Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation
Abstract
:1. Introduction
2. Results
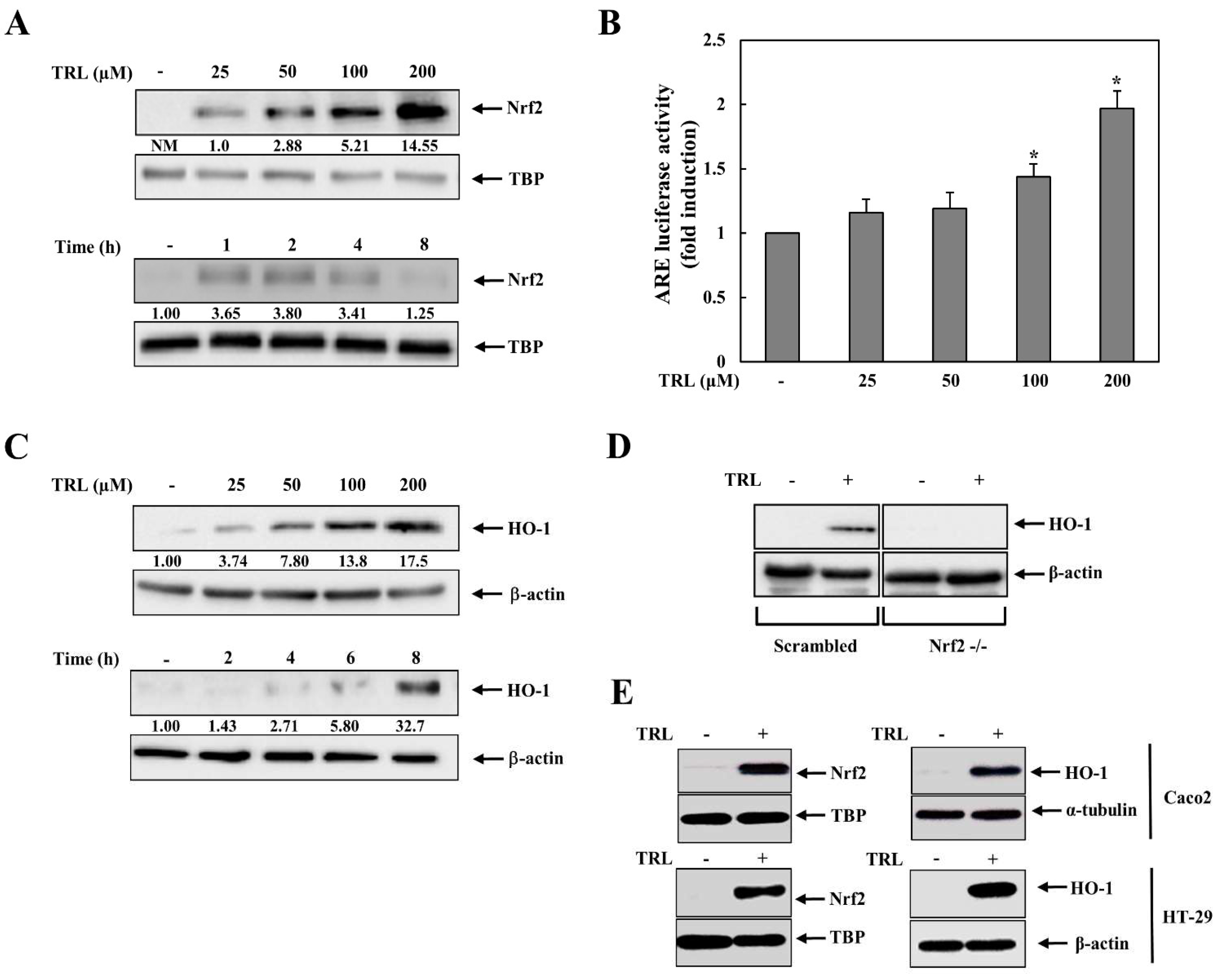
2.1. TRL Activates the Nrf2-HO-1 Pathway in Human Colon Carcinoma Cells
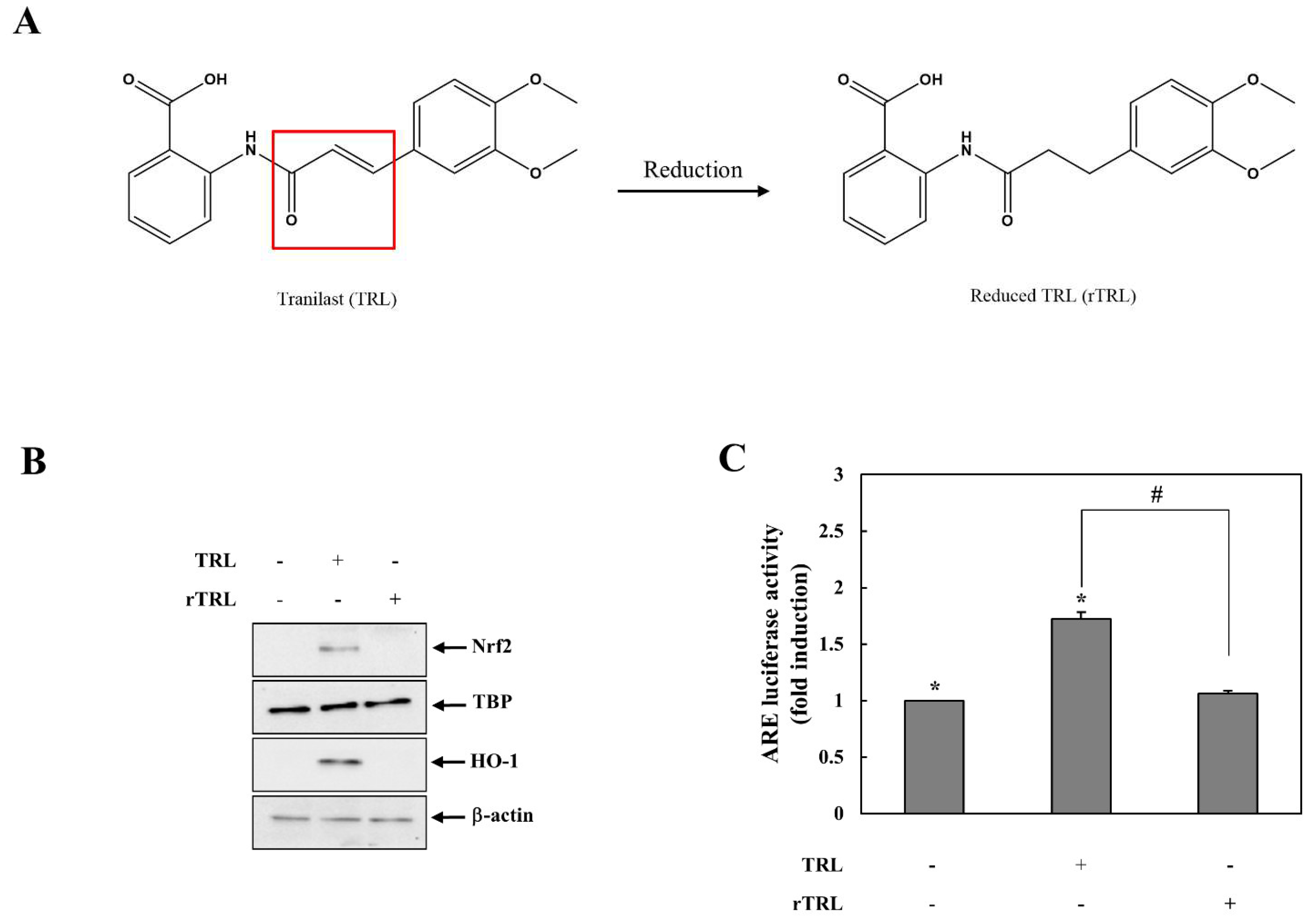
2.2. Reduced TRL Is Unable to Activate Nrf2
2.3. TRL Is Reactive with Thiol Group(s) in KEAP1 to Activate Nrf2
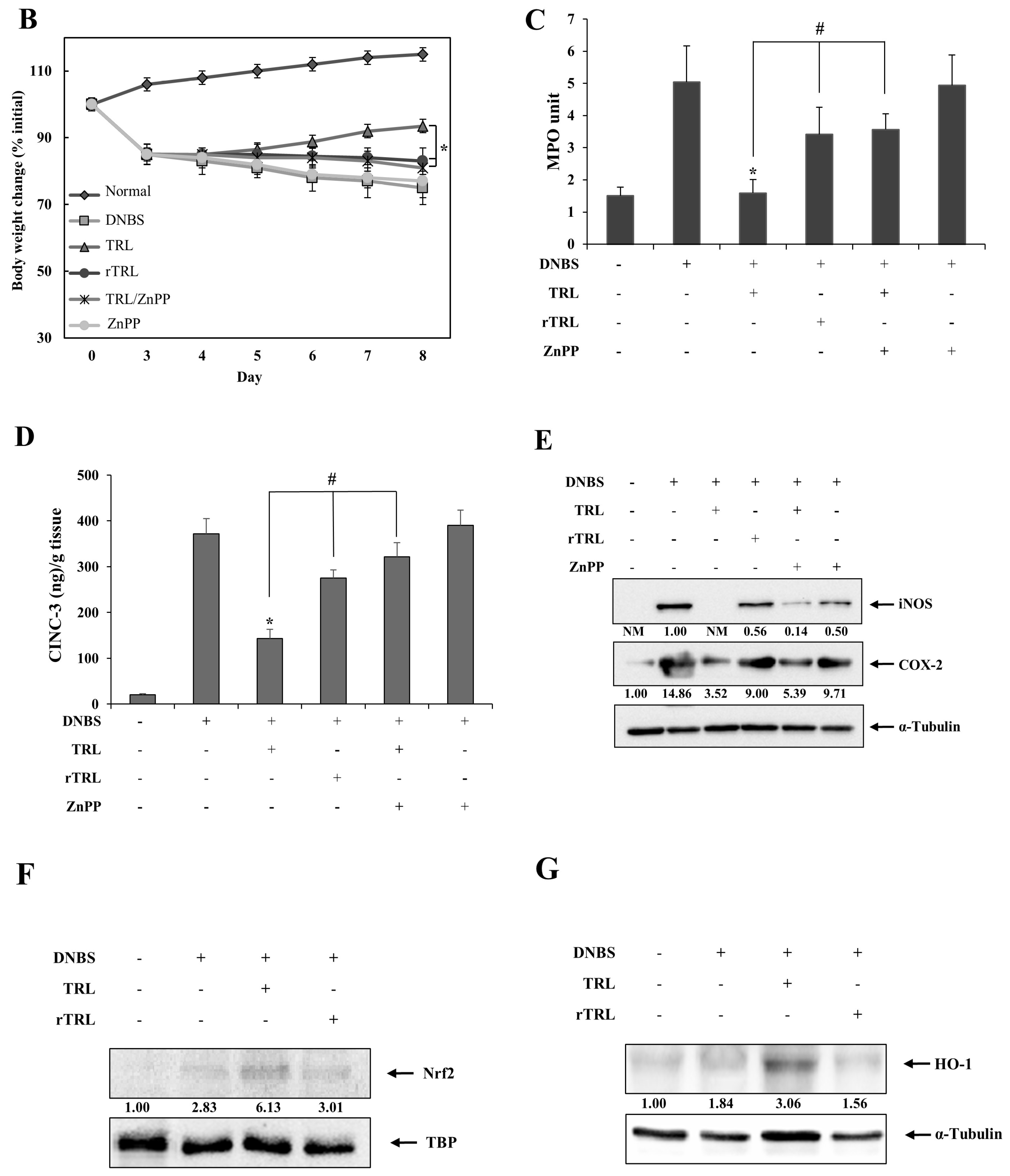
2.4. TRL Amelioration of Rat Colitis Is Partly Compromised by Either Chemical Reduction or an HO-1 Inhibitor
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Reduction of TRL
4.3. TRL-NAC Reaction
4.4. Cell Culture and Transient Transfection
4.5. Immunoblot Analysis
4.6. Precipitation of Biotin-Labeled Proteins and Coimmunoprecipitation
4.7. ELISA of CINC-3
4.8. Animals
4.9. DNBS-Induced Rat Colitis
4.10. Evaluation of Anti-Colitic Effects
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inglis, J.J.; Criado, G.; Andrews, M.; Feldmann, M.; Williams, R.O.; Selley, M.L. The anti-allergic drug, N-(3′,4′-dimethoxycinnamonyl) anthranilic acid, exhibits potent anti-inflammatory and analgesic properties in arthritis. Rheumatology 2007, 46, 1428–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darakhshan, S.; Pour, A.B. Tranilast: A review of its therapeutic applications. Pharmacol. Res. 2015, 91, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Gokhale, R.; Cho, J.H. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin. Microbiol. Rev. 2002, 15, 79–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.; Stallmach, A. Etiology and pathogenesis of inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2005, 51, 127–145. [Google Scholar]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.Q.; Li, J.; Huang, H.P.; Hao, W.D.; Duan, L.P.; Wei, X.T. Protective effects