Synthesis, Characterization and In Vitro Evaluation of Hybrid Monomeric Peptides Suited for Multimodal Imaging by PET/OI: Extending the Concept of Charge—Cell Binding Correlation
Abstract
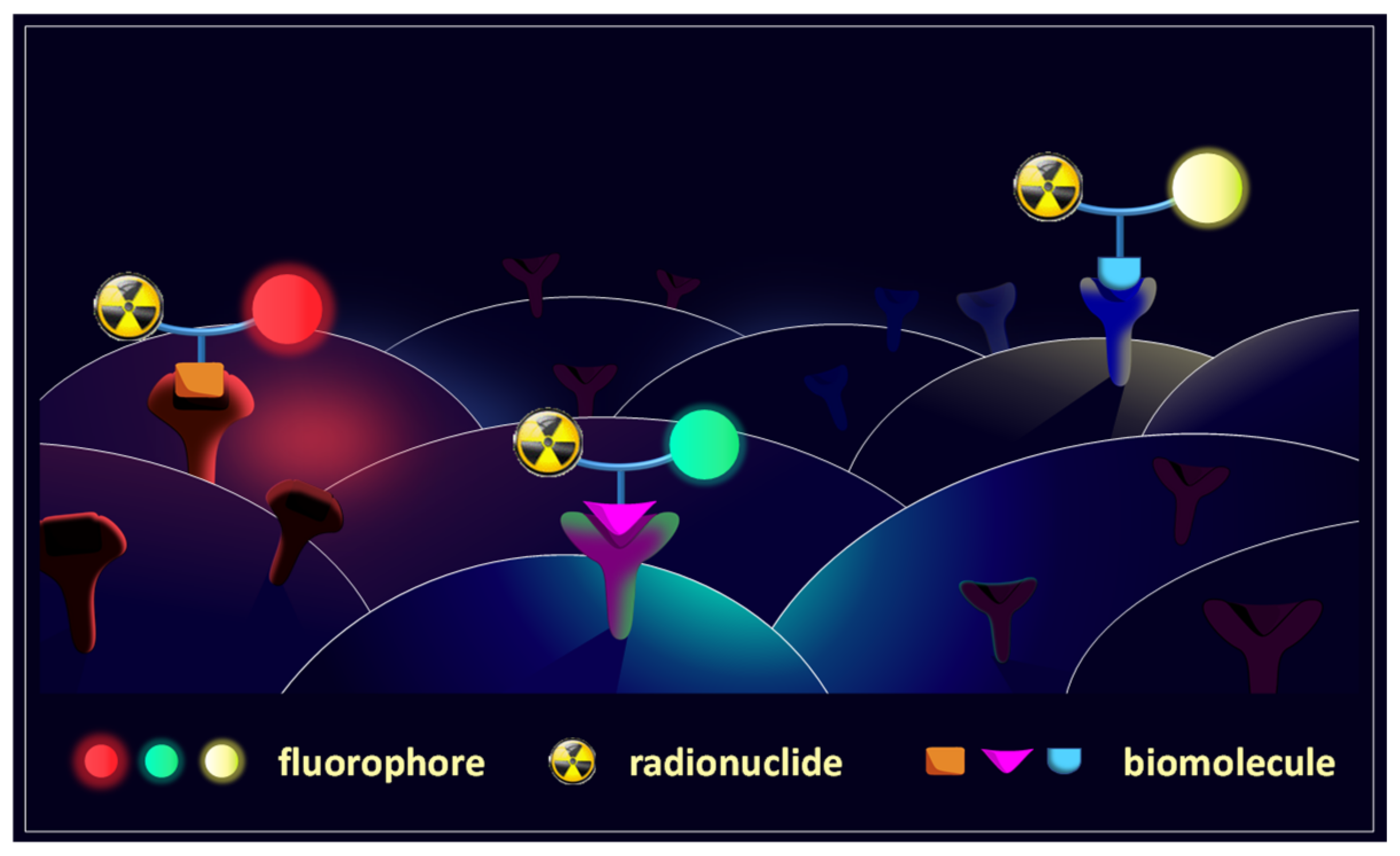
1. Introduction
2. Results and Discussion
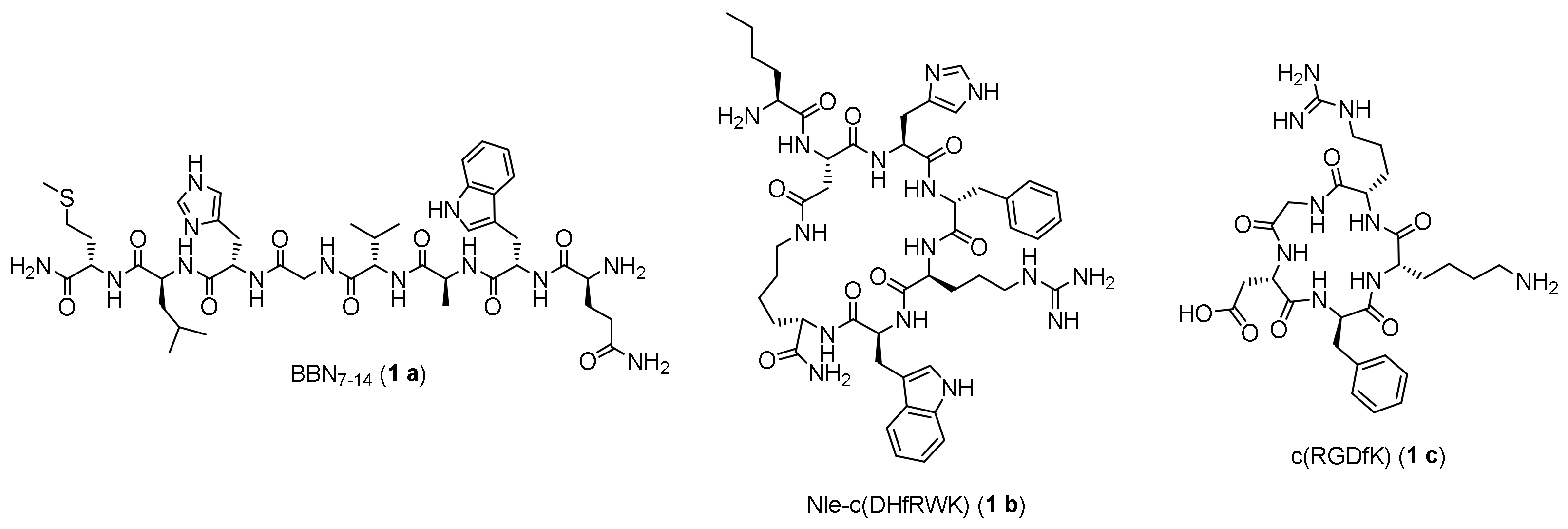
2.1. Synthesis of the Maleimide-Modified Peptide Units 3 a-c
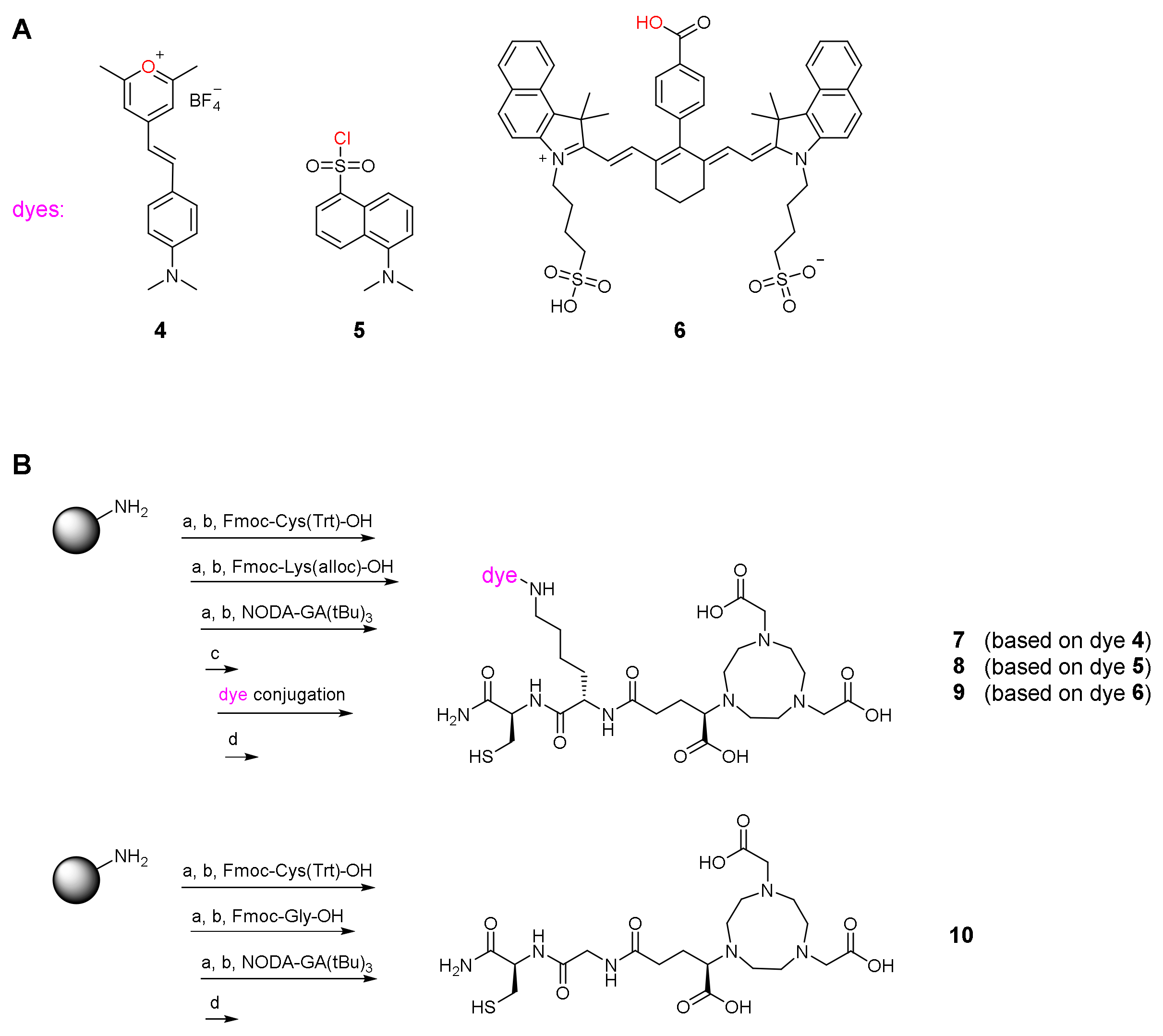
2.2. Synthesis of the Hybrid Multimodal Imaging Units 7-10
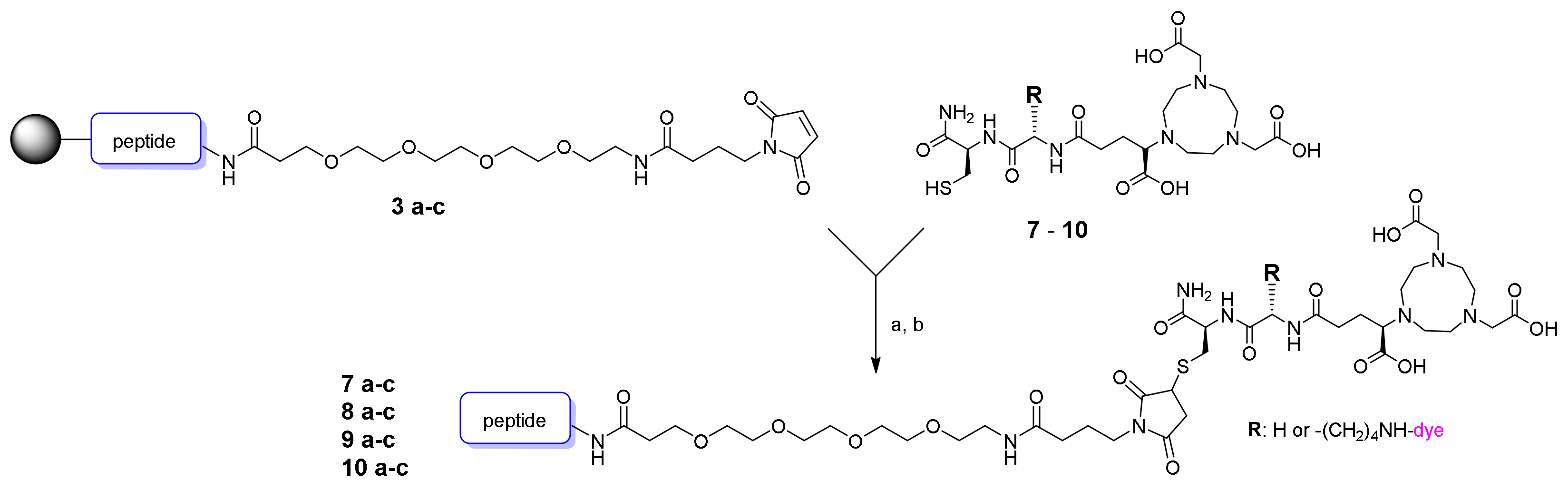
2.3. Synthesis of the MIU-Peptide-Bioconjugates 7 a-c, 8 a-c, 9 a-c and 10 a-c
2.4. Determination of Photophysical Properties of the MIU-Peptide-Bioconjugates 7 a-c, 8 a-c and 9 a-c
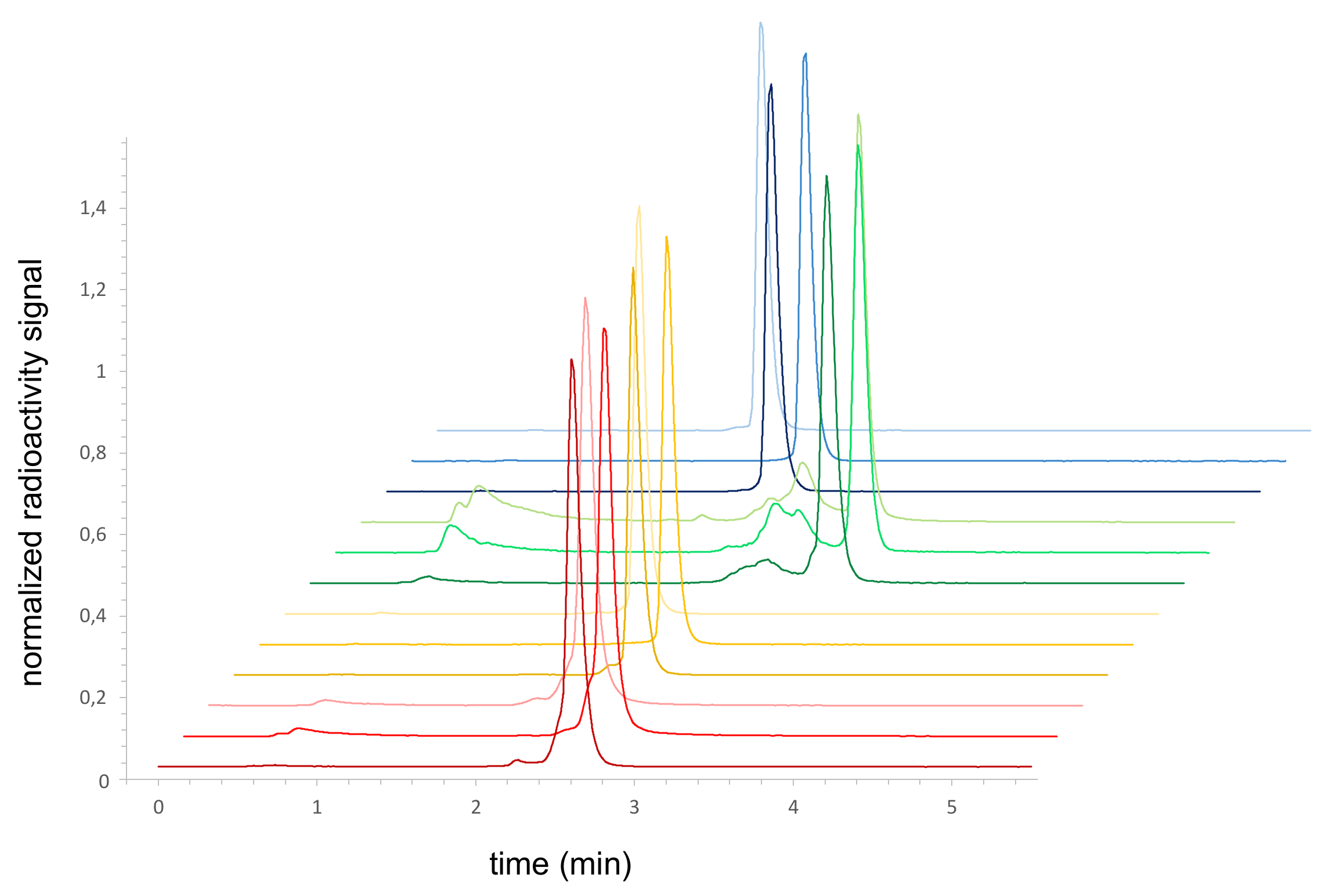
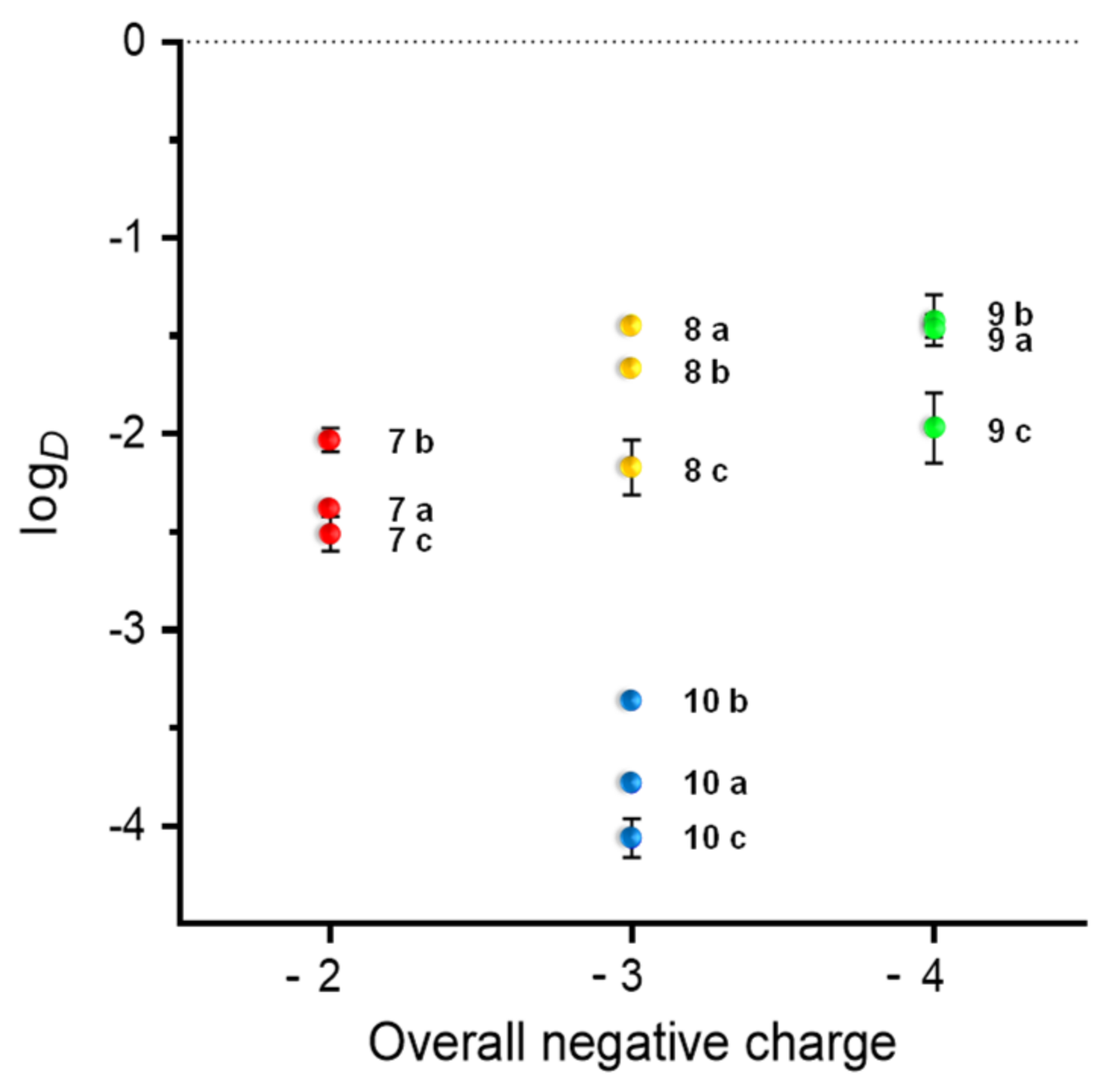
2.5. Radiolabeling of the MIU-Peptide-Bioconjugates 7 a-c, 8 a-c, 9 a-c and 10 a-c and Determination of the Lipophilicity/Hydrophilicity of the Labeled Agents
2.6. Receptor-Specific Cell Staining and Fluorescence Microscopy
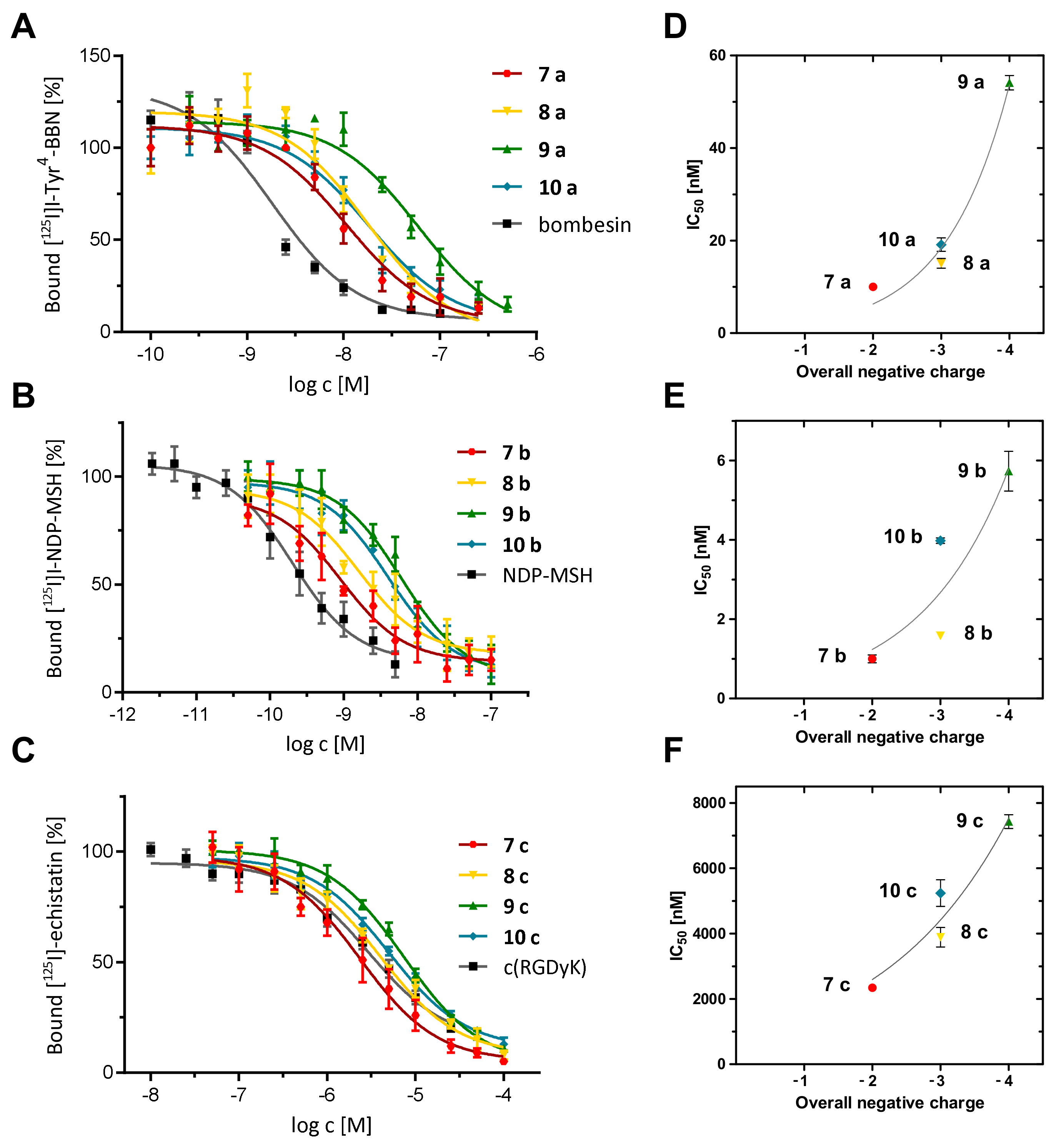
2.7. Determination of the In Vitro Receptor Binding Affinities of the Hybrid Peptide-MIU-Bioconjugates
3. Materials and Methods
3.1. Materials and Methods
3.2. General Synthesis of Peptides
3.3. General Synthesis of the Multimodal Imaging Units (MIUs) 7-10
3.4. General Synthesis of the Peptide-MIU-Conjugates 7 a-10 a, 7 b-10 b and 7 c-10 c
3.5. Radiochemistry
3.6. LogD Determination
3.7. Cell Culture
3.8. In Vitro Competitive Displacement Assays
3.9. Confocal Fluorescence Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mankoff, D. A Definition of Molecular Imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Imaging of tumour characteristics and molecular pathways with PET: Developments over the last decade towards personalised cancer therapy. Int. J. Radiat. Oncol. 2018, 102, 1165–1182. [Google Scholar] [CrossRef]
