Growth Inhibition of Triple-Negative Breast Cancer: The Role of Spatiotemporal Delivery of Neoadjuvant Doxorubicin and Cisplatin
Abstract
:1. Introduction
2. Results
2.1. Nanoparticle Characterization
2.2. Cell Line Characterization
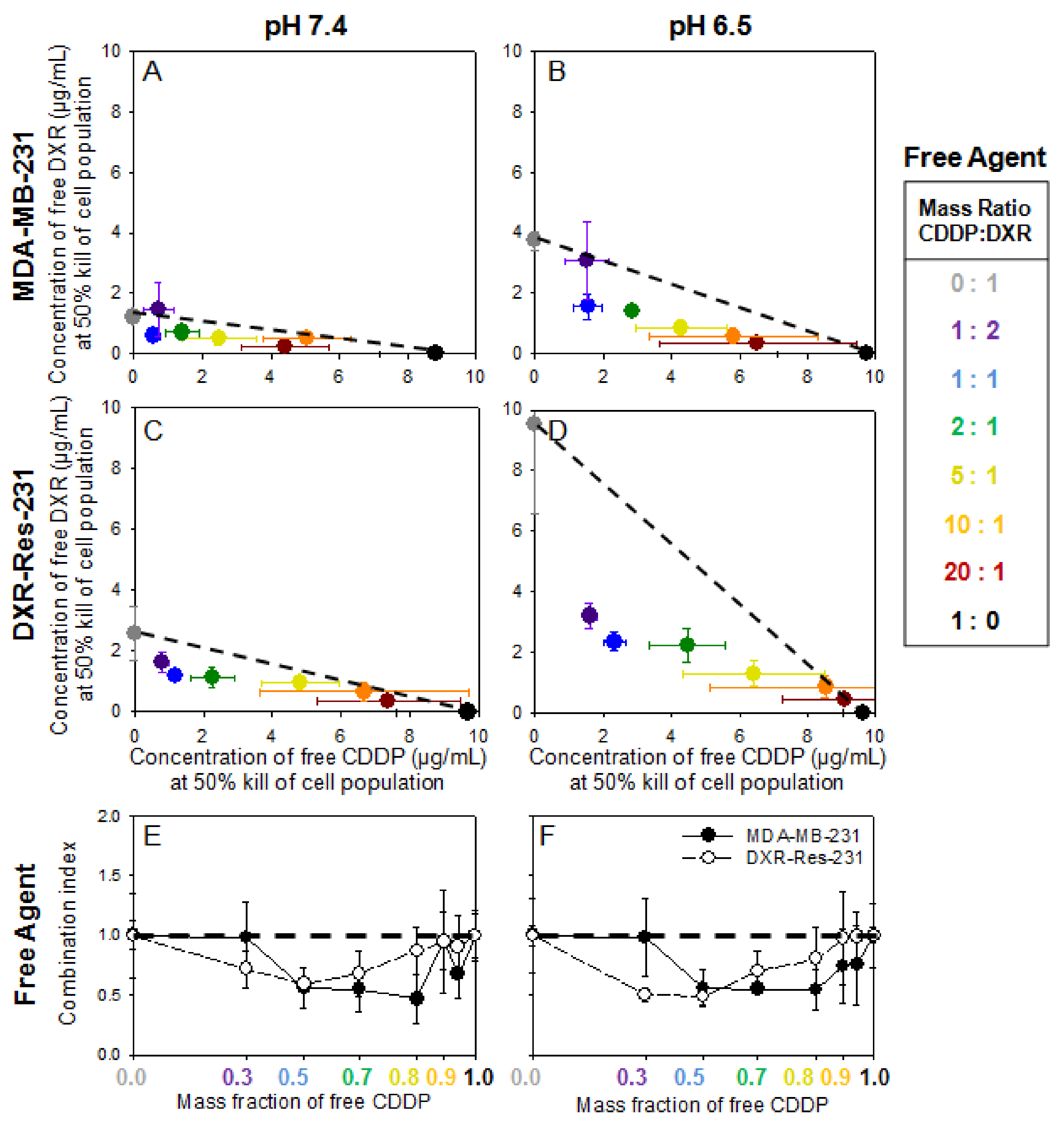
2.3. Cell Monolayers—Treatment with Free Agents
Justification for Combination of Agents for Synergistic Cytotoxic Effects—The Inhibiting Role of Extracellular Acidity
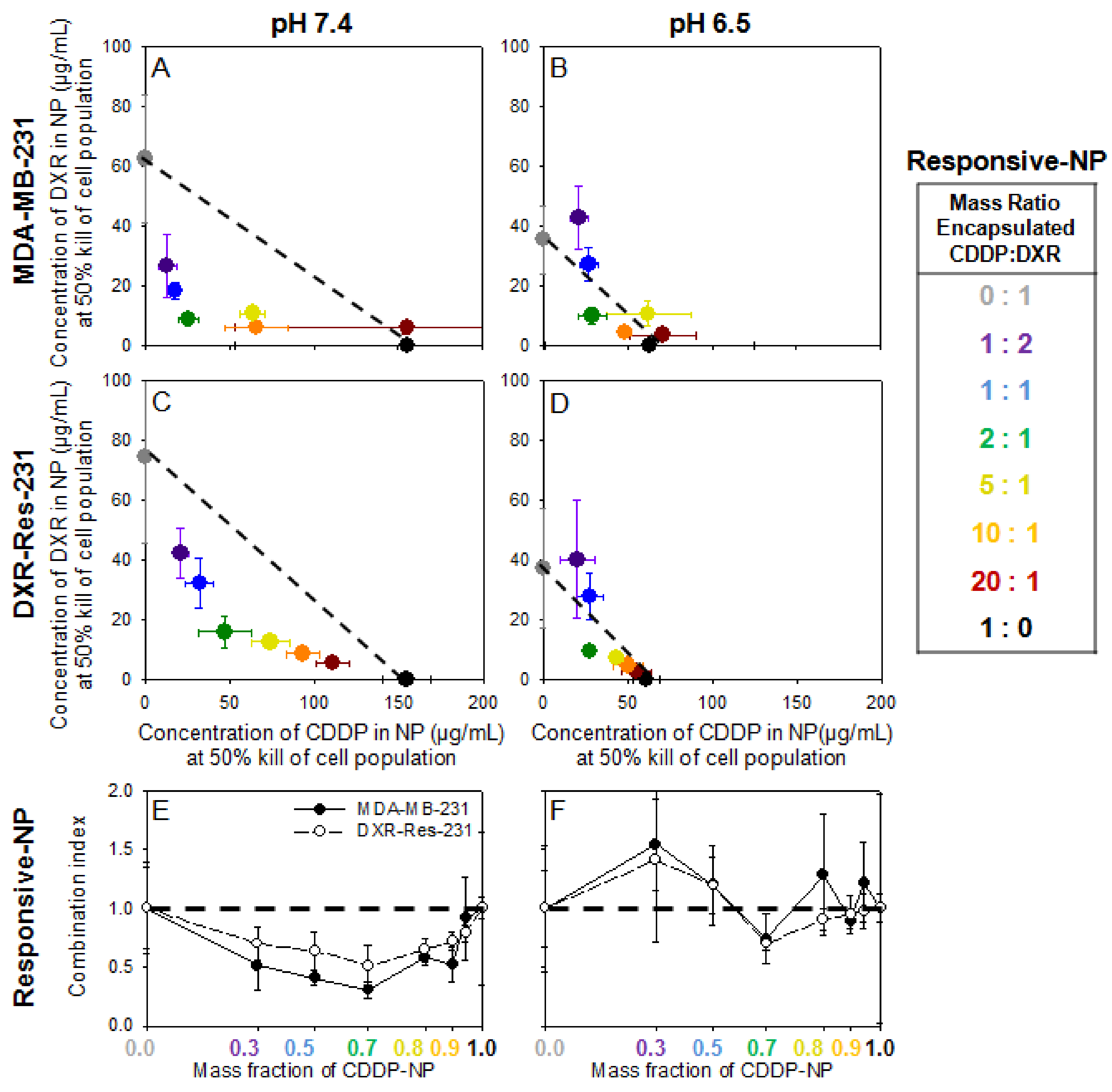
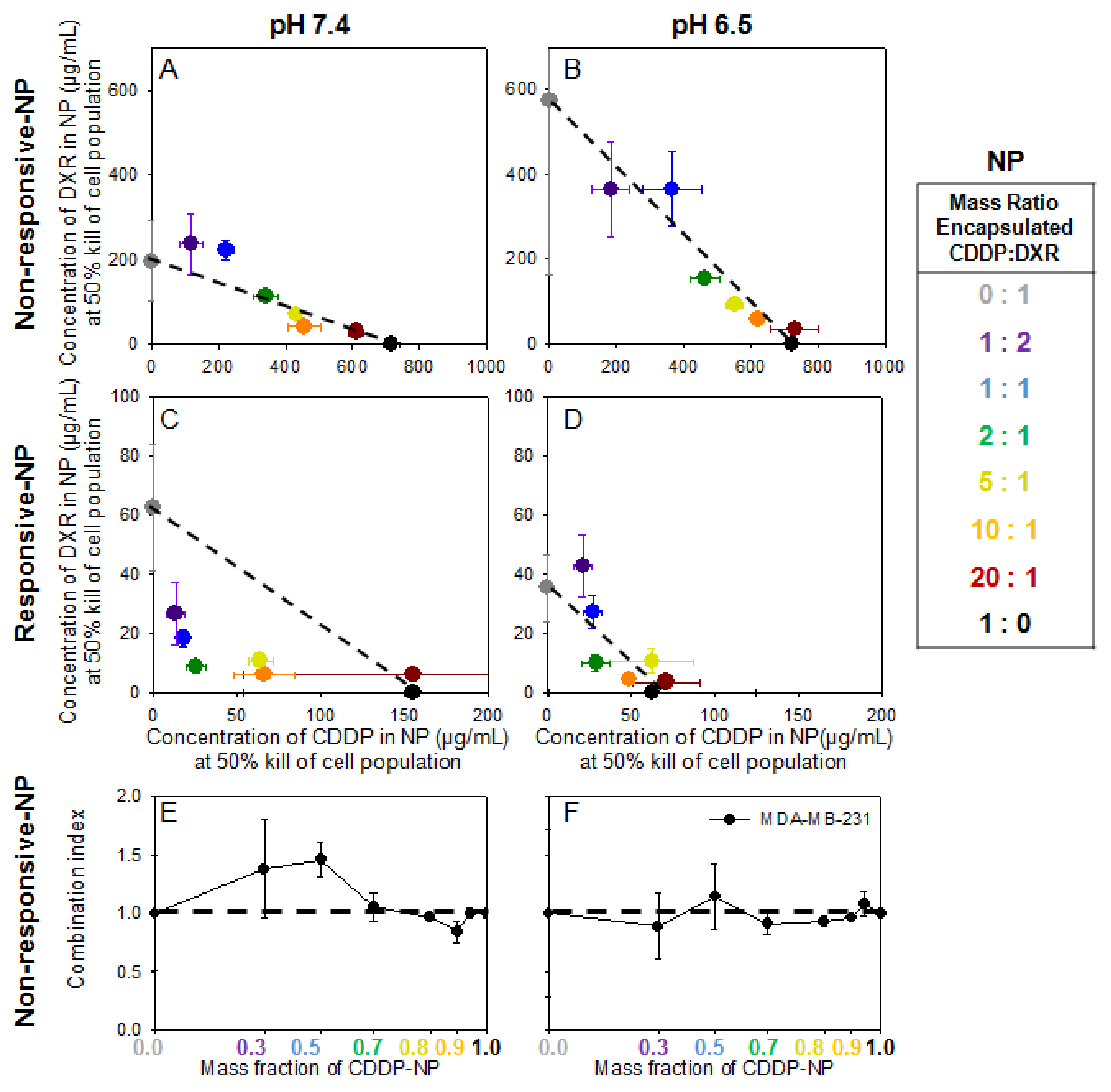
2.4. Cell Monolayers—Treatment with Agents in NP Forms
Activation of Responsive-NP Properties in the Acidic Extracellular Environment Improves Efficacy
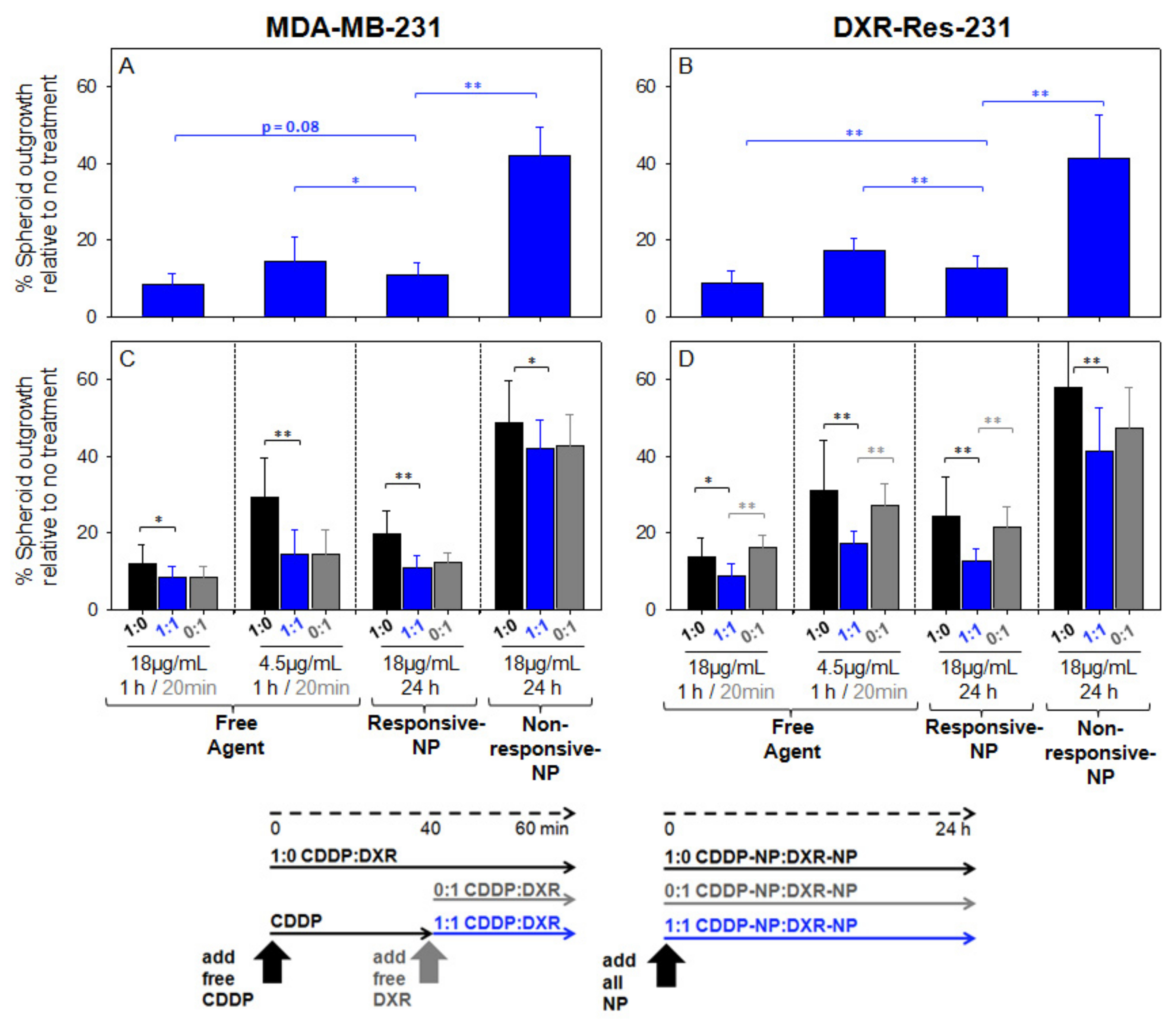
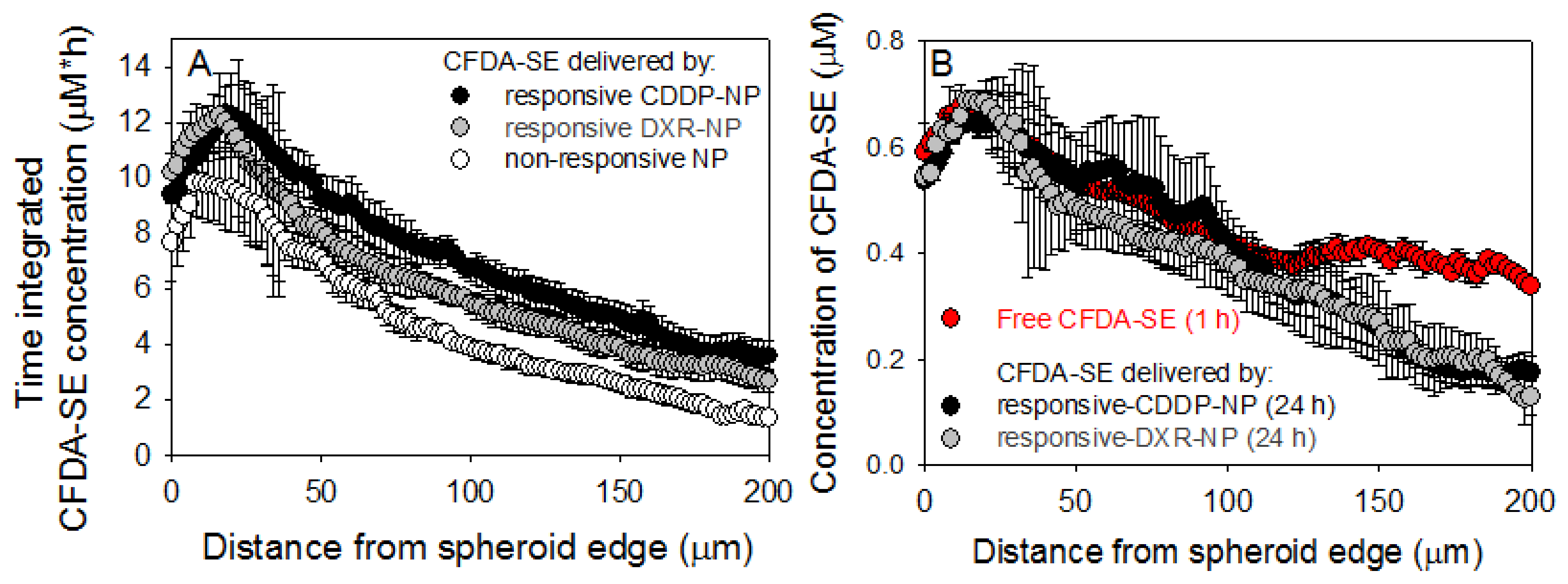
2.5. Spheroid Characterization and Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines
4.3. Development of Doxorubicin-Resistant MDA-MB-231 Cell Line (DXR-Res-231)
4.4. Lipid Nanoparticle (NP) Preparation and Characterization
4.5. Cell Monolayers—Treatment with Single Agents
4.6. Cell Monolayers—Treatment with Combination of Agents
4.7. Spheroid Formation
4.8. Spheroids—Treatment with Single and Combination of Agents
4.9. Spatiotemporal Profiles of Agents in Spheroids
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ovcaricek, T.; Frkovic, S.G.; Matos, E.; Mozina, B.; Borstnar, S. Triple negative breast cancer—Prognostic factors and survival. Radiol. Oncol. 2011, 45, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.D.; Pietenpol, J.A. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast 2015, 24 (Suppl. 2), S36–S40. