Abstract
Folium Sennae (FS), a popular laxative (Senna), contains polyphenolic anthranoids, whose conjugation metabolites are probable modulators of multidrug resistance-associated proteins (MRPs) and breast cancer resistance protein (BCRP). We suspected that the combined use of FS might alter the pharmacokinetics of various medicines transported by MRPs or BCRP. This study investigated the effect of FS on the pharmacokinetics of methotrexate (MTX), an anticancer drug and a probe substrate of MRPs/BCRP. Rats were orally administered MTX alone and with two dosage regimens of FS in a parallel design. The results show that 5.0 g/kg of FS significantly increased the AUC0–2880, AUC720–2880 and MRT of MTX by 45%, 102% and 42%, and the seventh dose of 2.5 g/kg of FS significantly enhanced the AUC720–2880 and MRT by 78% and 42%, respectively. Mechanism studies indicated that the metabolites of FS (FSM) inhibited MRP 2 and BCRP. In conclusion, the combined use of FS increased the systemic exposure and MRT of MTX through inhibition on MRP 2 and BCRP.
1. Introduction
Constipation is a common symptom for the elderly and women, and deteriorates their quality of life [1,2,3]. Moreover, numerous medicines have side effect of constipation in patients [4,5,6,7]. Folium Sennae (FS), the leaves of Cassia angustifolia VAHL (Fabaceae), is a popular laxative (Senna) worldwide in clinical practice and also a common component in the food supplements claimed for weight control [8,9,10,11]. FS contains dianthrone glycosides, namely sennosides A and B, together with other anthraquinones, such as rhein, aloe-emodin and chrysophanol [8,12], which have shown beneficial biological effects including anti-microbial, anti-fungal, anti-inflammatory, anti-oxidant, anti-diabetic and anti-tumor activities [13,14,15,16].
In recent decades, drug transporters have well been recognized as crucial determinants governing the absorption, distribution and excretion of drugs as well as playing important roles in drug-drug, supplement-drug and food-drug interactions [17,18,19,20,21,22,23]. FS and its polyphenolic anthraquinones have exhibited modulations on a number of drug transporters, such as P-glycoprotein (P-gp) [24,25], organic anion-transporting polypeptides (OATPs) and organic anion transporters (OATs), which were reported by in vitro studies [23,26,27]. However, in recent decades there was a consensus that polyphenols were rapidly and extensively metabolized, then mainly existed as conjugated metabolites in the circulation and organs [28,29,30]. Moreover, our pharmacokinetic study of FS reported that the major molecules in the plasma were rhein, rhein sulfate/glucuronide (S/G) and aloe-emodin S/G, which were all acids existing as anions in the serum [24]. The transports of these anionic metabolites were often mediated by modulate multidrug resistance-associated protein 2 (MRP 2) and breast cancer resistance protein (BCRP) [24,31]. Therefore, we hypothesized that these acidic metabolites of FS might modulate MRPs and BCRP.
MRPs are distributed widely in the body, and transport a number of anticancer drugs such as methotrexate (MTX), vinblastine, 5-fluorouracil, doxorubicine and etoposide [32,33,34]. BCRP is located in brain, breast cells, liver, intestine, kidney, placenta, amd testis [19], and transports a number of anticancer drugs such as MTX, daunorubicin, doxorubicin, gefitinib, imatinib, ireinotecan, mitoxantrone and sunitinib [35]. This study investigated the effect of FS on the pharmacokinetics of MTX, a dicarboxylate immunosuppressant and anticancer drug used as a probe substrate of MRPs/BCRP, in rats [36,37]. Furthermore, cell lines were employed to verify the transporter-based relevant mechanisms.
2. Results
2.1. FS-MTX Pharmacokinetic Interaction and Relevant Mechanisms
2.1.1. Effect of FS on MTX Pharmacokinetics in Rats
The serum MTX profiles after oral dosing of MTX without and with single dose of 5.0 g/kg of FS and the seventh dose of 2.5 g/kg of FS are shown in Figure 1. The pharmacokinetic parameters of MTX after various treatments are listed in Table 1. When 5.0 g/kg of FS was coadministered, AUC720–2880, AUC0–2880 and MRT of MTX were significantly increased by 102, 45 and 42%, respectively, whereas AUC0–240 was decreased by 26%, and Cmax was not affected. On other hand, after pretreatment with seven doses of FS (2.5 g/kg), the AUC 720–2880 and MRT of MTX was significantly increased by 78 and 42%, respectively, whereas AUC0–240 was decreased by 25% and Cmax was not affected.