of tranilast on oxazolone-induced rat colitis through a mast cell-dependent pathway. Dig. Liver Dis. 2016, 48, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Kato, K.; Tsukada, R.; Suzuki, H.; Kaneko, Y.; Kojo, Y.; Sato, H.; Onoue, S. Protective effects of tranilast on experimental colitis in rats. Biomed. Pharmacother. 2017, 90, 842–849. [Google Scholar] [CrossRef]
- Sun, X.; Suzuki, K.; Nagata, M.; Kawauchi, Y.; Yano, M.; Ohkoshi, S.; Matsuda, Y.; Kawachi, H.; Watanabe, K.; Asakura, H.; et al. Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol. Int. 2010, 60, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Crespi, M.; Dulbecco, P.; De Ceglie, A.; Conio, M. Strictures in Crohn’s Disease: From Pathophysiology to Treatment. Dig. Dis. Sci. 2020, 65, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Oshitani, N.; Yamagami, H.; Watanabe, K.; Higuchi, K.; Arakawa, T. Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn’s disease. Gut 2007, 56, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Tkachev, V.O.; Menshchikova, E.B.; Zenkov, N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochem. Biokhimiia 2011, 76, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Sihvola, V.; Levonen, A.L. Keap1 as the redox sensor of the antioxidant response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cha, Y.N.; Surh, Y.J. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat. Res. 2010, 690, 12–23. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Kim, S.; Ju, S.; Lee, H.; Jeong, S.; Yoo, J.W.; Yoon, I.S.; Jung, Y. Colon-Targeted Delivery Facilitates the Therapeutic Switching of Sofalcone, a Gastroprotective Agent, to an Anticolitic Drug via Nrf2 Activation. Mol. Pharm. 2019, 16, 4007–4016. [Google Scholar] [CrossRef]