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Javia, A.; Shetty, S.; Bardoliwala, D.; Maiti, K.; Banerjee, S.; Khopade, A.; Misra, A.; Sawant, K.; Bhowmick, S. Triple negative breast cancer and non-small cell lung cancer: Clinical challenges and nano-formulation approaches. J. Control. Release 2021, 337, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.R.; Metzger-Filho, O.; Sarmento, R.M.B.; Bines, J. Neoadjuvant Treatment of Stage IIB/III Triple Negative Breast Cancer with Cyclophosphamide, Doxorubicin, and Cisplatin (CAP Regimen): A Single Arm, Single Center Phase II Study (GBECAM 2008/02). Front. Oncol. 2017, 7, 329. [Google Scholar] [CrossRef] [Green Version]
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.F.; la Valle, G.; del Mastro, L.; de Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Chen, P.; Yin, X.; Sun, J.; Li, H.; Ren, G. Efficacy and safety of neoadjuvant chemotherapy regimens for triple-negative breast cancer: A network meta-analysis. Aging 2019, 11, 6286–6311. [Google Scholar] [CrossRef]
- Sempkowski, M.; Locke, T.; Stras, S.; Zhu, C.; Sofou, S. Liposome-Based Approaches for Delivery of Mainstream Chemotherapeutics: Preparation Methods, Liposome Designs, Therapeutic Efficacy. Crit. Rev. Oncol. 2014, 19, 177–221. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, M.; Zeng, J.; Feng, J.; Yu, L. Meta-analysis of clinical trials comparing the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN). Medicine 2018, 97, e13169. [Google Scholar] [CrossRef]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Basu, S.; Chen, W.; Tchou, J.; Mavi, A.; Cermik, T.; Czerniecki, B.; Schnall, M.; Alavi, A. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters. Cancer 2008, 112, 995–1000. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin. Radiat. Oncol. 2004, 14, 198–206. [Google Scholar] [CrossRef]