- Pysz, M.A.; Gambhir, S.S.; Willmann, J.K. Molecular imaging: Current status and emerging strategies. Clin. Radiol. 2010, 65, 500–516. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Zhao, T.; Li, Y.; Huang, G.; Sumer, B.D.; Gao, J. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials 2017, 157, 62–75. [Google Scholar] [CrossRef]
- Derlin, T.; Grünwald, V.; Steinbach, J.; Wester, H.J.; Ross, T.L. Molecular Imaging in Oncology Using Positron Emission Tomography. Dtsch. Arztebl. Int. 2018, 115, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Haris, M.; Yadav, S.K.; Rizwan, A.; Singh, A.; Wang, E.; Hariharan, H.; Reddy, R.; Marincola, F.M. Molecular magnetic resonance imaging in cancer. J. Transl. Med. 2015, 13, 313–329. [Google Scholar] [CrossRef]
- Abikhzer, G.; Keidar, Z. SPECT/CTand tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, S67–S80. [Google Scholar] [CrossRef]
- Endo, K.; Oriuchi, N.; Higuchi, T.; Iida, Y.; Hanaoka, H.; Miyakubo, M.; Ishikita, T.; Koyama, K. PET and PET/CT using 18F-FDG in the diagnosis and management of cancer patients. Int. J. Clin. Oncol. 2006, 11, 286–296. [Google Scholar] [CrossRef]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet 2019, 20, 354–367. [Google Scholar] [CrossRef]
- van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in—Human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Rieffel, J.; Chitgupi, U.; Lovell, J.F. Recent Advances in Higher-Order, Multimodal, Biomedical Imaging Agents. Small 2015, 11, 4445–4461. [Google Scholar] [CrossRef]
- Ahn, S.H.; Boros, E. Nuclear and Optical Bimodal Imaging Probes Using Sequential Assembly: A Perspective. Cancer Biother. Radiol. 2018, 33, 308–315. [Google Scholar] [CrossRef]
