Evaluation of 64Cu-Labeled New Anti-EGFR Antibody NCAB001 with Intraperitoneal Injection for Early PET Diagnosis of Pancreatic Cancer in Orthotopic Tumor-Xenografted Mice and Nonhuman Primates
Abstract
:1. Introduction
2. Results
2.1. Radiochemical Purity and Cell-Binding Property Evaluation
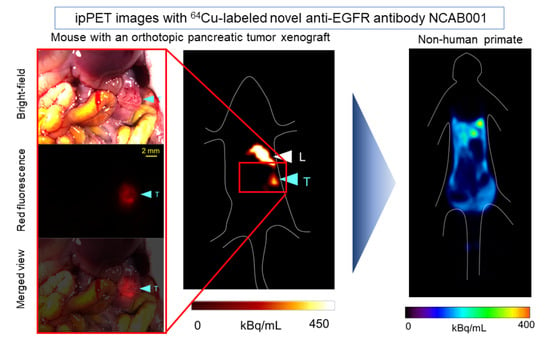
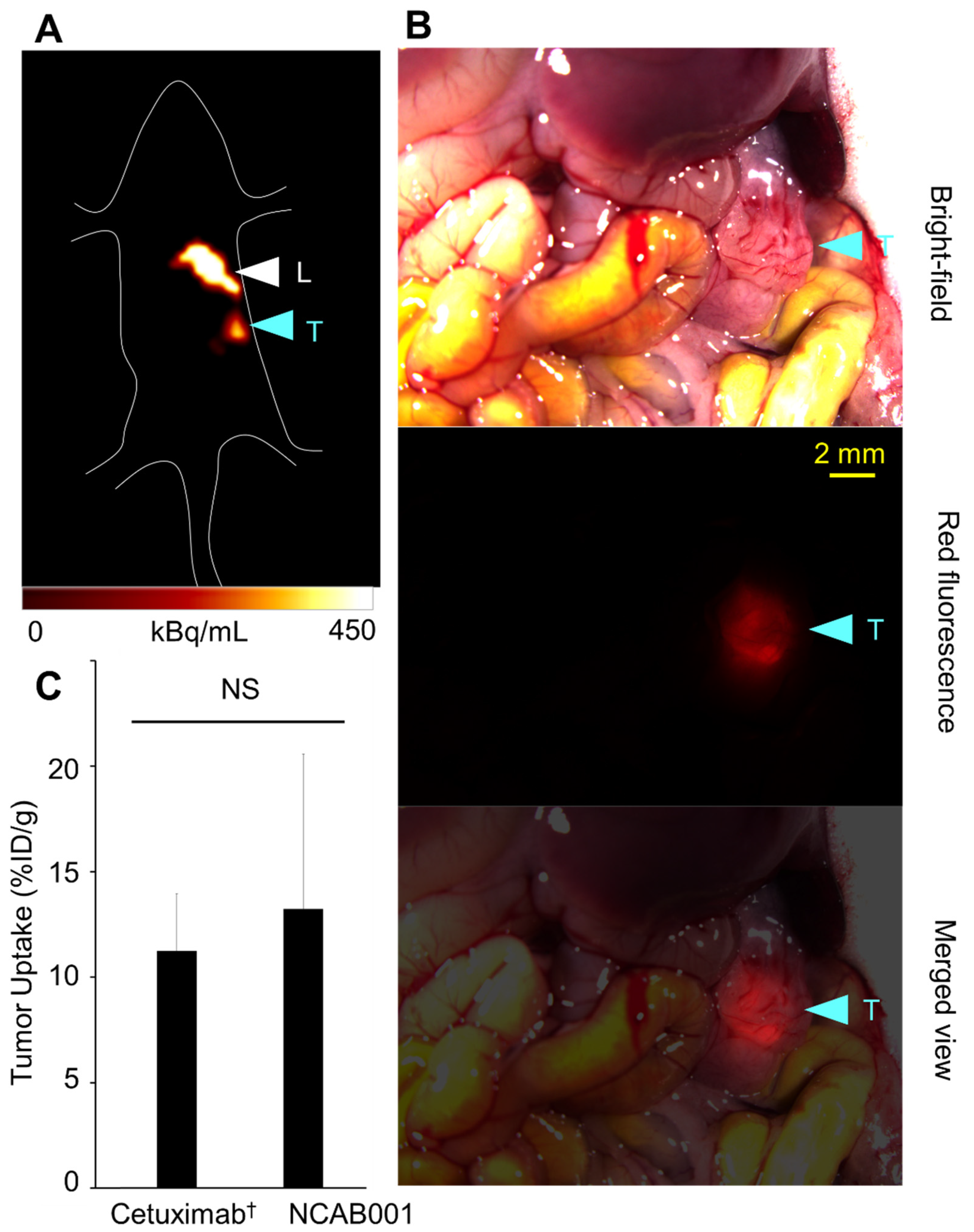
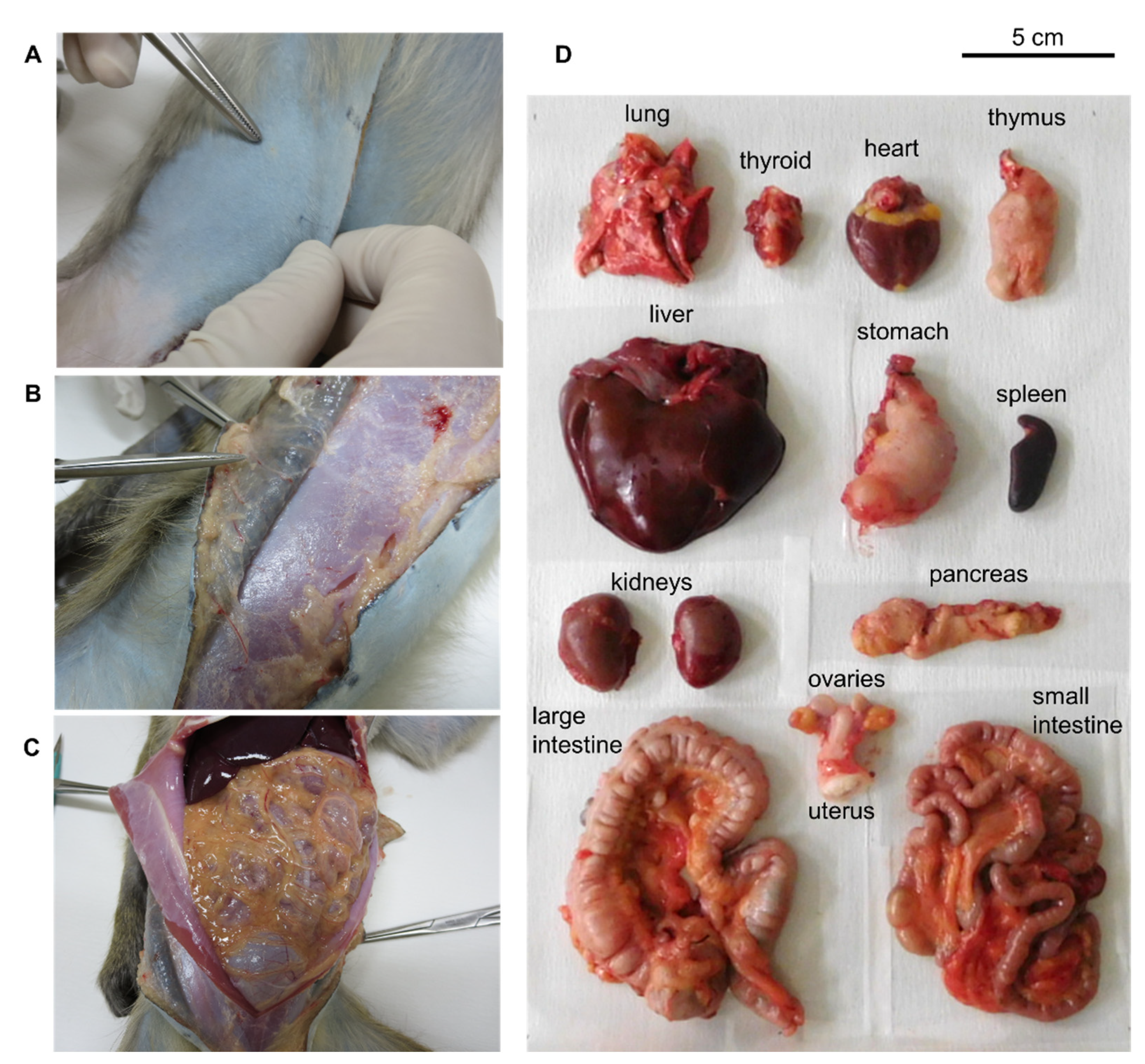
2.2. ipPET Imaging and Biodistribution in Mice
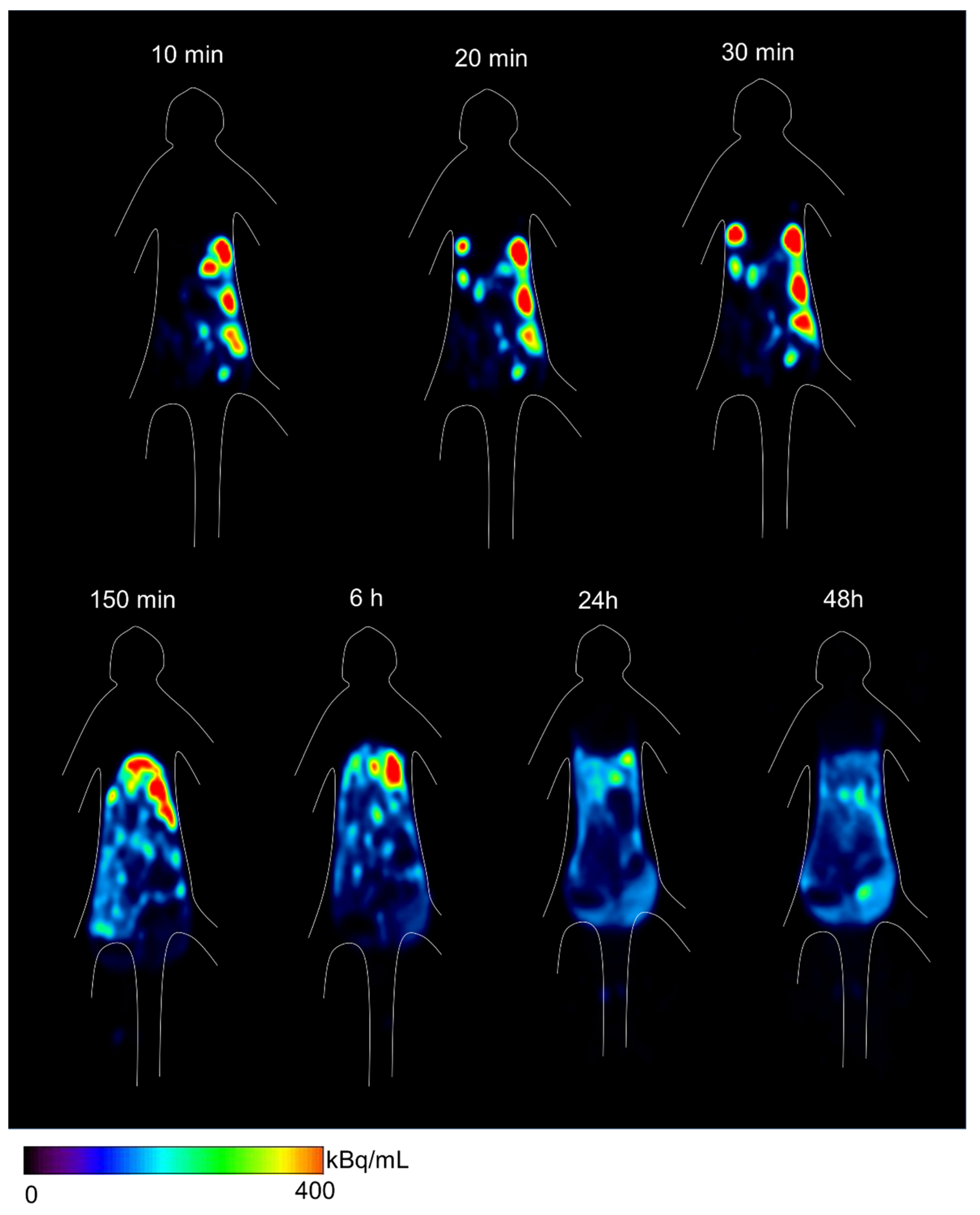
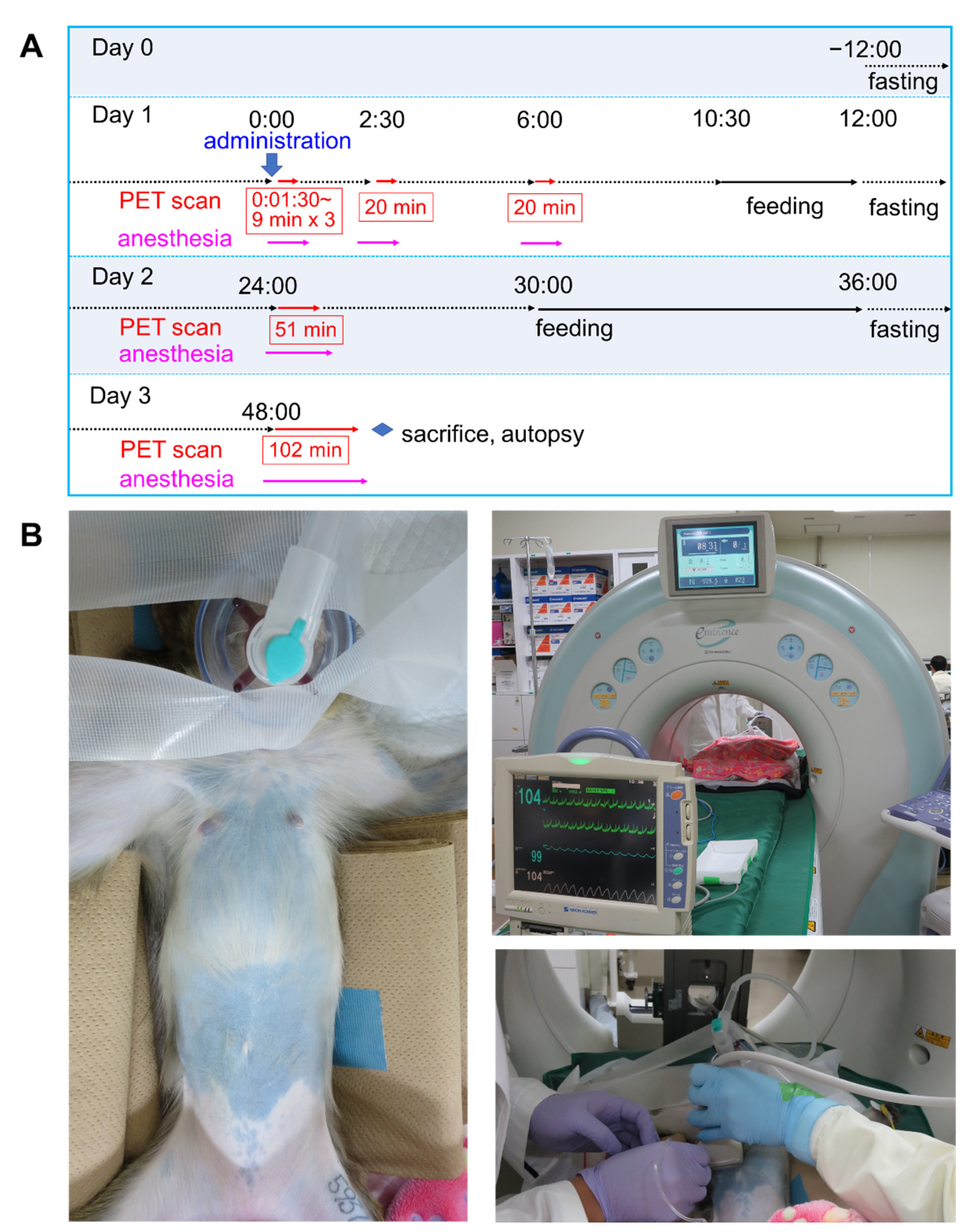
2.3. ipPET Imaging, Absorbed Radiation Dose Estimations, and Pharmacological Safety Assessments in Monkeys
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Experimental Animals
4.2. Manufacturing and Quality Control of NCAB001
4.3. Radiolabeling and Cell-Binding Assay
4.4. Imaging and Biodistribution of 64Cu-NCAB001 in Mice
4.5. 64Cu-NCAB001 ipPET Imaging in Monkeys
4.6. Image Analysis and Absorbed Radiation Dose Estimations from Monkey ipPET Images