- Sempkowski, M.; Zhu, C.; Menzenski, M.; Kevrekidis, Y.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Triggered ligand clustering on lipid nanoparticles enables selective targeting and killing of untargetable cancer cells: The case for ‘sticky patches’. Langmuir 2016, 32, 8329–8338. [Google Scholar] [CrossRef]
- Stras, S.; Holeran, T.; Howe, A.; Sofou, S. Interstitial Release of Cisplatin from Triggerable Liposomes Enhances Efficacy Against Triple Negative Breast Cancer Solid Tumor Analogues. Mol. Pharm. 2016, 13, 3224–3233. [Google Scholar] [CrossRef]
- Prasad, A.; Nair, R.; Bhatavdekar, O.; Sempkowski, M.; Josefsson, A.; Pancheco-Torres, J.; Bhujwalla, Z.M.; Gabrielson, K.; Sgouros, G.; Sofou, S. Transport-oriented engineering of liposomes for delivery of α-particle radiotherapy: Inhibition of solid tumor progression and onset delay of spontaneous metastases. Eur. J. Nucl. Med. Mol. Imaging 2021, in press. [Google Scholar] [CrossRef]
- Stras, S.; Howe, A.; Prasad, A.; Salerno, D.; Bhatavdekar, O.; Sofou, S. Growth of Metastatic Triple-Negative Breast Cancer Is Inhibited by Deep Tumor-Penetrating and Slow Tumor-Clearing Chemotherapy: The Case of Tumor-Adhering Liposomes with Interstitial Drug Release. Mol. Pharm. 2020, 17, 118–131. [Google Scholar] [CrossRef]
- Bandekar, A.; Sofou, S. Floret-shaped solid domains on giant fluid lipid vesicles induced by pH. Langmuir 2012, 28, 4113–4122. [Google Scholar] [CrossRef]