- Ni, D.; Ehlerding, E.B.; Cai, W. Multimodality Imaging Agents with PET as the Fundamental Pillar. Angew. Chem. Int. Ed. 2019, 58, 2570–2579. [Google Scholar] [CrossRef]
- Christensen, A.; Juhl, K.; Persson, M.; Charabi, B.W.; Mortensen, J.; Kiss, K.; Lelkaitis, G.; Rubek, N.; von Buchwald, C.; Kjær, A. uPAR-targeted optical near-infrared (NIR) fluorescence imaging and PET for image-guided surgery in head and neck cancer: Proof-of-concept in orthotopic xenograft model. Oncotarget 2017, 8, 15407–15419. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Dancer, P.A.; Portal, C.; Denat, F.; Prignon, A.L.; Goncalves, V. Design of Bimodal Ligands of Neurotensin Receptor 1 for Positron Emission Tomography Imaging and Fluorescence-Guided Surgery of Pancreatic Cancer. J. Med. Chem. 2020, 63, 2426–2433. [Google Scholar] [CrossRef]
- Baranski, A.C.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendörfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11-Derived Dual-Labeled PSMA inhibitors for preoperative PET imaging and precise fluorescence-guided surgery of prostate cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef]
- Kang, N.Y.; Lee, J.Y.; Lee, S.H.; Song, I.H.; Hwang, Y.H.; Kim, M.J.; Phue, W.H.; Agrawalla, B.K.; Wan, S.Y.D.; Lalic, J.; et al. Multimodal Imaging Probe Development for Pancreatic βCells: From Fluorescence to PET. J. Am. Chem. Soc. 2020, 142, 3430–3439. [Google Scholar] [CrossRef]
- Ahn, S.H.; Thach, D.; Vaughn, B.A.; Alford, V.M.; Preston, A.N.; Laughlin, S.T.; Boros, E. Linear Desferrichrome-Linked Silicon-Rhodamine Antibody Conjugate Enables Targeted Multimodal Imaging of HER2 in Vitro and in Vivo. Mol. Pharm. 2019, 16, 1412–1420. [Google Scholar] [CrossRef]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef]
