol. 1995, 277, 275–280. [Google Scholar] [CrossRef]
- Bileviciute-Ljungar, I.; Spetea, M. Contralateral, ipsilateral and bilateral treatments with the κ-opioid receptor agonist U-50,488H in mononeuropathic rats. Eur. J. Pharmacol. 2004, 494, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Dykstra, L.A.; Gmerek, D.E.; Winger, G.; Woods, J.H. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J. Pharmacol. Exp. Ther. 1987, 242, 413–420. [Google Scholar]
- Butelman, E.R.; Harris, T.J.; Kreek, M.-J. Effects of E-2078, a stable dynorphin A(1-8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology 1999, 147, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.C.H.; Johnson, M.D.; Butelman, E.R.; Willmont, K.J.; Mosberg, H.I.; Woods, J.H. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J. Pharmacol. Exp. Ther. 1999, 291, 1113–1120. [Google Scholar] [PubMed]
- Peng, X.; Neumeyer, J.L. Kappa receptor bivalent ligands. Curr. Top. Med. Chem. 2007, 7, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Calo’, G.; Rizzi, A.; Cifani, C.; Di Bonaventura, M.V.M.; Regoli, D.; Massi, M.; Salvadori, S.; Lambert, D.G.; Guerrini, R. UFP-112 a potent and long-lasting agonist selective for the nociceptin/orphanin FQ receptor. CNS Neurosci. Ther. 2010, 17, 178–198. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, A.; Spagnolo, B.; Wainford, R.; Fischetti, C.; Guerrini, R.; Marzola, G.; Baldisserotto, A.; Salvadori, S.; Regoli, D.; Kapusta, D.R.; et al. In vitro and in vivo studies on UFP-112, a novel potent and long lasting agonist selective for the nociceptin/orphanin FQ receptor. Peptides 2007, 28, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Calò, G.; Guerrini, R.; Ko, M.-C. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain 2010, 148, 107–113. [Google Scholar] [CrossRef]
- Ko, M.-C.; Woods, J.H.; Fantegrossi, W.E.; Galuska, C.M.; Wichmann, J.; Prinssen, E.P. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology 2009, 34, 2088–2096. [Google Scholar] [CrossRef]
- Varty, G.B.; Lu, S.X.; Morgan, C.A.; Cohen-Williams, M.E.; Hodgson, R.A.; Smith-Torhan, A.; Zhang, H.; Fawzi, A.B.; Graziano, M.P.; Ho, G.D.; et al. The anxiolytic-like effects of the novel, orally active nociceptin opioid receptor agonist 8-[bis(2-methylphenyl)methyl]-3-phenyl-8-azabicyclo[3.2.1]octan-3-ol (SCH 221510). J. Pharmacol. Exp. Ther. 2008, 326, 672–682. [Google Scholar] [CrossRef]
- Lin, A.P.; Ko, M.-C. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem. Neurosci. 2012, 4, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, F.M.; Wichmann, J.; Higelin, J.; Py-Lang, G.; Kratzeisen, C.; Malherbe, P.; Kilpatrick, G.; Jenck, F. Pharmacological characterization of the novel nonpeptide orphanin FQ/nociceptin receptor agonist Ro 64-6198: Rapid and reversible desensitization of the ORL1 receptor in vitro and lack of tolerance in vivo. J. Pharmacol. Exp. Ther. 2001, 298, 812–819. [Google Scholar]
- Reiss, D.; Wichmann, J.; Tekeshima, H.; Kieffer, B.L.; Ouagazzal, A.-M. Effects of nociceptin/orphanin FQ receptor (NOP) agonist, Ro64-6198, on reactivity to acute pain in mice: Comparison to morphine. Eur. J. Pharmacol. 2008, 579, 141–148. [Google Scholar] [CrossRef]
- Obara, I.; Przewlocki, R.; Przewlocka, B. Spinal and local peripheral antiallodynic activity of Ro64-6198 in neuropathic pain in the rat. Pain 2005, 116, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Schuller, A.G.; King, M.A.; Zhang, J.; Bolan, E.; Pan, Y.; Morgan, D.; Chang, A.; Czick, M.E.; Unterwald, E.M.; Pasternak, G.W.; et al. Retention of heroin and morphine–6β–glucuronide analgesia in a new line of mice lacking exon 1 of MOR–1. Nat. Neurosci. 1999, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.H.; Liu, H.C.; Cavalli, A.; Yang, W.; Chen, Y.F.; Wei, L.N. mu Opioid receptor knockout in mice: Effects on ligand-induced analgesia and morphine lethality. Brain Res. Mol. Brain Res. 1998, 54, 321–326. [Google Scholar] [CrossRef]
- Zhu, Y.; King, M.A.; Schuller, A.G.; Nitsche, J.F.; Reidl, M.; Elde, R.P.; Unterwald, E.; Pasternak, G.W.; Pintar, J.E. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron 1999, 24, 243–252. [Google Scholar] [CrossRef]
- Simonin, F.; Valverde, O.; Smadja, C.; Slowe, S.; Kitchen, I.; Dierich, A.; Le Meur, M.; Roques, B.P.; Maldonado, R.; Kieffer, B.L. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998, 17, 886–897. [Google Scholar] [CrossRef]
- Matthes, H.W.; Smadja, C.; Valverde, O.; Vonesch, J.L.; Foutz, A.S.; Boudinot, E.; Denavit-Saubie, M.; Severini, C.; Negri, L.; Roques, B.P.; et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J. Neurosci. 1998, 18, 7285–7295. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, Y.; Vanderah, T.W.; Burkey, T.H.; Ossipov, M.H.; Kovelowski, C.J.; Sora, I.; Uhl, G.R.; Zhang, X.; Rice, K.C.; Roeske, W.R.; et al. Delta-opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur. J. Pharmacol. 2000, 388, 241–248. [Google Scholar] [CrossRef]
- Lefebvre, R. Opioid peptides and their receptors. In Comparative Veterinary Pharmacology, Toxicology and Theraphy; Van Miert, A.S.J.P.A.M., Bogaert, M.G., Debackere, M., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 447–453. [Google Scholar] [CrossRef]