4.7. Pharmacological Safety Assessment in Monkeys
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Lowery, M.A.; O’Reilly, E.M. Novel therapeutics for pancreatic adenocarcinoma. Hematol. Clin. N. Am. 2015, 29, 777–787. [Google Scholar] [CrossRef]
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Ariyama, J.; Suyama, M.; Satoh, K.; Sai, J. Imaging of small pancreatic ductal adenocarcinoma. Pancreas 1998, 16, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Egawa, S.; Takeda, K.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Matsuno, S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004, 28, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Kim, M.-H.; Lee, T.Y.; Kwon, S.; Oh, H.-C.; Lee, S.S.; Seo, D.W.; Lee, S.K. Clinicopathological aspects of 542 cases of pancreatic cancer: A special emphasis on small pancreatic cancer. J. Korean Med. Sci. 2007, 22, S79–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukutake, N.; Ueno, M.; Hiraoka, N.; Shimada, K.; Shiraishi, K.; Saruki, N.; Ito, T.; Yamakado, M.; Ono, N.; Imaizumi, A.; et al. A novel multivariate index for pancreatic cancer detection based on the plasma free amino acid profile. PLoS ONE 2015, 10, e0132223. [Google Scholar] [CrossRef]
- Honda, K.; Kobayashi, M.; Okusaka, T.; Rinaudo, J.A.; Huang, Y.; Marsh, T.; Sanada, M.; Sasajima, Y.; Nakamori, S.; Shimahara, M.; et al. Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein-AII isoforms. Sci. Rep. 2015, 5, 15921. [Google Scholar] [CrossRef] [PubMed]
- Słotwiński, R.; Lech, G.; Słotwińska, S.M. MicroRNAs in pancreatic cancer diagnosis and therapy. Cent. Eur. J. Immunol. 2018, 43, 314–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, G.; Lu, L.; Qiu, Y.; Ni, Q.; Zhang, W.; Gao, Y.-T.; Risch, H.A.; Yu, H.; Jia, W. Plasma metabolite biomarkers for the detection of pancreatic cancer. J. Proteome Res. 2015, 14, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Zhang, B.; Ni, Q.; Yu, X.; Xu, J. Circulating biomarkers for early diagnosis of pancreatic cancer: Facts and hopes. Am. J. Cancer Res. 2018, 8, 332–353. [Google Scholar] [PubMed]