- Karve, S.; Kempegowda, G.B.; Sofou, S. Heterogeneous domains and membrane permeability in phosphatidylcholine- phosphatidic acid rigid vesicles as a function of pH and lipid chain mismatch. Langmuir 2008, 24, 5679–5688. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Vijayappa, S.; Kozin, S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol. Cancer Ther. 2006, 5, 1275–1279. [Google Scholar] [CrossRef] [Green Version]
- Bailey, A.L.; Cullis, P.R. Modulation of Membrane Fusion by Asymmetric Transbilayer Distributions of Amino Lipids. Biochemistry 1994, 33, 12573–12580. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, A.; Zhu, C.; Menzenski, M.Z.; Gomez, A.; Sempkowski, M.; Sofou, S. Masking and triggered unmasking of targeting ligands on liposomal chemotherapy selectively suppress tumor growth in vivo. Mol. Pharm. 2013, 10, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Sempkowski, M.; Holleran, T.; Linz, T.; Bertalan, T.; Josefsson, A.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Alpha-particle radiotherapy: For large solid tumors diffusion trumps targeting. Biomaterials 2017, 130, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stathopoulos, G.P.; Rigatos, S.K.; Stathopoulos, J. Liposomal cisplatin dose escalation for determining the maximum tolerated dose and dose-limiting toxicity: A phase I study. Anticancer Res. 2010, 30, 1317–1321. [Google Scholar] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharm. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef]