- Bergström, J.; Ahmed, M.; Li, J.; Ahmad, T.; Kreicbergs, A.; Spetea, M. Opioid peptides and receptors in joint tissues: Study in the rat. J. Orthop. Res. 2006, 24, 1193–1199. [Google Scholar] [CrossRef]
- Callahan, P.; Pasternak, G.W. Opiates, opioid peptides, and their receptors. J. Cardiothorac. Anesth. 1987, 1, 569–576. [Google Scholar] [CrossRef]
- Chaturvedi, K. Opioid peptides, opioid receptors and mechanism of down regulation. Indian J. Exp. Boil. 2003, 41, 5–13. [Google Scholar]
- Snyder, S.H.; Childers, S.R. Opiate receptors and opioid peptides. Annu. Rev. Neurosci. 1979, 2, 35–64. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Struck, J.; Hartmann, O.; Maisel, A.S.; Rehfeldt, M.; Magrini, L.; Melander, O.; Bergmann, A.; Di Somma, S. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J. Nephrol. 2015, 28, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Shoureshi, P.; Kozak, K.; Szynskie, L.; Baron, A.; Lecaude, S.; Dores, R.M. Tracking the evolution of the proenkephalin gene in tetrapods. Gen. Comp. Endocrinol. 2007, 153, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Nunez, V.; Gonzalez Sarmiento, R.; Rodriguez, R.E. Characterization of zebrafish proenkephalin reveals novel opioid sequences. Brain Res. Mol. Brain Res. 2003, 114, 31–39. [Google Scholar] [CrossRef]
- Merg, F.; Filliol, D.; Usynin, I.; Bazov, I.; Bark, N.; Hurd, Y.L.; Yakovleva, T.; Kieffer, B.L.; Bakalkin, G. Big dynorphin as a putative endogenous ligand for the kappa-opioid receptor. J. Neurochem. 2006, 97, 292–301. [Google Scholar] [CrossRef]
- Gein, S. Dynorphins in regulation of immune system functions. Biochemistry 2014, 79, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Diamant, M.; Henricks, P.A.; Nijkamp, F.P.; de Wied, D. Beta-endorphin and related peptides suppress phorbol myristate acetate-induced respiratory burst in human polymorphonuclear leukocytes. Life Sci. 1989, 45, 1537–1545. [Google Scholar] [CrossRef]
- Lolait, S.J.; Clements, J.A.; Markwick, A.J.; Cheng, C.; McNally, M.; Smith, I.; Funder, J.W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J. Clin. Investig. 1986, 77, 1776–1779. [Google Scholar] [CrossRef]
- Bodnar, R.J. Endogenous opiates and behavior: 2012. Peptides 2013, 50, 55–95. [Google Scholar] [CrossRef]
- Fine, P.G.; Portenoy, R.K. A Clinical Guide to Opioid Analgesia; The McGraw-Hill Companies: New York, NY, USA, 2004; pp. 1–7. [Google Scholar]
- AMH. Analgesics (Ch 3). In Australian Medicines Handbook (AMH). Available online: https://amhonline.amh.net.au/chapters/chap-03?menu=vertical (accessed on 22 October 2017).
- Pasternak, G.W. Opiate pharmacology and relief of pain. J. Clin. Oncol. 2014, 32, 1655–1661. [Google Scholar] [CrossRef]
- Pena, D.A.; Duarte, M.L.; Pramio, D.T.; Devi, L.A.; Schechtman, D. Exploring morphine-triggered PKC-targets and their interaction with signaling pathways leading to pain via TrkA. Proteomes 2018, 6, 39. [Google Scholar] [CrossRef]
- Law, P.Y. Opioid receptor signal transduction mechanism. In The Opiate Receptors, 2nd ed.; Pasternak, G.W., Ed.; Humana Press: New York, NY, USA, 2011; pp. 195–238. [Google Scholar]
- Witkowski, G.; Szulczyk, P. Opioid μ receptor activation inhibits sodium currents in prefrontal cortical neurons via a protein kinase A- and C-dependent mechanism. Brain Res. 2006, 1094, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Seseã, A.E.; Vega, R.; Soto, E.; Seseña, E. Activation of μ-opioid receptors inhibits calcium-currents in the vestibular afferent neurons of the rat through a cAMP dependent mechanism. Front. Cell. Neurosci. 2014, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, Y.; Huang, L.; Xu, S.; Li, J.; Yang, L.; Wang, L.; Xing, C.; Wang, X.; Peng, Y. L-3-n-Butylphthalide regulates proliferation, migration, and differentiation of neural stem cell in vitro and promotes neurogenesis in APP/PS1 mouse model by regulating BDNF/TrkB/CREB/Akt pathway. Neurotox. Res. 2018, 34, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhan, C.; Feng, M.; Leblanc, M.; Ke, E.; Yeddula, N.; Verma, I.M. Targeting CREB Pathway Suppresses Small Cell Lung Cancer. Mol. Cancer Res. 2018, 16, 825–832. [Google Scholar] [CrossRef]
- Smith, T.H.; Grider, J.R.; Dewey, W.L.; Akbarali, H.I. Morphine decreases enteric neuron excitability via inhibition of sodium channels. PLoS ONE 2012, 7, e45251. [Google Scholar] [CrossRef]
- Shirahama, M.; Ushio, S.; Egashira, N.; Yamamoto, S.; Sada, H.; Masuguchi, K.; Kawashiri, T.; Oishi, R. Inhibition of Ca2+/Calmodulin-Dependent protein kinase II reverses oxaliplatin-induced mechanical allodynia in rats. Mol. Pain 2012, 8, 26. [Google Scholar] [CrossRef]
- Hudson, C.; Kimura, T.E.; Duggirala, A.; Sala-Newby, G.B.; Newby, A.C.; Bond, M. Dual Role of CREB in the regulation of VSMC Proliferation: Mode of activation determines pro-or anti-mitogenic function. Sci. Rep. 2018, 8, 4904. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Law, P.-Y.; Loh, H.H. The other side of the opioid story: Modulation of cell growth and survival signaling. Curr. Med. Chem. 2008, 15, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Knapp, C.M. Opiates. In Encyclopedia of the Human Brain; Academic Press: New York, NY, USA, 2002; pp. 729–739. [Google Scholar]