- Nazmy, E.A.; El-Khouly, O.A.; Atef, H.; Said, E. Sulforaphane protects against sodium valproate–induced acute liver injury. Can. J. Physiol. Pharmacol. 2017, 95, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Kim, W.; Jeong, S.; Lee, Y.; Nam, J.; Lee, S.; Jung, Y. Oxidized 5-aminosalicylic acid activates Nrf2-HO-1 pathway by covalently binding to Keap1: Implication in anti-inflammatory actions of 5-aminosalicylic acid. Free Radic. Biol. Med. 2017, 108, 715–724. [Google Scholar] [CrossRef]
- Cocco, M.; Pellegrini, C.; Martinez-Banaclocha, H.; Giorgis, M.; Marini, E.; Costale, A.; Miglio, G.; Fornai, M.; Antonioli, L.; Lopez-Castejon, G.; et al. Development of an Acrylate Derivative Targeting the NLRP3 Inflammasome for the Treatment of Inflammatory Bowel Disease. J. Med. Chem. 2017, 60, 3656–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Kim, W.; Yum, S.; Hong, S.; Oh, J.E.; Lee, J.W.; Kwak, M.K.; Park, E.J.; Na, D.H.; Jung, Y. Caffeic acid phenethyl ester activation of Nrf2 pathway is enhanced under oxidative state: Structural analysis and potential as a pathologically targeted therapeutic agent in treatment of colonic inflammation. Free Radic. Biol. Med. 2013, 65, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Jeong, S.O.; Koo, B.S.; Ha, H.Y.; Lee, K.M.; Chung, H.T. Tranilast, an orally active anti-allergic drug, up-regulates the anti-inflammatory heme oxygenase-1 expression but down-regulates the pro-inflammatory cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW264.7 macrophages. Biochem. Biophys. Res. Commun. 2008, 371, 361–365. [Google Scholar] [CrossRef]