- Macpherson, I.R.; Evans, T.J. New approaches in the management of advanced breast cancer—Role of combination treatment with liposomal doxorubicin. Breast Cancer 2009, 1, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; He, C.; Kron, S.J.; Lin, W. Nanoparticle formulations of cisplatin for cancer therapy. WIREs Nanomed. Nanobiotechnol. 2016, 8, 776–791. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; He, C.; Wang, A.Z.; Lin, W. Application of liposomal technologies for delivery of platinum analogs in oncology. Int. J. Nanomed. 2013, 8, 3309–3319. [Google Scholar]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Lee, H.; Shields, A.F.; Siegel, B.A.; Miller, K.D.; Krop, I.; Ma, C.X.; LoRusso, P.M.; Munster, P.N.; Campbell, K.; Gaddy, D.F.; et al. 64Cu-MM-302 Positron Emission Tomography Quantifies Variability of Enhanced Permeability and Retention of Nanoparticles in Relation to Treatment Response in Patients with Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 4190–4202. [Google Scholar] [CrossRef] [Green Version]
- Sirois, I.; Aguilar-Mahecha, A.; Lafleur, J.; Fowler, E.; Vu, V.; Scriver, M.; Buchanan, M.; Chabot, C.; Ramanathan, A.; Balachandran, B.; et al. A Unique Morphological Phenotype in Chemoresistant Triple-Negative Breast Cancer Reveals Metabolic Reprogramming and PLIN4 Expression as a Molecular Vulnerability. Mol. Cancer Res. 2019, 17, 2492–2507. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.C.; Torga, G.; Sun, Y.; Axelrod, R.; Pienta, K.J.; Sturm, J.C.; Austin, R.H. The role of heterogeneous environment and docetaxel gradient in the emergence of polyploid, mesenchymal and resistant prostate cancer cells. Clin. Exp. Metastasis 2019, 36, 97–108. [Google Scholar] [CrossRef]