- Winters, B.; Gregoriou, G.C.; Kissiwaa, S.A.; Wells, O.A.; Medagoda, D.I.; Hermes, S.M.; Burford, N.T.; Alt, A.; Aicher, S.A.; Bagley, E.E. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat. Commun. 2017, 8, 14611. [Google Scholar] [CrossRef] [PubMed]
- Ghelardini, C.; Di Cesare Mannelli, L.; Bianchi, E. The pharmacological basis of opioids. Clin. Cases Miner. Bone Metab. 2015, 12, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Amano, T.; Kasakura, A.; Uhl, G.R.; Sora, I.; Sakai, N.; Kuzumaki, N.; Suzuki, T.; Narita, M. μ-Opioid receptor-independent fashion of the suppression of sodium currents by μ-opioid analgesics in thalamic neurons. Neurosci. Lett. 2009, 453, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.L.; Lane, J.R.; Coudrat, T.; Sexton, P.; Christopoulos, A.; Canals, M. Biased agonism of endogenous opioid peptides at the μ-Opioid receptor. Mol. Pharmacol. 2015, 88, 335–346. [Google Scholar] [CrossRef]
- Wisler, J.W.; Xiao, K.; Thomsen, A.; Lefkowitz, R.J. Recent developments in biased agonism. Curr. Opin. Cell Biol. 2014, 27, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Biased agonism. F1000 Biol. Rep. 2009, 1, 87. [Google Scholar] [CrossRef]
- Kenakin, T.; Watson, C.; Muniz-Medina, V.; Christopoulos, A.; Novick, S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 2012, 3, 193–203. [Google Scholar] [CrossRef]
- Al-Hasani, R.; Bruchas, M.R. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 2011, 115, 1363–1381. [Google Scholar] [CrossRef]
- Molinari, P.; Vezzi, V.; Sbraccia, M.; Grò, C.; Riitano, D.; Ambrosio, C.; Casella, I.; Costa, T. Morphine-like opiates selectively antagonize receptor-arrestin interactions. J. Biol. Chem. 2010, 285, 12522–12535. [Google Scholar] [CrossRef]
- Bohn, L.M.; Lefkowitz, R.J.; Gainetdinov, R.R.; Peppel, K.; Caron, M.G.; Lin, F.T. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999, 286, 2495–2498. [Google Scholar] [CrossRef]
- Soergel, D.G.; Subach, R.A.; Burnham, N.; Lark, M.W.; James, I.E.; Sadler, B.M.; Skobieranda, F.; Violin, J.D.; Webster, L.R. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014, 155, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- DeWire, S.; Yamashita, D.S.; Rominger, D.H.; Liu, G.; Cowan, C.L.; Graczyk, T.M.; Chen, X.-T.; Pitis, P.M.; Gotchev, D.; Yuan, C.; et al. A G Protein-Biased Ligand at the μ-Opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 2013, 344, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Raehal, K.M.; Walker, J.K.L.; Bohn, L. Morphine Side Effects in β-Arrestin 2 Knockout Mice. J. Pharmacol. Exp. Ther. 2005, 314, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Groer, C.E.; Tidgewell, K.; Moyer, R.A.; Harding, W.W.; Rothman, R.B.; Prisinzano, T.E.; Bohn, L.M. An opioid agonist that does not induce μ-Opioid receptor—arrestin interactions or receptor internalization. Mol. Pharmacol. 2007, 71, 549–557. [Google Scholar] [CrossRef]
- Maguma, H.T.; Dewey, W.L.; Akbarali, H.I. Differences in the characteristics of tolerance to μ-opioid receptor agonists in the colon from wild type and β-arrestin2 knockout mice. Eur. J. Pharmacol. 2012, 685, 133–140. [Google Scholar] [CrossRef]
- Spetea, M.; Eans, S.O.; Ganno, M.L.; Lantero, A.; Mairegger, M.; Toll, L.; Schmidhammer, H.; McLaughlin, J.P. Selective κ receptor partial agonist HS666 produces potent antinociception without inducing aversion after i.c.v. administration in mice. Br. J. Pharmacol. 2017, 174, 2444–2456. [Google Scholar] [CrossRef]
- Morgenweck, J.; Frankowski, K.J.; Prisinzano, T.; Aubé, J.; Bohn, L.M. Investigation of the role of βarrestin2 in kappa opioid receptor modulation in a mouse model of pruritus. Neuropharmacology 2015, 99, 600–609. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, M.; Gupta, A.; Page, C.; Gan, T.J.; Bergese, S.D. Evaluating oliceridine as a treatment option for moderate to severe acute post-operative pain in adults. Expert Opin. Pharmacother. 2021, 1–9. [Google Scholar] [CrossRef]
- Hammer, G.B.; Khanna, A.K.; Michalsky, C.; Wase, L.; Demitrack, M.A.; Little, R.; Fossler, M.J.; Ayad, S. Oliceridine exhibits improved tolerability compared to morphine at equianalgesic conditions: Exploratory analysis from two phase 3 randomized placebo and active controlled trials. Pain Ther. 2021, 1–11. [Google Scholar] [CrossRef]
- Devereaux, A.L.; Mercer, S.L.; Cunningham, C.W. DARK classics in chemical neuroscience: Morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407. [Google Scholar] [CrossRef]
- TG. Principles of Nonsteroidal Anti-Inflammatory Drug Use for Musculoskeletal Conditions in Adults. In eTG Complete Melbourne: Therapeutic Guidelines Limited. Available online: https://tgldcdp.tg.org.au/index (accessed on 29 December 2020).
- CDC. Prescription Opioids: Side Effects. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/drugoverdose/opioids/prescribed.html (accessed on 29 December 2020).
- WHO. Guidelines for the Psychosocially Assisted Pharmacological Treatment of Opioid Dependence. World Health Organization, Geneva. 2009. Available online: http://apps.who.int/iris/bitstream/10665/43948/1/9789241547543_eng.pdf (accessed on 26 October 2021).