- Oliveira-Cunha, M.; Newman, W.G.; Siriwardena, A.K. Epidermal growth factor receptor in pancreatic cancer. Cancers 2011, 3, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, J.; Backen, A.C.; Pihlak, R.; Lamarca, A.; Frizziero, M.; Tariq, N.-U.; Hubner, R.A.; Valle, J.W.; Amir, E.; McNamara, M.G. Targeting the epidermal growth factor receptor in addition to chemotherapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. Int. J. Mol. Sci. 2017, 18, 909. [Google Scholar] [CrossRef]
- Tummers, W.S.; Bs, S.E.M.; Teraphongphom, N.T.; Gomez, A.; Steinberg, I.; Huland, D.; Hong, S.; Kothapalli, S.-R.; Hasan, A.; Ertsey, R.; et al. Intraoperative pancreatic cancer detection using tumor-specific multimodality molecular imaging. Ann. Surg. Oncol. 2018, 25, 1880–1888. [Google Scholar] [CrossRef]
- Yoshii, Y.; Yoshimoto, M.; Matsumoto, H.; Tashima, H.; Iwao, Y.; Takuwa, H.; Yoshida, E.; Wakizaka, H.; Yamaya, T.; Zhang, M.-R.; et al. Integrated treatment using intraperitoneal radioimmunotherapy and positron emission tomography-guided surgery with 64Cu-labeled cetuximab to treat early- and late-phase peritoneal dissemination in human gastrointestinal cancer xenografts. Oncotarget 2018, 9, 28935–28950. [Google Scholar] [CrossRef]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Oe, Y.; Zhang, M.-R.; Nagatsu, K.; Sugyo, A.; Tsuji, A.; Higashi, T. 64Cu-intraperitoneal radioimmunotherapy: A novel approach for adjuvant treatment in a clinically relevant preclinical model of pancreatic cancer. J. Nucl. Med. 2019, 60, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, Y.; Tashima, H.; Iwao, Y.; Yoshida, E.; Wakizaka, H.; Akamatsu, G.; Yamaya, T.; Matsumoto, H.; Yoshimoto, M.; Igarashi, C.; et al. Immuno-OpenPET: A novel approach for early diagnosis and image-guided surgery for small resectable pancreatic cancer. Sci. Rep. 2020, 10, 4143. [Google Scholar] [CrossRef]
- Erbitux [Package Insert]; Eli Lilly and Co.: Indianapolis, IN, USA, 2004.