- Zhang, S.; Mercado-Uribe, I.; Xing, Z.; Sun, B.; Kuang, J.; Liu, J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene 2014, 33, 116–128. [Google Scholar] [CrossRef]
- Woo, J.; Chiu, G.N.C.; Karlsson, G.; Wasan, E.; Ickenstein, L.; Edwards, K.; Bally, M.B. Use of a passive equilibration methodology to encapsulate cisplatin into preformed thermosensitive liposomes. Int. J. Pharm. 2008, 349, 38–46. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Mateen, A.; Adil, A.R.; Maken, R.N.; Hashmi, Q.A.; Abdullah, F.; Duraishi, A.M. Neoadjuvant cisplatin and doxorubicin in locally advanced triple negative breast cancer. J. Clin. Oncol. 2016, 34 (Suppl. 15), e12511. [Google Scholar] [CrossRef]
- Saltzman, M.W. Engineering Principles for Drug Therapy; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
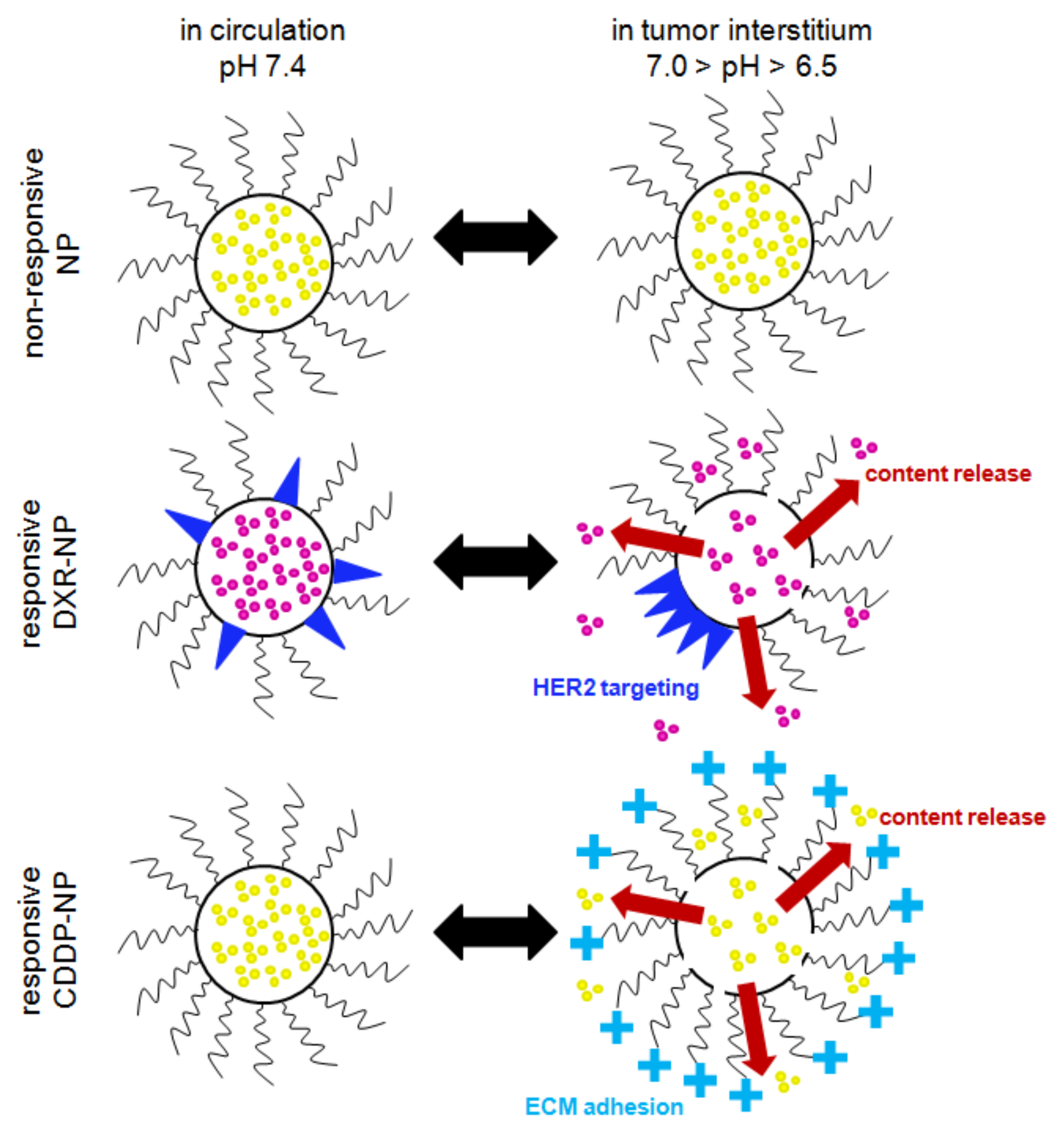
| DXR-NP (n = 5) | Size, nm (PDI) | Zeta Potential (mV) | % Loading Efficiency | Drug-to-Lipid Ratio (w/w) | % of Cell Associated DXR (6 h Incubation) with MDA-MB-231 Cells | Release Kinetics Fitting Parameters y = y∞ + exp(−t/τ 1/2 ) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 6.5 | ||||||||||
| pH 7.4 | pH 6.0 | pH 7.4 | pH 6.5 | y ∞ (%) | τ1/2 (min) | y ∞ (%) | τ1/2 (min) | ||||
| Responsive | 162 ± 19 (0.11 ± 0.06) | −5.91 ± 0.60 | −5.74 ± 0.89 | 61 ± 4 | 0.066 ± 0.011 | 1.09 ± 0.18 | 1.71 ± 0.24 | 90 ± 1.2 | 66 ± 31 | 70 ± 1.3 | 21 ± 4.2 |
| Non-Responsive | 123 ± 6 (0.09 ± 0.05) | −4.57 ± 0.63 | −4.15 ± 0.52 | 71 ± 8 | 0.090 ± 0.016 | 0.79 ± 0.16 | 0.84 ± 0.31 | 90 ± 1.3 | 120 ± 56 | 90 ± 0.9 | 54 ± 18 |