- Collin, E.; Poulain, P.; Gauvain-Piquard, A.; Petit, G.; Pichard-Leandri, E. Is disease progression the major factor in morphine ‘tolerance’ in cancer pain treatment? Pain 1993, 55, 319–326. [Google Scholar] [CrossRef]
- Freye, E.; Anderson-Hillemacher, A.; Ritzdorf, I.; Levy, J.V. Opioid rotation from high-dose morphine to transdermal buprenorphine (Transtec®) in chronic pain patients. Pain Pract. 2007, 7, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Rhondali, W.; Tremellat, F.; LeDoux, M.; Ciais, J.-F.; Bruera, E.; Filbet, M. Methadone rotation for cancer patients with refractory pain in a palliative care unit: An observational study. J. Palliat. Med. 2013, 16, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Kowalski, G. Long-term administration of high doses of transdermal buprenorphine in cancer patients with severe neuropathic pain. OncoTargets Ther. 2015, 8, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Sutou, I.; Nakatani, T.; Hashimoto, T.; Saito, Y. Fentanyl Tolerance in the Treatment of Cancer Pain: A Case of Successful Opioid Switching from Fentanyl to Oxycodone at a Reduced Equivalent Dose. J. Pain Palliat. Care Pharmacother. 2015, 29, 161–165. [Google Scholar] [CrossRef]
- Shinde, S.; Gordon, P.; Sharma, P.; Gross, J.; Davis, M.P. Use of non-opioid analgesics as adjuvants to opioid analgesia for cancer pain management in an inpatient palliative unit: Does this improve pain control and reduce opioid requirements? Support. Care Cancer 2014, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Raptis, E.; Vadalouca, A.; Stavropoulou, E.; Argyra, E.; Melemeni, A.; Siafaka, I. Pregabalin Vs. Opioids for the treatment of neuropathic cancer pain: A prospective, head-to-head, randomized, open-label study. Pain Pract. 2013, 14, 32–42. [Google Scholar] [CrossRef]
- Abdelhamid, E.E.; Sultana, M.; Portoghese, P.S.; Takemori, A.E. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991, 258, 299–303. [Google Scholar] [PubMed]
- Fundytus, M.E.; Schiller, P.W.; Shapiro, M.; Weltrowska, G.; Coderre, T.J. Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[psi]. Eur. J. Pharmacol. 1995, 286, 105–108. [Google Scholar] [CrossRef]
- Hepburn, M.J.; Little, P.J.; Gingras, J.; Kuhn, C.M. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J. Pharmacol. Exp. Ther. 1997, 281, 1350–1356. [Google Scholar]
- Roy, S.; Guo, X.; Kelschenbach, J.; Liu, Y.; Loh, H.H. In vivo activation of a mutant-opioid receptor by naltrexone produces a potent analgesic effect but no tolerance: Role of-Receptor activation and-receptor blockade in morphine tolerance. J. Neurosci. 2005, 25, 3229–3233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imam, M.Z.; Kuo, A.; Ghassabian, S.; Smith, M.T. Progress in understanding mechanisms of opioid-induced gastrointestinal adverse effects and respiratory depression. Neuropharmacology 2018, 131, 238–255. [Google Scholar] [CrossRef]
- Bie, B.; Pan, Z.Z. Trafficking of central opioid receptors and descending pain inhibition. Mol. Pain 2007, 3, 37. [Google Scholar] [CrossRef]
- Bs, A.C.B.; Whistler, J.L. How to design an opioid drug that causes reduced tolerance and dependence. Ann. Neurol. 2010, 67, 559–569. [Google Scholar] [CrossRef]
- Viet, C.T.; Dang, D.; Aouizerat, B.E.; Miaskowski, C.; Ye, Y.; Viet, D.T.; Ono, K.; Schmidt, B.L. OPRM1 methylation contributes to opioid tolerance in cancer patients. J. Pain 2017, 18, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Groer, C.E.; Schmid, C.L.; Jaeger, A.M.; Bohn, L.M. Agonist-directed Interactions with Specific β-Arrestins Determine μ-Opioid Receptor Trafficking, Ubiquitination, and Dephosphorylation. J. Biol. Chem. 2011, 286, 31731–31741. [Google Scholar] [CrossRef] [PubMed]
- Arttamangkul, S.; Heinz, D.A.; Bunzow, J.R.; Song, X.; Williams, J.T. Cellular tolerance at the µ-opioid receptor is phosphorylation dependent. eLife 2018, 7, e34989. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.; Whistler, J.L. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr. Opin. Neurobiol. 2007, 17, 556–564. [Google Scholar] [CrossRef]
- Bourova, L.; Vosahlikova, M.; Kagan, D.; Dlouha, K.; Novotny, J.; Svoboda, P. Long-term adaptation to high doses of morphine causes desensitization of mu-OR- and delta-OR-stimulated G-protein response in forebrain cortex but does not decrease the amount of G-protein alpha subunits. Med. Sci. Monit. 2010, 16, 260–270. [Google Scholar]
- Gupta, A.; Mulder, J.; Gomes, I.; Rozenfeld, R.; Bushlin, I.; Ong, E.; Lim, M.; Maillet, E.; Junek, M.; Cahill, C.M. Increased abundance of opioid receptor heteromers following chronic morphine administration. Sci. Signal. 2010, 3, ra54. [Google Scholar] [CrossRef]
- Leah, P.M.; Heath, E.M.L.; Balleine, B.W.; Christie, M. Chronic morphine reduces surface expression of δ-Opioid receptors in subregions of rostral striatum. Neurochem. Res. 2015, 41, 500–509. [Google Scholar] [CrossRef]
- Paul, A.; Gueven, N.; Dietis, N. Profiling the effects of repetitive morphine administration on motor behavior in rats. Molecules 2021, 26, 4355. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Gueven, N.; Dietis, N. Morphine dosing strategy plays a key role in the generation and duration of the produced antinociceptive tolerance. Neuropharmacology 2017, 121, 158–166. [Google Scholar] [CrossRef] [PubMed]
- McQuay, H. Central analgesics. Acta Neurochir. Suppl. 1987, 38, 41–43. [Google Scholar]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Gårdmark, M.; Höglund, A.U.; Hammarlund-Udenaes, M. Aspects on Tail-Flick, Hot-Plate and electrical stimulation tests for morphine antinociception. Pharmacol. Toxicol. 1998, 83, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Labianca, R.; Sarzi-Puttini, P.; Zuccaro, S.M.; Cherubino, P.; Vellucci, R.; Fornasari, D. Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin. Drug Investig. 2012, 32, 53–63. [Google Scholar] [CrossRef]
- Juurlink, D.N.; Dhalla, I.A. Dependence and addiction during chronic opioid therapy. J. Med. Toxicol. 2012, 8, 393–399. [Google Scholar] [CrossRef]
- Ballantyne, J.C.; LaForge, S.K. Opioid dependence and addiction during opioid treatment of chronic pain. Pain 2007, 129, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Aghajanian, G.K. Molecular and Cellular Basis of Addiction. Science 1997, 278, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Counts, S.E.; Mufson, E.J. Chapter 12-Locus coeruleus. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 425–438. [Google Scholar]
- Kosten, T.R.; George, T.P. The neurobiology of opioid dependence: Implications for treatment. Sci. Pract. Perspect. 2002, 1, 13–20. [Google Scholar] [CrossRef]
- Listos, J.; Łupina, M.; Talarek, S.; Mazur, A.; Orzelska-Górka, J.; Kotlińska, J. The mechanisms involved in morphine addiction: An overview. Int. J. Mol. Sci. 2019, 20, 4302. [Google Scholar] [CrossRef] [PubMed]
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef] [PubMed]
- (PHE) Public Health England; Faculty of Medicine of the Royal College of Anaesthetists; Royal College of General Practitioners; The British Pain Society. Managing Persistent Pain in Secure Settings. 2013. Available online: http://www.nta.nhs.uk/uploads/persistentpain.pdf (accessed on 24 September 2016).
- NOUGG. Canadian Guideline for Safe and Effective Use of Opioids for Chronic Non-Cancer Pain. Canada. National Opioid Use Guideline Group (NOUGG). 2010. Available online: http://nationalpaincentre.mcmaster.ca/opioid/ (accessed on 24 September 2016).