- Hübner, R.; Cheng, X.; Wängler, B.; Wängler, C. Functional Hybrid Molecules for the Visualization of Cancer: PESIN-Homodimers Combined with Multimodal Molecular Imaging Probes for Positron Emission Tomography and Optical Imaging: Suited for Tracking of GRPR-Positive Malignant Tissue. Chem. Eur. J. 2020, 26, 16349–16356. [Google Scholar] [CrossRef]
- Hübner, R.; von Kiedrowski, V.; Benkert, V.; Wängler, B.; Schirrmacher, R.; Krämer, R.; Wängler, C. Hybrid Multimodal Imaging Synthons for Chemoselective and Efficient Biomolecule Modification with Chelator and Near-Infrared Fluorescent Cyanine Dye. Pharmaceuticals 2020, 13, 250. [Google Scholar] [CrossRef]
- Hübner, R.; Paretzki, A.; von Kiedrowski, V.; Maspero, M.; Cheng, X.; Davarci, G.; Braun, D.; Damerow, H.; Judmann, B.; Filippou, V.; et al. PESIN Conjugates for Multimodal Imaging: Can Multimerization Compensate Charge Influences on Cell Binding Properties? A Case Study. Pharmaceuticals 2021, 14, 531. [Google Scholar] [CrossRef]
- Salazar-Onfray, F.; Lopez, M.; Lundqvist, A.; Aguirre, A.; Escobar, A.; Serrano, A.; Korenblit, C.; Petersson, M.; Chhajlani, V.; Larsson, O.; et al. Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Brit. J. Cancer 2002, 87, 414–422. [Google Scholar] [CrossRef]
- Brooks, P.C.; Montgomery, A.M.P.; Flosenfeld, M.; Reisfeld, R.A.; Flu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 Antagonists Promote Tumor Regression by Inducing Apoptosis of Angiogenic Blood Vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Haskell-Luevano, C.; Nikiforovich, G.; Sharma, S.D.; Yang, Y.K.; Dickinson, C.; Hruby, V.J.; Gantz, I. Biological and Conformational Examination of Stereochemical Modifications Using the Template Melanotropin Peptide, Ac-Nle-c[Asp-His-Phe-Arg-Trp- Ala-Lys]-NH2, on Human Melanocortin Receptors. J. Med. Chem. 1997, 40, 1738–1748. [Google Scholar] [CrossRef]