| CDDP-NP (n = 5) | Size, nm (PDI) | Zeta Potential (mV) | % Loading Efficiency ╪ | Drug-to-Lipid Ratio (w/w) | Release Kinetics Fitting Parameters y = y∞ + exp(−t/τ 1/2 ) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 6.5 | |||||||||
| pH 7.4 | pH 6.5 | pH 6.0 | y ∞ (%) | τ1/2 (min) | y ∞ (%) | τ1/2 (min) | ||||
| Responsive | 123 ± 5 (0.12 ± 0.05) | −2.06 ± 0.41 | −0.97 ± 0.46 | −0.11± 0.47 | 5.8 ± 0.98 | 0.090 ± 0.011 | 87 ± 1.5 | 150 ± 45 | 71 ± 0.5 | 131 ± 7 |
| Non-Responsive | 115 ± 6 (0.10 ± 0.04) | −4.57 ± 0.63 | −4.48 ± 0.56 | −4.15 ± 0.52 | 6.2 ± 0.73 | 0.103 ± 0.054 | 89 ± 0.3 | 133 ± 10 | 88 ± 0.3 | 157 ± 14 |
| Cell Line Characterization | Doubling Time (h) | HER-2 Expression, Receptors per Cell (KD, nM) | IC50 of Free DXR (µg/mL) | IC50 of Free CDDP (µg/mL) | ||
|---|---|---|---|---|---|---|
| pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | |||
| MDA-MB-231 (ATCC) | >36 ± 3 | >83,345 ± 10,117 (8.45 ± 3.81) | >1.20 ± 0.14 | >3.74 ± 0.31 | >8.82 ± 1.66 | >9.73 ± 2.63 |
| DXR-Res-231 | 34 ± 4 | 77,202 ± 7166 (8.00 ± 2.80) | 2.57 ± 0.90 | 9.52 ± 2.95 | 9.67 ± 2.07 | 9.63 ± 0.61 |
| Degree of Resistance (IC50 Resistant/IC50 Naïve) | 2.1 ± 0.8 | 2.5 ± 0.8 | 1.1 ± 0.3 | 1.0 ± 0.3 | ||
| IC50 of Responsive-DXR-NP (µg/mL) | IC50 of Responsive-CDDP-NP (µg/mL) | IC50 of Non-Responsive-DXR-NP (µg/mL) | IC50 of Non-Responsive-CDDP-NP (µg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | pH 7.4 | pH 6.5 | |
| MDA-MB-231 (ATCC) | 62 ± 21 | 35 ± 11 | 155 ± 101 | 63 ± 62 | 195 ± 67 | 575 ± 290 | 716 ± 25 | 721 ± 8 |