- American Society of Anesthesiologists Task Force on Chronic Pain Management. American Society of Regional Anesthesia and Pain Medicine Practice Guidelines for Chronic Pain Management. Anesthesiology 2010, 112, 810–833. [Google Scholar] [CrossRef]
- Trescot, A.M.; Helm, S.; Hansen, H.; Benyamin, R.; Glaser, S.E.; Adlaka, R.; Patel, S.; Manchikanti, L. Opioids in the management of chronic non-cancer pain: An update of American Society of the Interventional Pain Physicians’ (ASIPP) Guidelines. Pain Physician 2008, 11, S5–S62. [Google Scholar]
- Kirpalani, D. How to maximize patient safety when prescribing opioids. PM&R 2015, 7, S225–S235. [Google Scholar] [CrossRef]
- Stein, C.; Reinecke, H.; Sorgatz, H. Opioid use in chronic noncancer pain: Guidelines revisited. Curr. Opin. Anaesthesiol. 2010, 23, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Streicher, J.M.; Bilsky, E.J. Peripherally acting μ-Opioid receptor antagonists for the treatment of opioid-related side effects: Mechanism of action and clinical implications. J. Pharm. Pr. 2017, 31, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Opioid-Induced constipation: Challenges and therapeutic opportunities. Am. J. Gastroenterol. 2011, 106, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Ikarashi, N.; Hayakawa, A.; Haga, Y.; Fueki, A.; Kusunoki, Y.; Tajima, M.; Ochiai, W.; Machida, Y.; Sugiyama, K. Morphine-Induced constipation develops with increased aquaporin-3 expression in the colon via increased serotonin secretion. Toxicol. Sci. 2015, 145, 337–347. [Google Scholar] [CrossRef]
- Akbarali, H.I.; Inkisar, A.; Dewey, W.L. Site and mechanism of morphine tolerance in the gastrointestinal tract. Neurogastroenterol. Motil. 2014, 26, 1361–1367. [Google Scholar] [CrossRef]
- Swegle, J.M.; Logemann, C. Management of common opioid-induced adverse effects. Am. Fam. Physician 2006, 74, 1347–1354. [Google Scholar]
- Cherny, N.; Ripamonti, C.; Pereira, J.; Davis, C.; Fallon, M.; McQuay, H.; Mercadante, S.; Pasternak, G.; Ventafridda, V. For the expert working group of the european association of palliative care network strategies to manage the adverse effects of oral morphine: An evidence-based report. J. Clin. Oncol. 2001, 19, 2542–2554. [Google Scholar] [CrossRef]
- Prichard, D.; Norton, C.; Bharucha, A.E. Management of opioid-induced constipation. Br. J. Nurs. 2016, 25, S4–S5, S8–S11. [Google Scholar] [CrossRef]
- Meissner, W.; Schmidt, U.; Hartmann, M.; Kath, R.; Reinhart, K. Oral naloxone reverses opioid-associated constipation. Pain 2000, 84, 105–109. [Google Scholar] [CrossRef]
- Shimoyama, N.; Shimoyama, M. Treatment of constipation in chronic pain patients. Masui. Jpn. J. Anesthesiol. 2013, 62, 822–828. [Google Scholar]
- Burness, C.B.; Keating, G.M. Oxycodone/naloxone prolonged-release: A review of its use in the management of chronic pain while counteracting opioid-induced constipation. Drugs 2014, 74, 353–375. [Google Scholar] [CrossRef]
- Moore, R.A.; McQuay, H.J. Prevalence of opioid adverse events in chronic non-malignant pain: Systematic review of randomised trials of oral opioids. Arthritis Res. 2005, 7, R1046–R1051. [Google Scholar] [CrossRef]
- Watcha, M.F.; White, P.F. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology 1992, 77, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, N.; Heuser, F.; Saglam, M.; Schulz, C.M.; Wagner, K.J.; Taki, M.; Kochs, E.F.; Jahn, K.; Brandt, T.; Glasauer, S.; et al. Opioid-inducednausea involves a vestibular problem preventable by head-rest. PLoS ONE 2015, 10, e0135263. [Google Scholar] [CrossRef]
- Rojas, C.; Slusher, B.S. Mechanisms and latest clinical studies of new NK1 receptor antagonists for chemotherapy-induced nausea and vomiting: Rolapitant and NEPA (netupitant/palonosetron). Cancer Treat. Rev. 2015, 41, 904–913. [Google Scholar] [CrossRef]
- Rojas, C.; Raje, M.; Tsukamoto, T.; Slusher, B.S. Molecular mechanisms of 5-HT3 and NK1 receptor antagonists in prevention of emesis. Eur. J. Pharmacol. 2014, 722, 26–37. [Google Scholar] [CrossRef]
- Koju, R.B.; Gurung, B.S.; Dongol, Y. Prophylactic administration of ondansetron in prevention of intrathecal morphine-induced pruritus and post-operative nausea and vomiting in patients undergoing caesarean section. BMC Anesthesiol. 2015, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.A.; Martin, P.; Jackson, K.A. Opioid substitution to reduce adverse effects in cancer pain management. Med. J. Aust. 1999, 170, 68–71. [Google Scholar] [CrossRef]
- De Stoutz, N.D.; Bruera, E.; Suarez-Almazor, M. Opioid rotation for toxicity reduction in terminal cancer patients. J. Pain Symptom Manag. 1995, 10, 378–384. [Google Scholar] [CrossRef]
- Sultan, P.; Gutierrez, M.C.; Carvalho, B. Neuraxial morphine and respiratory depression. Drugs 2011, 71, 1807–1819. [Google Scholar] [CrossRef]
- Kuo, A.; Wyse, B.D.; Meutermans, W.; Smith, M.T. In vivo profiling of seven common opioids for antinociception, constipation and respiratory depression: No two opioids have the same profile. Br. J. Pharmacol. 2014, 172, 532–548. [Google Scholar] [CrossRef]
- Boom, M.; Niesters, M.; Sarton, E.; Aarts, L.; Smith, T.W.; Dahan, A. Non-analgesic effects of opioids: Opioid-induced respiratory depression. Curr. Pharm. Des. 2012, 18, 5994–6004. [Google Scholar] [CrossRef]
- Kamei, J.; Ohsawa, M.; Hayashi, S.-S.; Nakanishi, Y. Effect of chronic pain on morphine-induced respiratory depression in mice. Neuroscience 2011, 174, 224–233. [Google Scholar] [CrossRef]
