5-Aminolevulinic Acid Triggered by Ultrasound Halts Tumor Proliferation in a Syngeneic Model of Breast Cancer
Abstract
:1. Introduction
2. Results
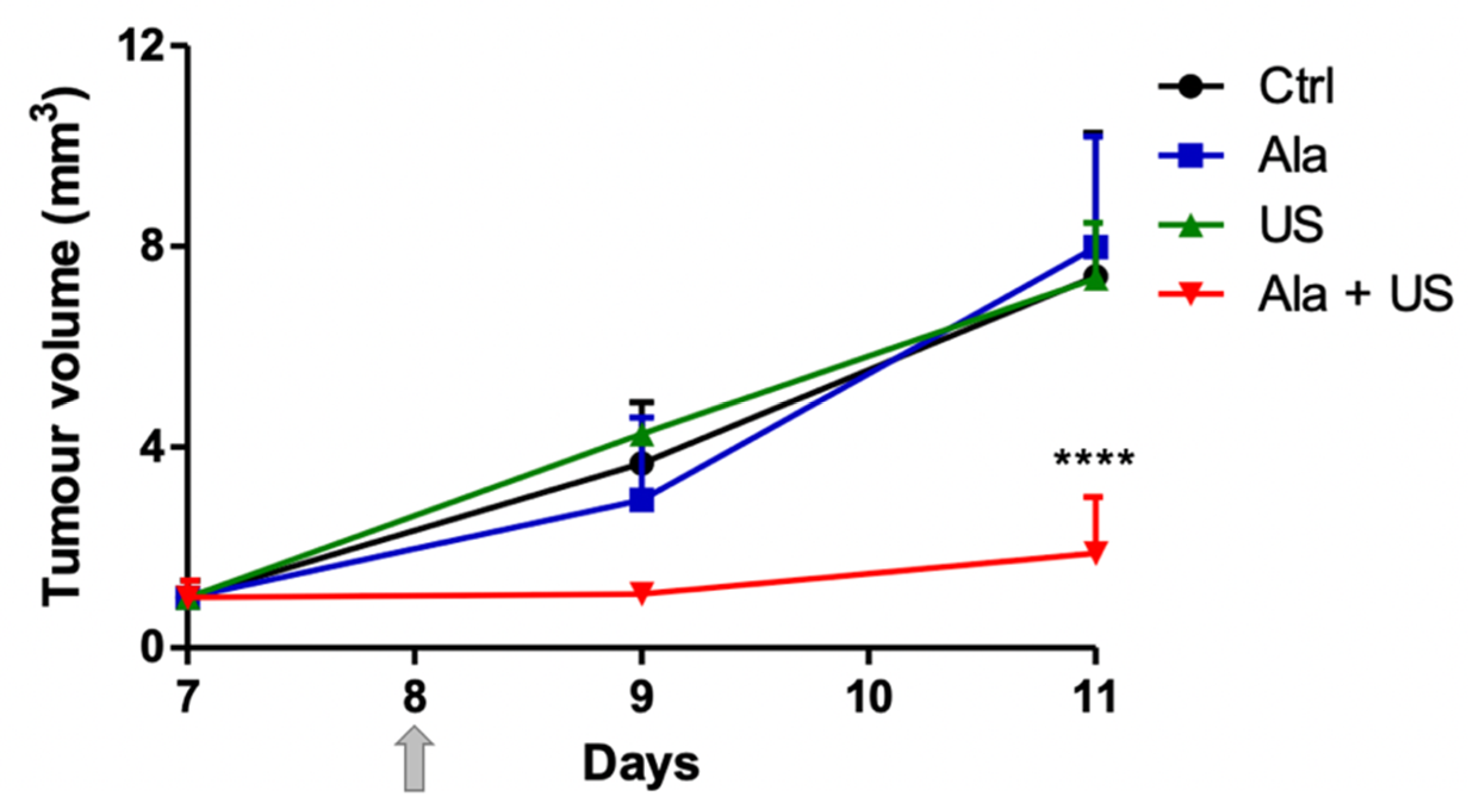
2.1. SDT Effect on Tumor Growth
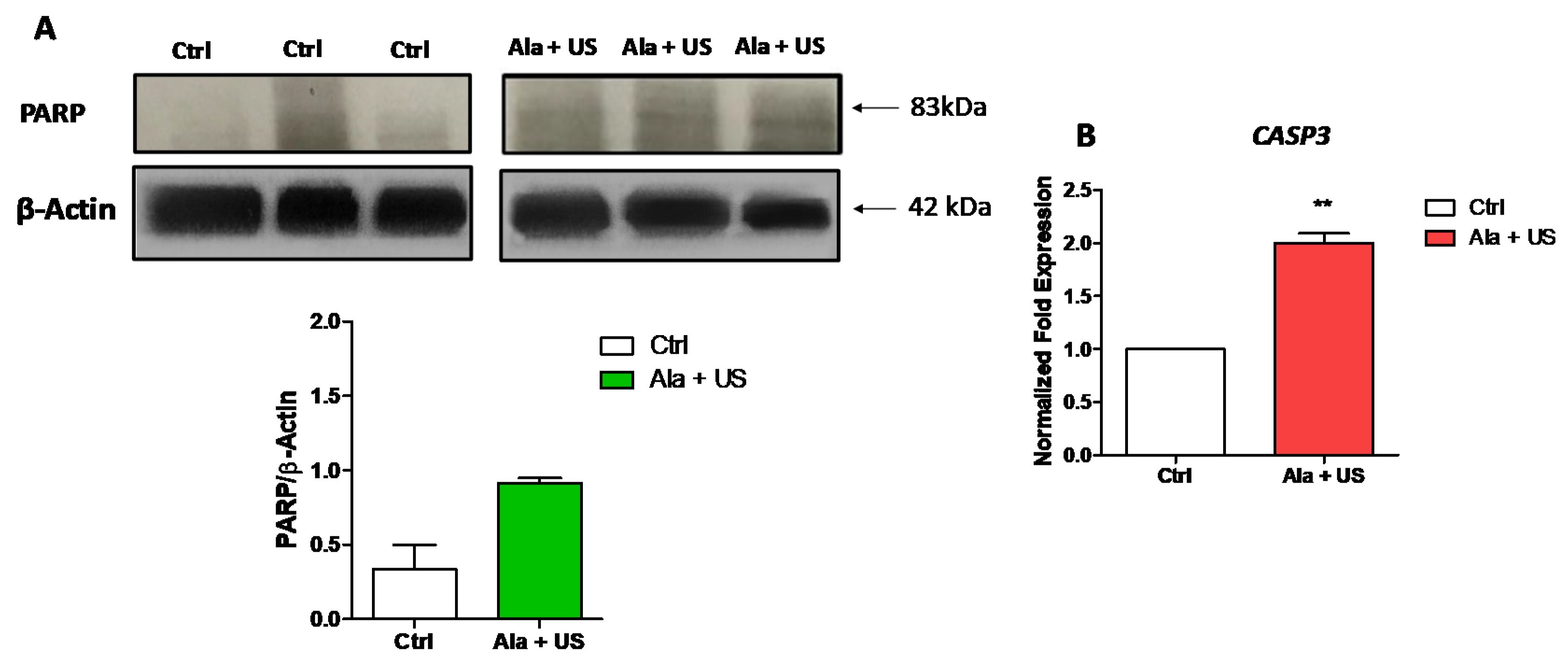
2.2. SDT Effect on PARP Cleavage and CASP3 mRNA Expression
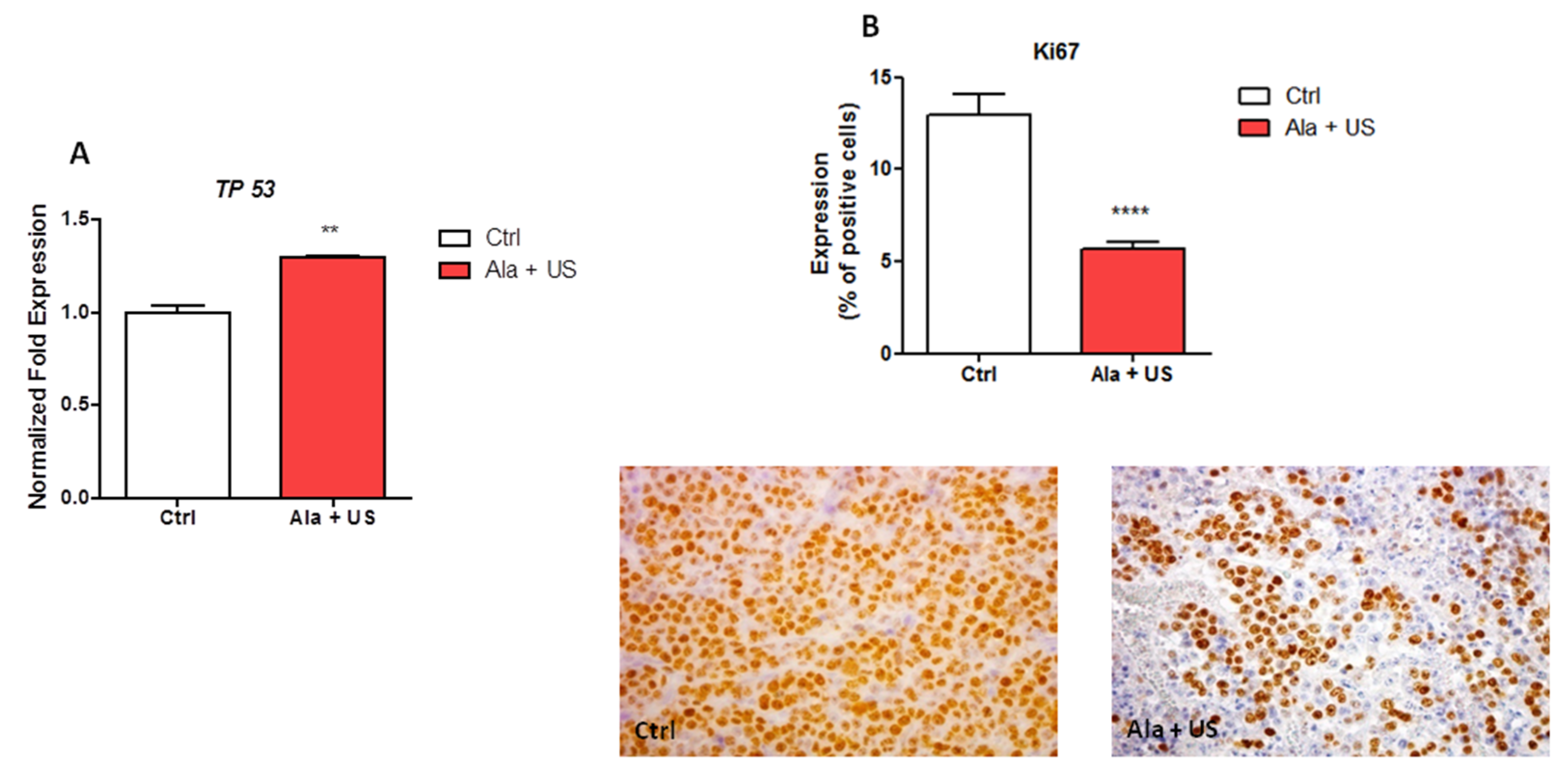
2.3. SDT Effect on TP53 mRNA Expression and on Ki67 Protein Expression
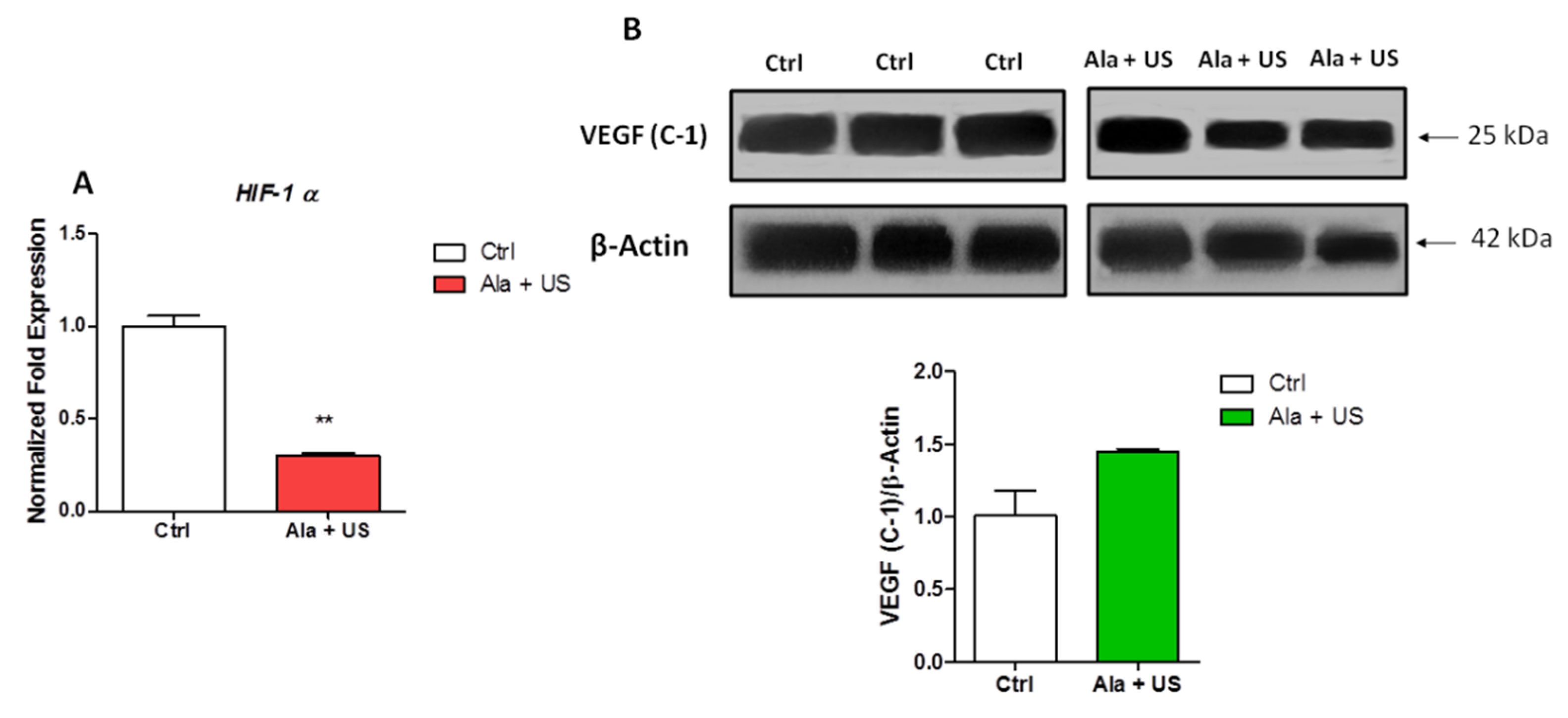
2.4. SDT Effect on HIF-1α mRNA Expression and VEGF Protein Expression
2.5. SDT Effect on NFE2L2 and NQO1 mRNA Expression
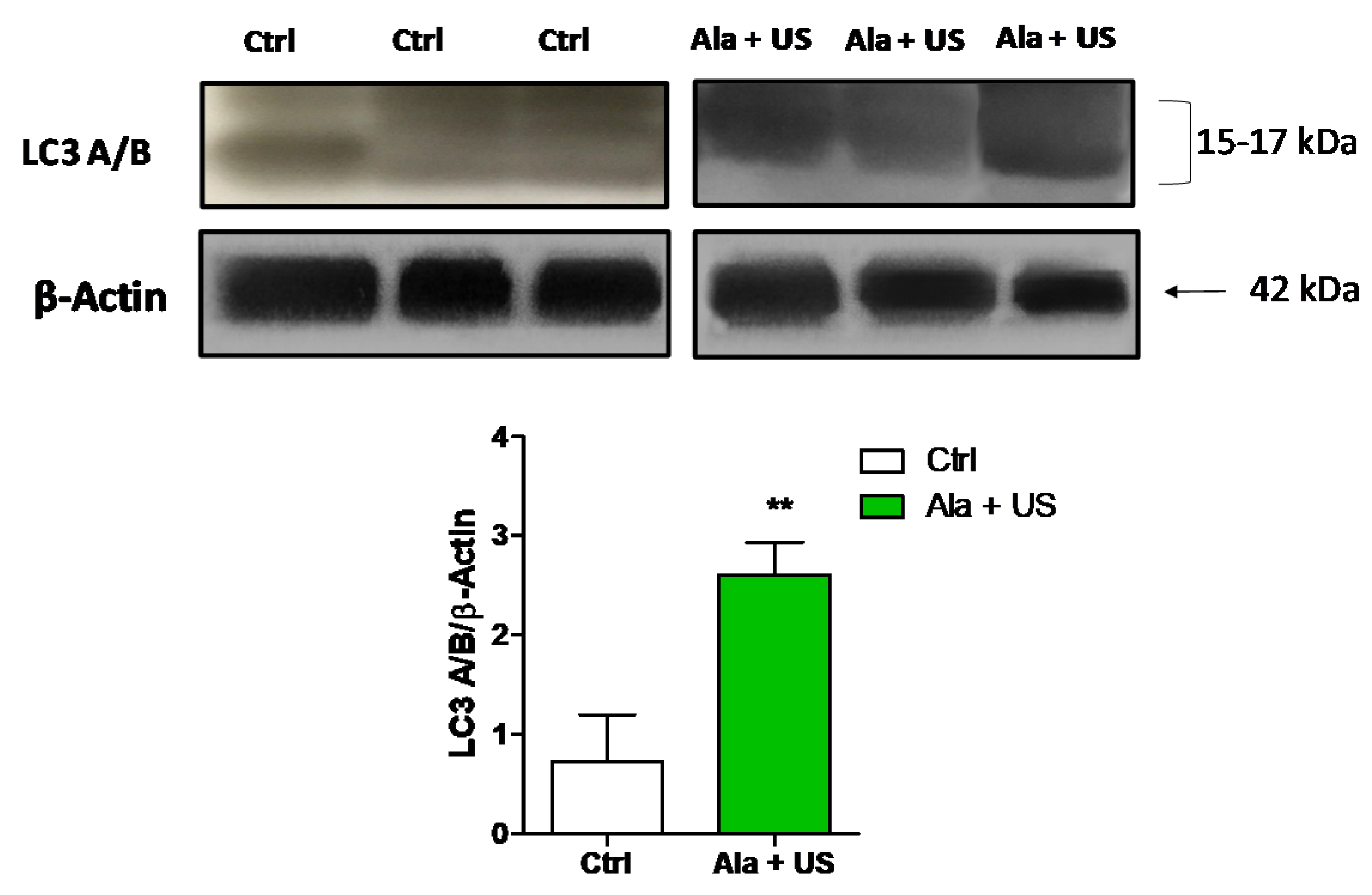
2.6. SDT Effect on LC3 A/B Protein Expression
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Sonodynamic Treatment
4.3. Western Blotting
4.4. RNA Isolation and SYBR Green Real-Time RT-PCR
4.5. Histopathological Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lafond, M.; Yoshizawa, S.; Umemura, S. Sonodynamic Therapy: Advances and Challenges in Clinical Translation: Clinical Translation of Sonodynamic Therapy. J. Ultrasound Med. 2019, 38, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Giuntini, F. Sonodynamic antimicrobial chemotherapy: First steps towards a sound approach for microbe inactivation. J. Photochem. Photobiol. B Biol. 2015, 150, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy—a review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Giuntini, F.; Foglietta, F.; Marucco, A.M.; Troia, A.; Dezhkunov, N.V.; Pozzoli, A.; Durando, G.; Fenoglio, I.; Serpe, L.; Canaparo, R. Insight into ultrasound-mediated reactive oxygen species