- Strohl, W.R.; Strohl, L.M. (Eds.) Cell line development. In Therapeutic Antibody Engineering; Woodhead Publishing: Cambridge, UK, 2012; pp. 421–595. [Google Scholar] [CrossRef]
- Guidance for Industry. Developing Medical Imaging Drugs and Biological Products. Part 1: Conducting Safety Assessments; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) and Center for Biologics Evaluation and Research (CBER): Bethesda, MD, USA, 2004.
- ICRP. ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context; ICRP Publication 118: 2012. Ann ICRP 41 (1/2); Elsevier: Amsterdam, The Netherland, 2012. [Google Scholar]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [Green Version]
- Martí-Climent, J.M.; Prieto, E.; Morán, V.; Sancho, L.; Rodríguez-Fraile, M.; Arbizu, J.; García-Velloso, M.J.; Richter, J.A. Effective dose estimation for oncological and neurological PET/CT procedures. EJNMMI Res. 2017, 7, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, R.G.; Noé, W.; Kopp, K.; Schlüter, M. Appropriate mammalian expression systems for biopharmaceuticals. Arzneim. Forsch. 1998, 48, 870–880. [Google Scholar]
- Achmad, A.; Hanaoka, H.; Yoshioka, H.; Yamamoto, S.; Tominaga, H.; Araki, T.; Ohshima, Y.; Oriuchi, N.; Endo, K. Predicting cetuximab accumulation in KRAS wild-type and KRAS mutant colorectal cancer using 64Cu-labeled cetuximab positron emission tomography. Cancer Sci. 2011, 103, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.-R.; Kurosawa, Y.; Saga, T. Evaluation of 89Zr-labeled human anti-CD147 monoclonal antibody as a positron emission tomography probe in a mouse model of pancreatic cancer. PLoS ONE 2013, 8, e61230. [Google Scholar] [CrossRef] [Green Version]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Okada, M.; Koizumi, M.; Satoh, H.; Kurosawa, G.; Kurosawa, Y.; Saga, T. Evaluation of efficacy of radioimmunotherapy with 90Y-labeled fully human anti-transferrin receptor monoclonal antibody in pancreatic cancer mouse models. PLoS ONE 2015, 10, e0123761. [Google Scholar] [CrossRef] [Green Version]
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.-R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A., Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef]
- Hirano, Y.; Nitta, M.; Nishikido, F.; Yoshida, E.; Inadama, N.; Yamaya, T. Induced radioactivity of a GSO scintillator by secondary fragments in carbon ion therapy and its effects on in-beam OpenPET imaging. Phys. Med. Biol. 2016, 61, 4870–4889. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, A.S.; Ice, R.D.; Beierwaltes, W.H. Author’s Reply: Radiation dosimetry of 131-I-19-iodocholesterol: The pitfalls of using tissue concentration data. J. Nucl. Med. 1975, 16, 248–249. [Google Scholar]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar]