- Hill, R.; Santhakumar, R.; Dewey, W.; Kelly, E.; Henderson, G. Fentanyl depression of respiration: Comparison with heroin and morphine. Br. J. Pharmacol. 2020, 177, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Manzke, T.; Guenther, U.; Ponimaskin, E.G.; Haller, M.; Dutschmann, M.; Schwarzacher, S.; Richter, D.W. 5-HT 4(a) Receptors avert opioid-induced breathing depression without loss of analgesia. Science 2003, 301, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Pattinson, K.T.S. Opioids and the control of respiration. Br. J. Anaesth. 2008, 100, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Manzke, T.; Niebert, M.; Koch, U.; Caley, A.; Vogelgesang, S.; Bischoff, A.-M.; Hülsmann, S.; Ponimaskin, E.; Müller, U.; Smart, T.; et al. Die von serotoninrezeptor 1A modulierte dephosphorylierung des glyzinrezeptors α3. Der. Schmerz 2011, 25, 272–281. [Google Scholar] [CrossRef]
- Dunn, E.B.; Wolfe, J.J. Finding an optimal dose: Considerations in accurate opioid dispensing. Vet. Hum. Toxicol. 2000, 42, 36–38. [Google Scholar]
- Lewanowitsch, T.; Irvine, R.J. Naloxone methiodide reverses opioid-induced respiratory depression and analgesia without withdrawal. Eur. J. Pharmacol. 2002, 445, 61–67. [Google Scholar] [CrossRef]
- Droney, J.M.; Gretton, S.K.; Sato, H.; Ross, J.R.; Branford, R.; Welsh, K.I.; Cookson, W.; Riley, J. Analgesia and central side-effects: Two separate dimensions of morphine response. Br. J. Clin. Pharmacol. 2013, 75, 1340–1350. [Google Scholar] [CrossRef]
- Babbini, M.; Davis, W.M. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br. J. Pharmacol. 1972, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Hollais, A.W.; Patti, C.L.; Zanin, K.A.; Fukushiro, D.F.; Berro, L.F.; Carvalho, R.C.; Kameda, S.R.; Frussa-Filho, R. Effects of acute and long-term typical or atypical neuroleptics on morphine-induced behavioural effects in mice. Clin. Exp. Pharmacol. Physiol. 2014, 41, 255–263. [Google Scholar] [CrossRef]
- Patti, C.L.; Frussa-Filho, R.; Silva, R.; Carvalho, R.C.; Kameda, S.R.; Takatsu-Coleman, A.L.; Cunha, J.L.; Abílio, V.C. Behavioral characterization of morphine effects on motor activity in mice. Pharmacol. Biochem. Behav. 2005, 81, 923–927. [Google Scholar] [CrossRef]
- Rodrı́guez-Arias, M.; Broseta, I.; Aguilar, M.; Miñarro, J. Lack of specific effects of selective D1 and D2 dopamine antagonists vs. risperidone on morphine-induced hyperactivity. Pharmacol. Biochem. Behav. 2000, 66, 189–197. [Google Scholar] [CrossRef]
- Domino, E.F.; Vasco, M.R.; Wilson, A.E. Mixed depressant and stimulant actions of morphine and their relationship to brain acetylcholine. Life Sci. 1976, 18, 361–376. [Google Scholar] [CrossRef][Green Version]
- Brady, L.S.; Holtzman, S.G. Locomotor activity in morphine-dependent and post-dependent rats. Pharmacol. Biochem. Behav. 1981, 14, 361–370. [Google Scholar] [CrossRef]
- Tulunay, F.C.; Ayhan, I.H.; Sparber, S.B. The effects of morphine and delta-9-tetrahydrocannabinol on motor activity in rats. Psychopharmacology 1982, 78, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.P.; Lam, H.A.; Maidment, N.T. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem. 2008, 79, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.E.; Crow, T.J. Turning behaviour as an index of the action of amphetamines and ephedrines on central dopamine-containing neurones. Br. J. Pharmacol. 1971, 43, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.; Blackburn, T.; Greenwood, D. An automatic apparatus for recording rotational behaviour in rats with brain lesions. Physiol. Behav. 1973, 11, 117–120. [Google Scholar] [CrossRef]
- Urs, N.M.; Daigle, T.L.; Caron, M.G. A Dopamine D1 receptor-dependent β-arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology 2010, 36, 551–558. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. Dopamine and the brainstem locomotor networks: From lamprey to human. Front. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Chastain, L.G.; Qu, H.; Bourke, C.H.; Iuvone, P.M.; Dobner, P.R.; Nemeroff, C.B.; Kinkead, B. Striatal dopamine receptor plasticity in neurotensin deficient mice. Behav. Brain Res. 2014, 280, 160–171. [Google Scholar] [CrossRef]
- Borgkvist, A.; Usiello, A.; Greengard, P.; Fisone, G. Activation of the cAMP/PKA/DARPP-32 signaling pathway is required for morphine psychomotor stimulation but not for morphine reward. Neuropsychopharmacology 2007, 32, 1995–2003. [Google Scholar] [CrossRef]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and functional basis of dopamine receptors and their role in neurogenesis: Possible implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 1179069518779829. [Google Scholar] [CrossRef]
- Horsfall, J.T.; Sprague, J.E. The pharmacology and toxicology of the ‘Holy Trinity’. Basic Clin. Pharmacol. Toxicol. 2016, 120, 115–119. [Google Scholar] [CrossRef]
- Anderson, E.; Hearing, M. Chapter 4-Neural circuit plasticity in addiction. In Neural Mechanisms of Addiction; Torregrossa, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 35–60. [Google Scholar]
- Oliva, I.; Wanat, M.J. Ventral tegmental area afferents and drug-dependent behaviors. Front. Psychiatry 2016, 7, 30. [Google Scholar] [CrossRef]
- Adinoff, B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry 2004, 12, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.L.; Ashby, C.R. Heterogeneity of the mesotelencephalic dopamine fibers: Physiology and pharmacology. Neurosci. Biobehav. Rev. 2000, 24, 115–118. [Google Scholar] [CrossRef]
- Lever, C.; Burton, S.; O’Keefe, J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006, 17, 111–133. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Wetsel, W.C. Frontiers in neuroscience assessments of cognitive deficits in mutant mice. In Animal Models of Cognitive Impairment; Levin, E.D., Buccafusco, J.J., Eds.; CRC Press/Taylor & Francis, Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2006. [Google Scholar]
- Alves, R.; de Carvalho, J.G.B.; Venditti, M.A.C. High-and Low-Rearing rats differ in the brain excitability controlled by the allosteric benzodiazepine site in the GABAA receptor. J. Behav. Brain Sci. 2012, 2, 315–325. [Google Scholar] [CrossRef]