generation by various metal-porphyrin complexes. Free. Radic. Biol. Med. 2018, 121, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Feril, L.B.; Ikeda-Dantsuji, Y. Sonodynamic therapy. Ultrasonics 2008, 48, 253–259. [Google Scholar] [CrossRef]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhou, X.; Gao, Y.; Zheng, B.; Tang, F.; Huang, J. Recent progress in development of new sonosensitizers for sonodynamic cancer therapy. Drug. Discov. Today 2014, 19, 502–509. [Google Scholar] [CrossRef]
- Collaud, S.; Juzeniene, A.; Moan, J.; Lange, N. On the Selectivity of 5-Aminolevulinic Acid-Induced Protoporphyrin IX Formation. Curr. Med. Chem.-Anti-Cancer Agents 2004, 4, 301–316. [Google Scholar] [CrossRef]
- Millon, S.R.; Ostrander, J.H.; Yazdanfar, S.; Brown, J.Q.; Bender, J.E.; Rajeha, A.; Ramanujam, N. Preferential accumulation of 5-aminolevulinic acid-induced protoporphyrin IX in breast cancer: A comprehensive study on six breast cell lines with varying phenotypes. J. Biomed. Opt. 2010, 15, 018002. [Google Scholar] [CrossRef] [Green Version]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef] [Green Version]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Leighton, T.G. What is ultrasound? Prog. Biophys. Mol. Biol. 2007, 93, 3–83. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, P.; Jiang, C.L.; Jia, D.X.; Du, X.X.; Zhou, J.H.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; et al. Sonodynamically induced anti-tumor effect of 5-aminolevulinic acid on pancreatic cancer cells. Ultrasound. Med. Biol. 2014, 40, 2671–2679. [Google Scholar] [CrossRef]