- Haubner, R.; Gratias, R.; Diefenbach, B.; Goodman, S.L.; Jonczyk, A.; Kessler, H. Structural and Functional Aspects of RGD-Containing Cyclic Pentapeptides as Highly Potent and Selective Integrin αVβ3 Antagonists. J. Am. Chem. Soc. 1996, 118, 7461–7472. [Google Scholar] [CrossRef]
- Hübner, R.; Benkert, V.; Cheng, X.; Wängler, B.; Krämer, R.; Wängler, C. Probing two PESIN-indocyanine-dye-conjugates: Significance of the used fluorophore. J. Mater. Chem. B 2020, 8, 1302–1309. [Google Scholar] [CrossRef]
- von Kiedrowski, V.; Hübner, R.; Kail, D.; Schirrmacher, R.; Wängler, C.; Wängler, B. Synthesis, characterization and optimization of in vitro properties of NIR-fluorescent cyclic a-MSH peptides for melanoma imaging. J. Mater. Chem. B 2020, 8, 10602–10608. [Google Scholar] [CrossRef]
- Cheng, X.; Hübner, R.; von Kiedrowski, V.; Fricker, G.; Schirrmacher, R.; Wängler, C.; Wängler, B. Design, Synthesis, In Vitro and In Vivo Evaluation of Heterobivalent SiFAlin-Modified Peptidic Radioligands Targeting Both Integrin αvβ3 and the MC1 Receptor—Suitable for the Specific Visualization of Melanomas? Pharmaceuticals 2021, 14, 547. [Google Scholar] [CrossRef]
- Schroeder, R.P.; Muller, C.; Reneman, S.; Melis, M.L.; Breeman, W.A.; de Blois, E.; Bangma, C.H.; Krenning, E.P.; van Weerden, W.M.; de Jong, M. A standardised study to compare prostate cancer targeting efficacy of five radiolabelled bombesin analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1386–1396. [Google Scholar] [CrossRef]