- Valverde, O.; Mantamadiotis, T.; Torrecilla, M.; Ugedo, L.; Pineda, J.; Bleckmann, S.; Gass, P.; Kretz, O.; Mitchell, J.M.; Schütz, G.; et al. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology 2004, 29, 1122–1133. [Google Scholar] [CrossRef]
- Contet, C.; Filliol, D.; Matifas, A.; Kieffer, B.L. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropsychopharmacology 2008, 54, 475–486. [Google Scholar] [CrossRef]
- Zan, G.-Y.; Wang, Q.; Wang, Y.-J.; Liu, Y.; Hang, A.; Shu, X.-H.; Liu, J.-G. Antagonism of κ opioid receptor in the nucleus accumbens prevents the depressive-like behaviors following prolonged morphine abstinence. Behav. Brain Res. 2015, 291, 334–341. [Google Scholar] [CrossRef]
- Aceves, M.; Bancroft, E.; Aceves, A.R.; Hook, M.A. Nor-binaltorphimine blocks the adverse effects of morphine after spinal cord injury. J. Neurotrauma 2017, 34, 1164–1174. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Paul, A.K.; Gueven, N.; Dietis, N. Age-dependent antinociception and behavioral inhibition by morphine. Pharmacol. Biochem. Behav. 2018, 168, 8–16. [Google Scholar] [CrossRef]
- Paul, A.K.; Gueven, N.; Dietis, N. Data on prolonged morphine-induced antinociception and behavioral inhibition in older rats. Data Brief. 2018, 19, 183–188. [Google Scholar] [CrossRef]
- Dominguez, J.E.; Habib, A. Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr. Opin. Anaesthesiol. 2013, 26, 288–295. [Google Scholar] [CrossRef]
- Raffaeli, W.; Marconi, G.; Fanelli, G.; Taddei, S.; Borghi, G.B.; Casati, A. Opioid-related side-effects after intrathecal morphine. Eur. J. Anaesthesiol. 2006, 23, 605–610. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Miyamoto, T.; Patapoutian, A. Why does morphine make you itch? Cell 2011, 147, 261–262. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.-C.; Sun, Y.-G.; Ross, M.; Kim, S.; Tsai, F.-F.; Li, Q.-F.; Jeffry, J.; Kim, J.-Y.; Loh, H.H.; et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell 2011, 147, 447–458. [Google Scholar] [CrossRef]
- Abe, K.; Kobayashi, K.; Yoshino, S.; Taguchi, K.; Nojima, H. Withdrawal of repeated morphine enhances histamine-induced scratching responses in mice. Drug Chem. Toxicol. 2015, 38, 167–173. [Google Scholar] [CrossRef]
- Roeckel, L.-A.; Le Coz, G.-M.; Gaveriaux-Ruff, C.; Simonin, F. Opioid-induced hyperalgesia: Cellular and molecular mechanisms. Neuroscience 2016, 338, 160–182. [Google Scholar] [CrossRef]
- Swartjes, M.; Mooren, R.A.G.; Waxman, A.R.; Arout, C.; Van De Wetering, K.; Hartigh, J.D.; Beijnen, J.H.; Kest, B.; Dahan, A. Morphine induces hyperalgesia without involvement of µ-Opioid receptor or morphine-3-glucuronide. Mol. Med. 2012, 18, 1320–1326. [Google Scholar] [CrossRef]
- Song, L.; Wu, C.; Zuo, Y. Melatonin prevents morphine-induced hyperalgesia and tolerance in rats: Role of protein kinase C and N-methyl-D-aspartate receptors. BMC Anesthesiol. 2015, 15, 12. [Google Scholar] [CrossRef]
- Elhabazi, K.; Ayachi, S.; Ilien, B.; Simonin, F. Assessment of morphine-induced hyperalgesia and analgesic tolerance in mice using thermal and mechanical nociceptive modalities. J. Vis. Exp. 2014, 89, e51264. [Google Scholar] [CrossRef]
- Van Rijn, R.; Whistler, J.L.; Waldhoer, M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 2010, 10, 73–79. [Google Scholar] [CrossRef]
- Walwyn, W.; John, S.; Maga, M.; Evans, C.J.; Hales, T.G. δ Receptors are required for full inhibitory coupling of μ receptors to voltage-dependent Ca2+ channels in dorsal root ganglion neurons. Mol. Pharmacol. 2009, 76, 134–143. [Google Scholar] [CrossRef]
- Alfaras-Melainis, K.; Gomes, I.; Rozenfeld, R.; Zachariou, V.; Devi, L. Modulation of opioid receptor function by protein-protein interactions. Front. Biosci. 2009, 14, 3594–3607. [Google Scholar] [CrossRef]
- Ballet, S.; Pietsch, M.; Abell, A.D. Multiple ligands in opioid research. Protein Pept. Lett. 2008, 15, 668–682. [Google Scholar] [CrossRef]
- Lezoualc, H.F.; Jockers, R. Bivalent ligands as specific pharmacological tools for G protein-coupled receptor dimers. Curr. Drug Discov. Technol. 2008, 5, 312–318. [Google Scholar] [CrossRef]
- Yamada, H.; Shimoyama, N.; Sora, I.; Uhl, G.R.; Fukuda, Y.; Moriya, H.; Shimoyama, M. Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. Brain Res. 2006, 1083, 61–69. [Google Scholar] [CrossRef]
- Abul-Husn, N.; Sutak, M.; Milne, B.; Jhamandas, K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of μ- and δ -opioid receptor antagonists. Br. J. Pharmacol. 2007, 151, 877–887. [Google Scholar] [CrossRef]
- McNaull, B.; Trang, T.; Sutak, M.; Jhamandas, K. Inhibition of tolerance to spinal morphine antinociception by low doses of opioid receptor antagonists. Eur. J. Pharmacol. 2007, 560, 132–141. [Google Scholar] [CrossRef]
- Dietis, N.; Guerrini, R.; Calo, G.; Salvadori, S.; Rowbotham, D.J.; Lambert, D.G. Simultaneous targeting of multiple opioid receptors: A strategy to improve side-effect profile. Br. J. Anaesth. 2009, 103, 38–49. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Schiller, P.W. Bi- or multifunctional opioid peptide drugs. Life Sci. 2010, 86, 598–603. [Google Scholar] [CrossRef]
- Toll, L.; Khroyan, T.V.; Polgar, W.E.; Jiang, F.; Olsen, C.; Zaveri, N.T. Comparison of the antinociceptive and antirewarding profiles of novel bifunctional nociceptin receptor/μ-opioid receptor ligands: Implications for therapeutic applications. J. Pharmacol. Exp. Ther. 2009, 331, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Turnaturi, R.; Aricò, G.; Ronsisvalle, G.; Parenti, C.; Pasquinucci, L. Multitarget opioid ligands in pain relief: New players in an old game. Eur. J. Med. Chem. 2016, 108, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Anand, J.P.; Montgomery, D. Multifunctional opioid ligands. Gastrointest. Regul. Pept. 2018, 247, 21–51. [Google Scholar] [CrossRef]