- Wood, A.K.W.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound. Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef] [Green Version]
- Krasovitski, B.; Frenkel, V.; Shoham, S.; Kimmel, E. Intramembrane cavitation as a unifying mechanism for ultrasound-induced bioeffects. Proc. Natl. Acad. Sci. USA 2011, 108, 3258–3263. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug. Deliv. Rev. 2008, 60, 1193–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suslick, K.S.; Flannigan, D.J. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Francovich, A.; Serpe, L. The bright side of sound: Perspectives on the biomedical application of sonoluminescence. Photochem. Photobiol. Sci. 2020, 19, 1114–1121. [Google Scholar] [CrossRef]
- Brazzale, C.; Canaparo, R.; Racca, L.; Foglietta, F.; Durando, G.; Fantozzi, R.; Caliceti, P.; Salmaso, S.; Serpe, L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Nanomedicine 2016, 11, 3053–3070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.-S.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, W.; Xu, Z.; Luo, Y.; Mitchell, D.; Moss, R.-W. Sonodynamic and photodynamic therapy in advanced breast carcinoma: A report of 3 cases. Integr. Cancer Ther. 2009, 8, 283–287. [Google Scholar] [CrossRef]
- Iniu, T.; Makit, K.; Miura, H.; Matsuda, A.; Kuchiike, D.; Kubo, K.; Mette, M.; Uto, Y.; Nishikata, T.; Hori, H.; et al. Case report: A breast cancer patient treated with GcMAF, sonodynamic therapy and hormone therapy. Anticancer. Res. 2014, 34, 4589–4593. [Google Scholar]