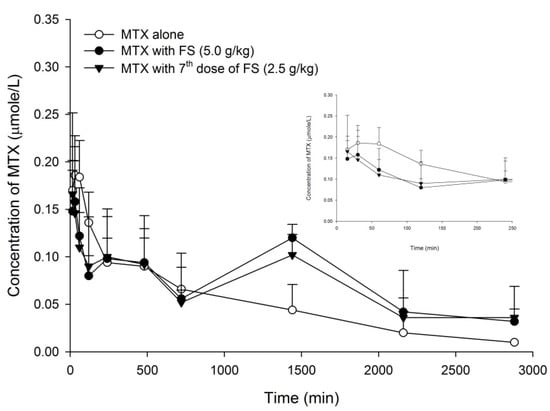
Figure 1.
Mean (±S.E.) serum concentration-time profiles of MTX after oral MTX alone (5.0 mg/kg, ○) and with single dose of FS (5 g/kg, ●), and the 7th dose of FS (2.5 g/kg, ▼) (n = 5 in each group).

Table 1.
Pharmacokinetic parameters of MTX after oral MTX alone (5.0 mg/kg) and with single dose of 5.0 g/kg of FS, and the 7th dose of 2.5 g/kg of FS.
2.1.2. Characterization of FSM
In order to mimic the real molecules present in the kidney, we have prepared FSM from rats. Characterization of FSM showed that for the study of influence on BCRP activity, the concentrations of rhein, rhein S/G and aloe-emodin S/G were 14.5, 7.2 and 0.6 μM, respectively; on the other hand, for the study of influence on MRP 2 activity, the concentrations of rhein, rhein S/G and aloe-emodin S/G were 25.0, 14.0 and 4.5 μM, respectively.
2.1.3. Effects of FS, FSM and Rhein on BCRP Activity
The accumulation of MXR in MDCKII-BCRP cells measured after 30-min incubation with tested agents is shown in Figure 2 and Figure 3. Figure 2 shows that FS at 2.5 mg/mL significantly enhanced the intracellular accumulation of MXR by 51%. As a positive control of BCRP inhibitor, Ko143, at 0.5 μM significantly enhanced the intracellular accumulation of MXR by 216%. Figure 3 indicates that when compared to those of blank serum specimen at corresponding concentrations, FSM at 1/4- and 1/2-fold serum concentrations markedly enhanced the intracellular accumulation of MXR by 27 and 42%, respectively. Conversely, 25 μM of rhein reduced the intracellular accumulation of MXR by 33.7%. As a positive control of BCRP inhibitor, Ko143 at 0.5 μM significantly enhanced the intracellular accumulation of MXR by 157%.
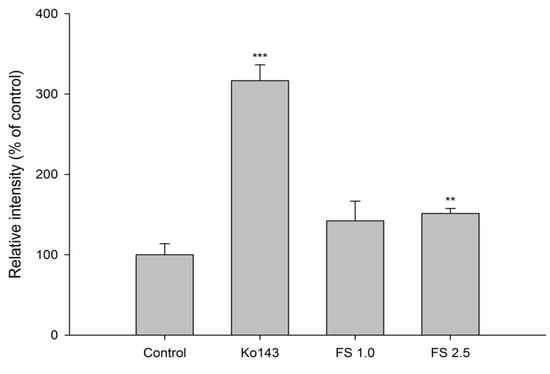
Figure 2.
Effects of FS (1.0 and 2.5 mg/mL) and Ko143 (0.5 μM) on the intracellular accumulation of MXR in MDCKII-BCRP cells. ** p < 0.01 and *** p < 0.001.
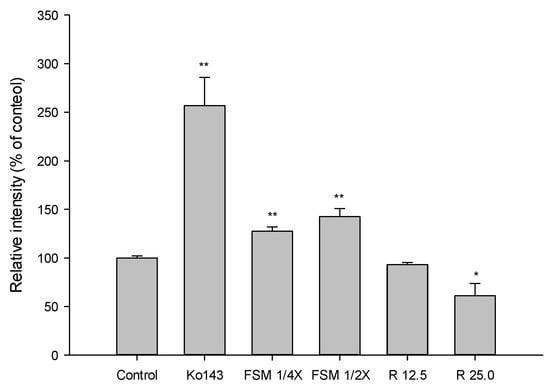
Figure 3.
Effects of FSM (1/4 and 1/2-fold serum concentrations), rhein (R, μM) and Ko143 (0.5 μM) on the intracellular accumulation of MXR in MDCKII-BCRP cells. * p < 0.05 and ** p < 0.01.