| DXR-Res-231 | 75 ± 30 | 37 ± 20 | 154 ± 15 | 61 ± 7 | Not measurable ╪ | 713 ± 52 | 761 ± 48 | |
| NP Compositions (Mole %) | pH- Triggered Content Release | ECM Adhesion | HER2 Targeting | 20PC | DPPS | Cholesterol | DSPE-18PEG | DPPE-Rhodamine | PEG-DAP | HER2-Targeting Lipopeptide | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DXR- NP | Responsive | + | − | + | 81 | 9 | 4.5 | 4 | 0.5 | − | 1 |
| Non-Responsive | − | − | − | 76.5 | − | 19 | 4 | 0.5 | − | − | |
| CDDP-NP | Responsive | + | + | − | 53 | 35 | 4.5 | − | 0.5 | 7 | − |
| Non-Responsive | − | − | − | 73.5 | − | 19 | 7 | 0.5 | − | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, D.; Sofou, S. Growth Inhibition of Triple-Negative Breast Cancer: The Role of Spatiotemporal Delivery of Neoadjuvant Doxorubicin and Cisplatin. Pharmaceuticals 2021, 14, 1035. https://doi.org/10.3390/ph14101035
Salerno D, Sofou S. Growth Inhibition of Triple-Negative Breast Cancer: The Role of Spatiotemporal Delivery of Neoadjuvant Doxorubicin and Cisplatin. Pharmaceuticals. 2021; 14(10):1035. https://doi.org/10.3390/ph14101035
Chicago/Turabian StyleSalerno, Dominick, and Stavroula Sofou. 2021. "Growth Inhibition of Triple-Negative Breast Cancer: The Role of Spatiotemporal Delivery of Neoadjuvant Doxorubicin and Cisplatin" Pharmaceuticals 14, no. 10: 1035. https://doi.org/10.3390/ph14101035
APA StyleSalerno, D., & Sofou, S. (2021). Growth Inhibition of Triple-Negative Breast Cancer: The Role of Spatiotemporal Delivery of Neoadjuvant Doxorubicin and Cisplatin. Pharmaceuticals, 14(10), 1035. https://doi.org/10.3390/ph14101035