- Son, S.; Kim, J.-H.; Wang, X.; Zhang, C.; Yoon, S.-A.; Shin, J.; Sharma, A.; Lee, M.H.; Chen, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, J.; Li, Z.; Chen, H.; Gao, Y. Recent progress in sono-photodynamic cancer therapy: From developed new sensitizers to nanotechnology-based efficacy-enhancing strategies. Acta. Pharm. Sin. B 2021, 11, 2197–2219. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Li, Y.; Hamblin, M.-R. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn. Ther. 2017, 19, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, P.; Liu, J.; Yang, Y.; Chen, Q.; Zhang, Y.; Zhang, H.; Wang, X. Application of 5-aminolevulinic acid-photodynamic therapy in common skin diseases. Transl. Biophotonics 2020, 2, e201900028. [Google Scholar] [CrossRef]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000, 14, 34–44. [Google Scholar]
- Mariño, G.; Niso-Santano, M.; Baehrecke, E.H.; Kroemer, G. Self-consumption: The interplay of autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2014, 15, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. In Autophagosome and Phagosome; Deretic, V., Totowa, N.J., Walker, J.M., Eds.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2008; Volume 445, pp. 77–88. [Google Scholar]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of Cell Damage by Ultrasound in Combination with Hematoporphyrin. Jpn. J. Cancer Research 1990, 81, 962–966. [Google Scholar] [CrossRef]
- Tachibana, K.; Kimura, N.; Okumura, M.; Eguchi, H.; Tachibana, S. Enhancement of cell killing of HL-60 cells by ultrasound in the presence of the photosensitizing drug Photofrin II. Cancer Lett. 1993, 72, 195–199. [Google Scholar] [CrossRef]