- Tomasch, M.; Schwed, J.S.; Kuczka, K.; dos Santos, S.M.; Harder, S.; Nusing, R.M.; Paulke, A.; Stark, H. Fluorescent Human EP3 Receptor Antagonists. ACS Med. Chem. Lett. 2012, 3, 774–779. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Mason, J.C.; Achilefu, S. Heptamethine Cyanine Dyes with a Robust C-C Bond at the Central Position of the Chromophore. J. Org. Chem. 2006, 71, 7862–7865. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgórski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The Thiol-Michael Addition Click Reaction: A Powerful and Widely Used Tool in Materials Chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Wängler, C.; Wängler, B.; Lehner, S.; Elsner, A.; Todica, A.; Bartenstein, P.; Hacker, M.; Schirrmacher, R. A Universally Applicable 68Ga-Labeling Technique for Proteins. J. Nucl. Med. 2011, 52, 586–591. [Google Scholar] [CrossRef]
- Cabelli, D.E.; Bielski, B.H.J. Kinetics and mechanism for the oxidation of ascorbic acid/ascorbate by HO2/O2- (hydroperoxyl/superoxide) radicals. A pulse radiolysis and stopped-flow photolysis study. J. Phys. Chem. 1983, 87, 1809–1812. [Google Scholar] [CrossRef]
- Läppchen, T.; Holland, J.P.; Kiefer, Y.; Bartholomä, M.D. Preparation and preclinical evaluation of a (68)Ga-labelled c(RGDfK) conjugate comprising the bifunctional chelator NODIA-Me. EJNMMI Radiopharm. Chem. 2018, 3, 6–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, S.; Czerwinski, A.; Valenzuela, F.; Pennington, M.; Liu, S. FITC-conjugated cyclic RGD peptides as fluorescent probes for staining integrin alphavbeta3/alphavbeta5 in tumor tissues. Bioconjug. Chem. 2014, 25, 1925–1941. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled Cyclic RGD Peptide Bioconjugates as Radiotracers Targeting Multiple Integrins. Bioconjug. Chem. 2015, 26, 1413–1438. [Google Scholar] [CrossRef]
- Wellings, D.A.; Atherton, E. Standard Fmoc Protocols. Methods Enzymol. 1997, 289, 44–53. [Google Scholar] [CrossRef]


| Peptide Unit | MIU | Resulting Conjugate | Yield [%] |
|---|---|---|---|