| Animal ID | Sex | Age (Months) | Body Weight (kg) | Administered Activity (MBq) |
|---|---|---|---|---|
| 5937366897 | Female | 26 | 2.5 | 31.0 |
| 4311432746 | Female | 22 | 2.7 | 31.1 |
| 9777568022 | Male | 27 | 2.5 | 29.3 |
| 8214867971 | Male | 27 | 2.5 | 31.3 |
| All | -- | 25.5 ± 2.4 | 2.5 ± 0.1 | 30.7 ± 0.9 |
| Target Organ | Total Estimated Absorbed Dose (mSv/MBq) | ||||||
|---|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | All | |||
| Adrenals | 6.76 × 10−3 | 7.36 × 10−3 | 3.75 × 10−3 | 5.03 × 10−3 | 5.73 × 10−3 | ± | 1.65 × 10−3 |
| Brain | 4.11 × 10−5 | 4.11 × 10−5 | 1.89 × 10−5 | 2.97 × 10−5 | 3.27 × 10−5 | ± | 1.07 × 10−5 |
| Breasts | 1.30 × 10−3 | 1.32 × 10−3 | 6.56 × 10−4 | 9.64 × 10−4 | 1.06 × 10−3 | ± | 3.15 × 10−4 |
| Gallbladder wall | 1.29 × 10−2 | 1.47 × 10−2 | 8.52 × 10−3 | 1.09 × 10−2 | 1.18 × 10−2 | ± | 2.66 × 10−3 |
| Lower large intestinal wall | 7.12 × 10−2 | 4.98 × 10−2 | 6.39 × 10−2 | 5.27 × 10−2 | 5.94 × 10−2 | ± | 9.94 × 10−3 |
| Small intestine | 5.18 × 10−2 | 8.74 × 10−2 | 9.59 × 10−2 | 1.10 × 10−1 | 8.63 × 10−2 | ± | 2.48 × 10−2 |
| Stomach wall | 3.72 × 10−3 | 4.43 × 10−3 | 3.01 × 10−3 | 3.51 × 10−3 | 3.67 × 10−3 | ± | 5.89 × 10−4 |
| Upper large intestinal wall | 3.38 × 10−2 | 6.49 × 10−2 | 8.05 × 10−2 | 6.70 × 10−2 | 6.16 × 10−2 | ± | 1.97 × 10−2 |
| Heart wall | 6.56 × 10−3 | 4.82 × 10−3 | 4.94 × 10−3 | 7.05 × 10−3 | 5.84 × 10−3 | ± | 1.13 × 10−3 |
| Kidneys | 4.05 × 10−2 | 5.96 × 10−2 | 4.82 × 10−2 | 4.89 × 10−2 | 4.93 × 10−2 | ± | 7.85 × 10−3 |
| Liver | 1.42 × 10−1 | 1.45 × 10−1 | 5.65 × 10−2 | 8.42 × 10−2 | 1.07 × 10−1 | ± | 4.37 × 10−2 |
| Lungs | 7.35 × 10−3 | 5.43 × 10−3 | 2.12 × 10−3 | 6.34 × 10−3 | 5.31 × 10−3 | ± | 2.27 × 10−3 |
| Muscle | 1.81 × 10−3 | 2.10 × 10−3 | 1.47 × 10−3 | 1.74 × 10−3 | 1.78 × 10−3 | ± | 2.59 × 10−4 |
| Ovaries | 5.64 × 10−3 | 7.35 × 10−3 | 6.50 × 10−3 | * | |||
| Pancreas | 3.37 × 10−2 | 3.56 × 10−2 | 2.71 × 10−2 | 3.00 × 10−2 | 3.16 × 10−2 | ± | 3.80 × 10−3 |
| Red marrow | 2.27 × 10−3 | 2.71 × 10−3 | 2.19 × 10−3 | 2.53 × 10−3 | 2.43 × 10−3 | ± | 2.39 × 10−4 |
| Osteogenic cells | 1.49 × 10−3 | 1.68 × 10−3 | 1.05 × 10−3 | 1.28 × 10−3 | 1.38 × 10−3 | ± | 2.71 × 10−4 |
| Skin | 8.60 × 10−4 | 9.67 × 10−4 | 6.13 × 10−4 | 7.58 × 10−4 | 8.00 × 10−4 | ± | 1.51 × 10−4 |
| Spleen | 2.49 × 10−3 | 3.03 × 10−3 | 1.95 × 10−3 | 2.25 × 10−3 | 2.43 × 10−3 | ± | 4.57 × 10−4 |