- Hachimine, K.; Shibaguchi, H.; Kuroki, M.; Yamada, H.; Kinugasa, T.; Nakae, Y.; Asano, R.; Sakata, I.; Yamashita, Y.; Shirakusa, T.; et al. Sonodynamic therapy of cancer using a novel porphyrin derivative, DCPH-P-Na(I), which is devoid of photosensitivity. Cancer Sci. 2007, 98, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Foglietta, F.; Canaparo, R.; Francovich, A.; Arena, F.; Civera, S.; Cravotto, G.; Frairia, R.; Serpe, L. Sonodynamic treatment as an innovative bimodal anticancer approach: Shock wave-mediated tumor growth inhibition in a syngeneic breast cancer model. Discov. Med. 2015, 20, 197–205. [Google Scholar] [PubMed]
- Han, X.; Song, Z.; Zhou, Y.; Zhang, Y.; Deng, Y.; Qin, J.; Zhang, T.; Jiang, Z. Mitochondria-targeted high-load sound-sensitive micelles for sonodynamic therapy to treat triple-negative breast cancer and inhibit metastasis. Mater. Sci. Eng. C 2021, 124, 112054. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Ma, A.; Yin, T.; Chen, Z.; Liang, R.; Qiu, Y.; Zheng, M.; Cai, L. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer. Biomaterials 2021, 269, 120639. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Q.; Deng, Z.; Pan, M.; Liu, X.; Wu, J.; Yan, F.; Zheng, H. IR-780 Dye as a Sonosensitizer for Sonodynamic Therapy of Breast Tumor. Sci. Rep. 2016, 6, 25968. [Google Scholar] [CrossRef] [Green Version]
- Pfeffer, C.; Singh, A. Apoptosis: A Target for Anticancer Therapy. IJMS 2018, 19, 448. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [Green Version]