| 3 a (BBN7-14-maleimide) | 7 (thio-pyrylium-NODA-GA) | 7 a (BBN7-14-pyrylium-NODA-GA) | 76 |
| 8 (thio-dansyl-NODA-GA) | 8 a (BBN7-14-dansyl-NODA-GA) | 30 | |
| 9 (thio-cyanine-NODA-GA) | 9 a (BBN7-14-cyanine-NODA-GA) | 36 | |
| 10 (thio-NODA-GA) | 10 a (BBN7-14-NODA-GA) | 54 | |
| 3 b (Nle-c(DHfRWK)-maleimide) | 7 (thio-pyrylium-NODA-GA) | 7 b (Nle-c(DHfRWK)-pyrylium-NODA-GA) | 70 |
| 8 (thio-dansyl-NODA-GA) | 8 b (Nle-c(DHfRWK)-dansyl-NODA-GA) | 47 | |
| 9 (thio-cyanine-NODA-GA) | 9 b (Nle-c(DHfRWK)-cyanine-NODA-GA) | 82 | |
| 10 (thio-NODA-GA) | 10 b (Nle-c(DHfRWK)-NODA-GA) | 52 | |
| 3 c (c(RGDfK)-maleimide) | 7 (thio-pyrylium-NODA-GA) | 7 c (c(RGDfK)-pyrylium-NODA-GA) | 38 |
| 8 (thio-dansyl-NODA-GA) | 8 c (c(RGDfK)-dansyl-NODA-GA) | 49 | |
| 9 (thio-cyanine-NODA-GA) | 9 c (c(RGDfK)-cyanine-NODA-GA) | 33 | |
| 10 (thio-NODA-GA) | 10 c (c(RGDfK)-NODA-GA) | 23 |
| Compound | λmax(abs) (nm) | log ε (M−1cm−1) | λmax(em) 1 (nm) | Stokes Shift (nm) |
|---|---|---|---|---|
| 73 | 440 | 4.16 | 600 | 160 |
| 7 a | 448 | 4.27 | 601 | 153 |
| 7 b | 450 | 4.16 | 597 | 147 |
| 7 c | 450 | 4.29 | 607 | 157 |
| 83 | - | - | 550 | - |
| 8 a | - | - | 549 | - |
| 8 b | - | - | 545 | - |
| 8 c | - | - | 543 | - |
| 92,3 | 720/790 | 4.80/4.61 | 800 | 10 |
| 9 a2 | 724/800 | 4.79/4.60 | 809 | 9 |
| 9 b2 | 726/802 | 4.78/4.57 | 807 | 5 |
| 9 c2 | 722/798 | 4.65/4.54 | 811 | 13 |
| Compound | logD | Overall Charge | IC50 (nM) |
|---|---|---|---|
| 74 | −2.04 ± 0.11 | −2 | - |
| 7 a | −2.38 ± 0.02 | −2 | 10.01 ± 0.67 1 |
| 7 b | −2.03 ± 0.06 | −2 | 1.00 ± 0.10 2 |
| 7 c | −2.51 ± 0.09 | −2 | 2338.00 ± 116.83 3 |
| 84 | −1.39 ± 0.04 | −3 | - |
| 8 a | −1.44 ± 0.01 | −3 | 15.09 ± 1.04 1 |
| 8 b | −1.66 ± 0.03 | −3 | 1.58 ± 0.04 2 |
| 8 c | −2.17 ± 0.14 | −3 | 3893.50 ± 295.89 3 |
| 94 | −1.29 ± 0.11 | −4 | - |