- Baillie, T.A. Targeted Covalent Inhibitors for Drug Design. Angew. Chem. Int. Ed. Engl. 2016, 55, 13408–13421. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S.; Dalvie, D.K. Drug discovery for a new generation of covalent drugs. Expert Opin. Drug Discov. 2012, 7, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.W.; Fu, B.; Chou, R.-H.; Yu, C. Blocking the interactions between calcium-bound S100A12 protein and the V domain of RAGE using tranilast. PLoS ONE 2016, 11, e0162000. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, e8689. [Google Scholar] [CrossRef]
- Huang, Y.-K.; Chou, R.-H.; Yu, C. Tranilast blocks the interaction between the protein S100A11 and receptor for advanced glycation end products (RAGE) V domain and inhibits cell proliferation. J. Biol. Chem. 2016, 291, 14300–14310. [Google Scholar] [CrossRef] [Green Version]
- Kusama, H.; Kikuchi, S.; Tazawa, S.; Katsuno, K.; Baba, Y.; Zhai, Y.L.; Nikaido, T.; Fujii, S. Tranilast inhibits the proliferation of human coronary smooth muscle cell through the activation of p21waf1. Atherosclerosis 1999, 143, 307–313. [Google Scholar] [CrossRef]
- Davis, B.K.; Philipson, C.; Hontecillas, R.; Eden, K.; Bassaganya-Riera, J.; Allen, I.C. Emerging significance of NLRs in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 2412–2432. [Google Scholar] [CrossRef]
- Schottelius, A.J.; Baldwin, A.S., Jr. A role for transcription factor NF-kappa B in intestinal inflammation. Int. J. Colorectal Dis. 1999, 14, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Duarte-Silva, M.; de Barros Cardoso, C.R. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Jarmuz, A.; Wasilewski, A.; Salaga, M.; Fichna, J. Role of transient receptor potential channels in intestinal inflammation and visceral pain: Novel targets in inflammatory bowel diseases. Inflamm. Bowel Dis. 2015, 21, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. The tea catechin epigallocatechin gallate inhibits NF-kappaB-mediated transcriptional activation by covalent modification. Arch. Biochem. Biophys. 2020, 695, 108620. [Google Scholar] [CrossRef]