- Cunningham, C.W.; Elballa, W.M.; Vold, S.U. Bifunctional opioid receptor ligands as novel analgesics. Neuropharmacology 2019, 151, 195–207. [Google Scholar] [CrossRef]
- Agnes, R.S.; Ying, J.; Kövér, K.E.; Lee, Y.S.; Davis, P.; Ma, S.-W.; Badghisi, H.; Porreca, F.; Lai, J.; Hruby, V.J. Structure–activity relationships of bifunctional cyclic disulfide peptides based on overlapping pharmacophores at opioid and cholecystokinin receptors. Peptides 2008, 29, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.V.; Lee, Y.S.; Salibay, C.; Davis, P.; Ma, S.-W.; Porreca, F.; Hruby, V.J. Novel analogues of bifunctional ligands for opioid and melanocortin 4 receptor. In Peptides for Youth; Springer: New York, NY, USA, 2009; pp. 195–196. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nair, P.; Jacobsen, N.E.; Kulkarni, V.; Davis, P.; Ma, S.-W.; Navratilova, E.; Yamamura, H.I.; Vanderah, T.W.; Porreca, F.; et al. Biological and conformational evaluation of bifunctional compounds for opioid receptor agonists and neurokinin 1 receptor antagonists possessing two penicillamines. J. Med. Chem. 2010, 53, 5491–5501. [Google Scholar] [CrossRef]
- Yekkirala, A.S.; Kalyuzhny, A.E.; Portoghese, P.S. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem. Biol. 2013, 8, 1412–1416. [Google Scholar] [CrossRef]
- La Rochelle, A.D.; Guillemyn, K.; Dumitrascuta, M.; Martin, C.; Utard, V.; Quillet, R.; Schneider, S.; Daubeuf, F.; Willemse, T.; Mampuys, P.; et al. A bifunctional-biased mu-opioid agonist–neuropeptide FF receptor antagonist as analgesic with improved acute and chronic side effects. Pain 2018, 159, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, T.M.; Linz, K.; Koch, T.; Christoph, T. Cebranopadol: A novel first-in-class potent analgesic acting via NOP and opioid receptors. Antisense Res. Appl. 2019, 254, 367–398. [Google Scholar] [CrossRef]
- Fujita, W.; Gomes, I.; Devi, L.A. Heteromers of μ-δ opioid receptors: New pharmacology and novel therapeutic possibilities. Br. J. Pharmacol. 2014, 172, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Jordan, B.A.; Gupta, A.; Trapaidze, N.; Nagy, V.; Devi, L.A. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J. Neurosci. 2000, 20, RC110. [Google Scholar] [CrossRef]
- Fujita, W.; Gomes, I.; Devi, L.A. Revolution in GPCR signalling: Opioid receptor heteromers as novel therapeutic targets: IUPHAR Review 10. Br. J. Pharmacol. 2014, 171, 4155–4176. [Google Scholar] [CrossRef]
- Gomes, I.; Fujita, W.; Gupta, A.; Saldanha, S.A.; Negri, A.; Pinello, C.E.; Eberhart, C.; Roberts, E.; Filizola, M.; Hodder, P.; et al. Identification of a-opioid receptor heteromer-biased agonist with antinociceptive activity. Proc. Natl. Acad. Sci. USA 2013, 110, 12072–12077. [Google Scholar] [CrossRef]
- Milan-Lobo, L.; Enquist, J.; Van Rijn, R.M.; Whistler, J.L. Anti-analgesic effect of the mu/delta opioid receptor heteromer revealed by ligand-biased antagonism. PLoS ONE 2013, 8, e58362. [Google Scholar] [CrossRef]
- Wallace, M.S.; Kosek, P.S.; Staats, P.; Fisher, R.; Schultz, D.M.; Leong, M. Phase II, Open-label, multicenter study of combined intrathecal morphine and ziconotide: Addition of ziconotide in patients receiving intrathecal morphine for severe chronic pain. Pain Med. 2008, 9, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Reig, E.; Abejón, D.; Krames, E.S. Chapter 35-Intrathecal non-opioid analgesics for the control of pain. In Neuromodulation; Krames, E.S., Peckham, P.H., Rezai, A.R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 467–481. [Google Scholar]
| Receptor Nomenclature 1 | Gene | Most Common Location in the CNS 2 | Most Common Roles and Functions | Selective Agonist 3 | Selective Antagonist 4 |
|---|---|---|---|---|---|
| μ, mu, MOP | OPRM1 | Thalamus, amygdala, dorsal horn, cerebral cortex, striatum, hippocampus, locus coeruleus | Analgesia, intestinal transit, feeding, mood, hormone secretion, thermoregulation, cardiovascular function | DAMGO, sufentanil, PL017 | CTAP, CTOP, β-FNA |
| δ, delta, DOP | OPRD1 | Olfactory bulb, thalamus, cortex, caudate putamen, nucleus accumbens (NAc), amygdala, dorsal horn | Analgesia, mood, gastrointestinal motility, behaviour, cardiovascular regulation | DPDPE, [D-Ala2]deltorphin I, [D-Ala2]deltorphin II SNC80 | Naltrindole, TIPPᴪ, Naltriben |
| κ, kappa, KOP | OPRK1 | Olfactory bulb, NAc, cerebral cortex, claustrum, amygdala, caudate nucleus, hypothalamus, subthalamic nucleus, thalamus, corpus callosum. | Analgesia in inflammation, diuresis, feeding, neuroprotection, neuroendocrine functions | Enadoline, U50488, U69593, salvinorin A | norBNI, GNTI |
| N/OFQ, NOP | OPRL1 | Hippocampus, hypothalamus, amygdala, substantia nigra, dorsal horn, lateral septum | Spinal analgesia, anxiety, mood, memory, feeding, locomotor activity | UFP-102, Ro64-6198, N/OFQ-(1-13)-NH2, UFP-112 | UFP-101, SB 612111, J-113397, JTC-801 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. https://doi.org/10.3390/ph14111091
Paul AK, Smith CM, Rahmatullah M, Nissapatorn V, Wilairatana P, Spetea M, Gueven N, Dietis N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals. 2021; 14(11):1091. https://doi.org/10.3390/ph14111091
Chicago/Turabian StylePaul, Alok K., Craig M. Smith, Mohammed Rahmatullah, Veeranoot Nissapatorn, Polrat Wilairatana, Mariana Spetea, Nuri Gueven, and Nikolas Dietis. 2021. "Opioid Analgesia and Opioid-Induced Adverse Effects: A Review" Pharmaceuticals 14, no. 11: 1091. https://doi.org/10.3390/ph14111091
APA StylePaul, A. K., Smith, C. M., Rahmatullah, M., Nissapatorn, V., Wilairatana, P., Spetea, M., Gueven, N., & Dietis, N. (2021). Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals, 14(11), 1091. https://doi.org/10.3390/ph14111091