- Fischer, U.; Jänicke, R.U.; Schulze-Osthoff, K. Many cuts to ruin: A comprehensive update of caspase substrates. Cell Death Differ. 2003, 10, 76–100. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Penault-Llorca, F.; Radosevic-Robin, N. Ki67 assessment in breast cancer: An update. Pathology 2017, 49, 166–171. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Huang, Z.; Wu, J.; Huang, W.; Zhang, G. Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer. Cancer Biol. Med. 2019, 16, 575–586. [Google Scholar]
- Moazed, V.; Jafari, E.; Kalantari Khandani, B.; Nemati, A.; Roozdar, A.; Ben Razavi, S.A. Prognostic Significance of Reduction in Ki67 Index After Neoadjuvant Chemotherapy in Patients With Breast Cancer in Kerman Between 2009 And 2014. Iran. J. Pathol. 2018, 13, 71–77. [Google Scholar] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular Mechanisms Activating the Nrf2-Keap1 Pathway of Antioxidant Gene Regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Faraonio, R.; Vergara, P.; Di Marzo, D.; Pierantoni, M.G.; Napolitano, M.; Russo, T.; Cimino, F. p53 Suppresses the Nrf2-dependent Transcription of Antioxidant Response Genes. J. Biol. Chem. 2006, 281, 39776–39784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Wang, P.; Yang, S.; Zhang, K.; Liu, Q.; Wang, X. Sonodynamic therapy induces the interplay between apoptosis and autophagy in K562 cells through ROS. Int. J. Biochem. Cell Biology 2015, 60, 82–92. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Warren, S.; Adjemian, S.; Agostinis, P.; Martinez, A.B.; Chan, T.A.; Coukos, G.; Demaria, S.; Deutsch, E.; et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer 2020, 8, e000337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Bao, C.; Cai, X.; Jin, L.; Sun, L.; Lang, Y.; Li, L. Sonodynamic therapy-assisted immunotherapy: A novel modality for cancer treatment. Cancer Sci. 2018, 109, 1330–1345. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Jiang, X.; Sun, L.; Li, H.; Su, C.; Zhang, Y.; Xu, G.; Li, X.; Zhao, C.; Chen, Y.; et al. Continuous inertial cavitation evokes massive ROS for reinforcing sonodynamic therapy and immunogenic cell death against breast carcinoma. Nano Today 2021, 36, 101009. [Google Scholar] [CrossRef]
- Pagano, E.; Bergamo, A.; Carpi, S.; Donnini, S.; Notarbartolo di Villarosa, M.; Serpe, L.; Lucia, L. Preclinical models in oncological pharmacology: Limits and advantages. Pharmadvances 2021, 3, 402–420. [Google Scholar]
- Festing, M.-F.; Altman, D.-G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Varchi, G.; Foglietta, F.; Canaparo, R.; Ballestri, M.; Arena, F.; Sotgiu, G.; Guerrini, A.; Nanni, C.; Cicoria, G.; Cravotto, G.; et al. Engineered porphyrin loaded core-shell nanoparticles for selective sonodynamic anticancer treatment. Nanomedicine 2015, 10, 3483–3494. [Google Scholar] [CrossRef] [PubMed]
- Zeqiri, B.; Bickley, C.J. A new anechoic material for medical ultrasonic applications. Ultrasound Med. Biology 2000, 26, 481–485. [Google Scholar] [CrossRef]
- Serpe, L.; Canaparo, R.; Varchi, G.; Ballestri, M.; Federica Foglietta, F.; Sotgiu, G.; Guerrini, A.; Francovich, A.; Civera, P.; Frairia, R.; et al. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int. J. Nanomed. 2013, 8, 4247. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.