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018, 9, 2550. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Shin, D.H.; Kim, J.H.; Hong, S.; Choi, D.; Kim, Y.J.; Kwak, M.K.; Jung, Y. Caffeic acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase inhibition are involved in NFkappaB inhibitory effect: Structural analysis for NFkappaB inhibition. Eur. J. Pharmacol. 2010, 643, 21–28. [Google Scholar] [CrossRef]
- Andrews, N.C.; Faller, D.V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991, 19, 2499. [Google Scholar] [CrossRef]
- Hong, S.; Yum, S.; Yoo, H.J.; Kang, S.; Yoon, J.H.; Min, D.; Kim, Y.M.; Jung, Y. Colon-targeted cell-permeable NFkappaB inhibitory peptide is orally active against experimental colitis. Mol. Pharm. 2012, 9, 1310–1319. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Kang, C.; Park, S.; Ju, S.; Yoo, J.-W.; Yoon, I.-S.; Yun, H.; Jung, Y. Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation. Pharmaceuticals 2021, 14, 1092. https://doi.org/10.3390/ph14111092
Jeong S, Kang C, Park S, Ju S, Yoo J-W, Yoon I-S, Yun H, Jung Y. Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation. Pharmaceuticals. 2021; 14(11):1092. https://doi.org/10.3390/ph14111092
Chicago/Turabian StyleJeong, Seongkeun, Changyu Kang, Sohee Park, Sanghyun Ju, Jin-Wook Yoo, In-Soo Yoon, Hwayoung Yun, and Yunjin Jung. 2021. "Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation" Pharmaceuticals 14, no. 11: 1092. https://doi.org/10.3390/ph14111092
APA StyleJeong, S., Kang, C., Park, S., Ju, S., Yoo, J.-W., Yoon, I.-S., Yun, H., & Jung, Y. (2021). Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation. Pharmaceuticals, 14(11), 1092. https://doi.org/10.3390/ph14111092