| 9 a | −1.45 ± 0.06 | −4 | 58.27 ± 3.66 1 |
| 9 b | −1.42 ± 0.13 | −4 | 5.73 ± 0.50 2 |
| 9 c | −1.97 ± 0.18 | −4 | 7425.75 ± 207.44 3 |
| 104 | −3.35 ± 0.15 | −3 | - |
| 10 a | −3.78 ± 0.04 | −3 | 19.13 ± 1.44 1 |
| 10 b | −3.36 ± 0.02 | −3 | 3.98 ± 0.07 2 |
| 10 c | −4.06 ± 0.10 | −3 | 4560.67 ± 436.66 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maspero, M.; Cheng, X.; von Kiedrowski, V.; Dallanoce, C.; Wängler, B.; Hübner, R.; Wängler, C. Synthesis, Characterization and In Vitro Evaluation of Hybrid Monomeric Peptides Suited for Multimodal Imaging by PET/OI: Extending the Concept of Charge—Cell Binding Correlation. Pharmaceuticals 2021, 14, 989. https://doi.org/10.3390/ph14100989
Maspero M, Cheng X, von Kiedrowski V, Dallanoce C, Wängler B, Hübner R, Wängler C. Synthesis, Characterization and In Vitro Evaluation of Hybrid Monomeric Peptides Suited for Multimodal Imaging by PET/OI: Extending the Concept of Charge—Cell Binding Correlation. Pharmaceuticals. 2021; 14(10):989. https://doi.org/10.3390/ph14100989
Chicago/Turabian StyleMaspero, Marco, Xia Cheng, Valeska von Kiedrowski, Clelia Dallanoce, Björn Wängler, Ralph Hübner, and Carmen Wängler. 2021. "Synthesis, Characterization and In Vitro Evaluation of Hybrid Monomeric Peptides Suited for Multimodal Imaging by PET/OI: Extending the Concept of Charge—Cell Binding Correlation" Pharmaceuticals 14, no. 10: 989. https://doi.org/10.3390/ph14100989
APA StyleMaspero, M., Cheng, X., von Kiedrowski, V., Dallanoce, C., Wängler, B., Hübner, R., & Wängler, C. (2021). Synthesis, Characterization and In Vitro Evaluation of Hybrid Monomeric Peptides Suited for Multimodal Imaging by PET/OI: Extending the Concept of Charge—Cell Binding Correlation. Pharmaceuticals, 14(10), 989. https://doi.org/10.3390/ph14100989