| Testes | 4.42 × 10−4 | 4.37 × 10−4 | 4.40 × 10−4 | * | |||
| Thymus | 1.18 × 10−3 | 1.12 × 10−3 | 6.22 × 10−4 | 9.31 × 10−4 | 9.63 × 10−4 | ± | 2.51 × 10−4 |
| Thyroid | 2.62 × 10−4 | 2.58 × 10−4 | 1.11 × 10−4 | 1.74 × 10−4 | 2.01 × 10−4 | ± | 7.26 × 10−5 |
| Urinary bladder wall | 1.66 × 10−3 | 2.05 × 10−3 | 1.92 × 10−3 | 1.97 × 10−3 | 1.90 × 10−3 | ± | 1.69 × 10−4 |
| Uterus | 3.81 × 10−3 | 5.43 × 10−3 | 4.62 × 10−3 | * | |||
| Total body | 6.29 × 10−3 | 7.10 × 10−3 | 4.34 × 10−3 | 5.43 × 10−3 | 5.79 × 10−3 | ± | 1.18 × 10−3 |
| Effective dose equivalent | 2.32 × 10−2 | 2.75 × 10−2 | 2.32 × 10−2 | 2.49 × 10−2 | 2.47 × 10−2 | ± | 2.03 × 10−3 |
| Effective dose | 1.94 × 10−2 | 1.77 × 10−2 | 1.55 × 10−2 | 1.69 × 10−2 | 1.74 × 10−2 | ± | 1.63 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, H.; Watabe, T.; Igarashi, C.; Tachibana, T.; Hihara, F.; Waki, A.; Zhang, M.-R.; Tashima, H.; Yamaya, T.; Ooe, K.; et al. Evaluation of 64Cu-Labeled New Anti-EGFR Antibody NCAB001 with Intraperitoneal Injection for Early PET Diagnosis of Pancreatic Cancer in Orthotopic Tumor-Xenografted Mice and Nonhuman Primates. Pharmaceuticals 2021, 14, 950. https://doi.org/10.3390/ph14100950
Matsumoto H, Watabe T, Igarashi C, Tachibana T, Hihara F, Waki A, Zhang M-R, Tashima H, Yamaya T, Ooe K, et al. Evaluation of 64Cu-Labeled New Anti-EGFR Antibody NCAB001 with Intraperitoneal Injection for Early PET Diagnosis of Pancreatic Cancer in Orthotopic Tumor-Xenografted Mice and Nonhuman Primates. Pharmaceuticals. 2021; 14(10):950. https://doi.org/10.3390/ph14100950
Chicago/Turabian StyleMatsumoto, Hiroki, Tadashi Watabe, Chika Igarashi, Tomoko Tachibana, Fukiko Hihara, Atsuo Waki, Ming-Rong Zhang, Hideaki Tashima, Taiga Yamaya, Kazuhiro Ooe, and et al. 2021. "Evaluation of 64Cu-Labeled New Anti-EGFR Antibody NCAB001 with Intraperitoneal Injection for Early PET Diagnosis of Pancreatic Cancer in Orthotopic Tumor-Xenografted Mice and Nonhuman Primates" Pharmaceuticals 14, no. 10: 950. https://doi.org/10.3390/ph14100950
APA StyleMatsumoto, H., Watabe, T., Igarashi, C., Tachibana, T., Hihara, F., Waki, A., Zhang, M.-R., Tashima, H., Yamaya, T., Ooe, K., Shimosegawa, E., Hatazawa, J., Yoshida, S., Naito, K., Kurihara, H., Ueno, M., Ito, K., Higashi, T., & Yoshii, Y. (2021). Evaluation of 64Cu-Labeled New Anti-EGFR Antibody NCAB001 with Intraperitoneal Injection for Early PET Diagnosis of Pancreatic Cancer in Orthotopic Tumor-Xenografted Mice and Nonhuman Primates. Pharmaceuticals, 14(10), 950. https://doi.org/10.3390/ph14100950