-H.; Bay, B.-H.; Yip, G.; Selvarajan, S.; Tan, P.; Wu, J.; Lee, C.-H.; Li, K.-B. Immunohistochemical detection of Ki67 in breast cancer correlates with transcriptional regulation of genes related to apoptosis and cell death. Mod. Pathol. 2005, 18, 374–381. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglietta, F.; Gola, G.; Biasibetti, E.; Capucchio, M.T.; Bruni, I.; Francovich, A.; Durando, G.; Serpe, L.; Canaparo, R. 5-Aminolevulinic Acid Triggered by Ultrasound Halts Tumor Proliferation in a Syngeneic Model of Breast Cancer. Pharmaceuticals 2021, 14, 972. https://doi.org/10.3390/ph14100972
Foglietta F, Gola G, Biasibetti E, Capucchio MT, Bruni I, Francovich A, Durando G, Serpe L, Canaparo R. 5-Aminolevulinic Acid Triggered by Ultrasound Halts Tumor Proliferation in a Syngeneic Model of Breast Cancer. Pharmaceuticals. 2021; 14(10):972. https://doi.org/10.3390/ph14100972
Chicago/Turabian StyleFoglietta, Federica, Giulia Gola, Elena Biasibetti, Maria Teresa Capucchio, Iside Bruni, Andrea Francovich, Gianni Durando, Loredana Serpe, and Roberto Canaparo. 2021. "5-Aminolevulinic Acid Triggered by Ultrasound Halts Tumor Proliferation in a Syngeneic Model of Breast Cancer" Pharmaceuticals 14, no. 10: 972. https://doi.org/10.3390/ph14100972
APA StyleFoglietta, F., Gola, G., Biasibetti, E., Capucchio, M. T., Bruni, I., Francovich, A., Durando, G., Serpe, L., & Canaparo, R. (2021). 5-Aminolevulinic Acid Triggered by Ultrasound Halts Tumor Proliferation in a Syngeneic Model of Breast Cancer. Pharmaceuticals, 14(10), 972. https://doi.org/10.3390/ph14100972