2.1.4. Effect of FSM and Rhein on MRP 2 Activity
The accumulation of GSMF in MDCKII cells measured after 30-min incubation with tested agents is shown in Figure 4. The results indicate that FSM at 1/4- and 1/2- fold serum concentrations significantly increased the intracellular accumulation of GSMF by 31% and 33 %, respectively. In addition, 12.5 and 25 μM of rhein increased the intracellular accumulation of GSMF by 99 and 233%, respectively. As a positive control of MRP 2 inhibitor, MK571 at 10 μM significantly increased the intracellular accumulation of GSMF by 467%.
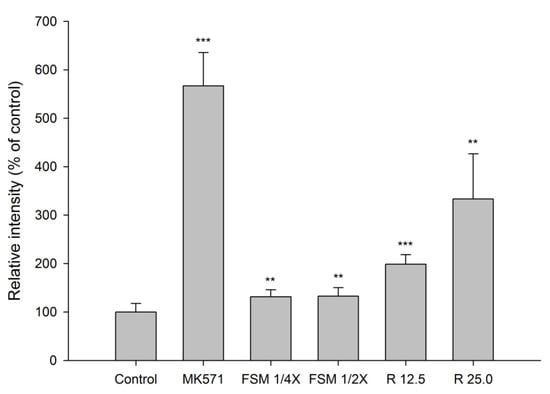
Figure 4.
Effects of FSM at 1/4 and 1/2-fold serum concentrations, rhein (R, μM) and MK571 (10 μM) on the intracellular accumulation of GSMF in MDCK II cells. ** p < 0.01 and *** p < 0.001.
3. Discussions
In the present study, MTX was selected as a probe for evaluating the influence of FS on the pharmacokinetics of MRPs/BCRP substrates. A seven-dose regimen of FS was intentionally designed to mimic the long-term use of FS. The results show that a single dose of FS (5.0 g/kg) increased the AUC0–2880, AUC720–2880 and MRT of MTX, and the seventh dose of FS (2.5 g/kg) enhanced the AUC720–2880 and MRT of MTX, indicating that both regimens of FS decreased the elimination of MTX. In addition, the AUC720–2880 were significantly increased by 102 and 78% following the coadministrations with single dose at 5.0 g/kg and repeated dose at 2.5 g/kg, respectively, indicating that FS decreased the elimination of MTX in a dose-dependent manner.
As shown in Figure 1, the profiles of MTX following co-administrations of FS showed prominent second peaks at 1440 min, which caused the elimination constants and half lives of MTX to be incalculable. That was why the parameters of elimination constants and half lives of MTX were not provided in Table 1. On other hand, the amounts of MTX excreted in urine were not measured in this study, and the inhibition on MTX excretion via the kidney could not be evaluated.
Based on the known pharmacokinetic of FS, the polyphenolic anthranoids were rapidly and extensively metabolized to conjugated metabolites [24]. For mimicking the virtual molecules interacting with MRP 2 and BCRP in the kidney, we had prepared FSM from rats, and the characterization of FSM showed that it contained rhein, rhein S/G and aloe-emodin S/G, mainly rhein, which were all acids existing as anions in the bloodstream and probable inhibitors of MRP 2/BCRP [38,39]. The results of transport assay showing that FSM increased the intracellular accumulation of GSMF (a substrate of MRP 2) and MXR (a substrate of BCRP) indicated that FSM inhibited MRP 2 and BCRP. Rhein, a major metabolite of sennoside A and B, was also evaluated by transport assay. Rhein showed inhibition on MRP 2 like FSM. However, regarding the modulations on BCRP, rhein showed activation, which was opposite to the inhibition by FSM. We assumed that the conjugated metabolites of polyphenols in FSM might overwhelm the activation effect of rhein, and resulted in a net effect of inhibition on BCRP. Taken together, these ex-vivo findings indicated that FSM inhibited MRP 2- and BCRP-mediated excretions of MTX in the kidney, which could in part explain the increased systemic exposure of MTX.
Concerning the absorption of MTX, observing the profiles closely found that the absorptions of MTX (shown in the right upper diagram in Figure 1) were similarly decreased by single-dose and repeated-dose regimens of FS. The early exposures (AUC0–240) of MTX were slightly decreased, but did not reach statistical significance. The results of the transport assay showed that FS inhibited BCRP. However, as a major constituent in FS [24], rhein showed activation on BCRP, which was opposite to FS. We speculated that it is because rhein was also a metabolite of sennosides A and B via micofloral biotransformation, and the concentration in the intestine was increased to become greater than FS [24,40,41]. Therefore, rhein might override FS and result in a net effect of activation on BCRP, which would lead to decreased absorption of MTX. In this study, although the absorption of MTX was not significantly decreased by FS, it clearly demonstrated that rhein was an activator of BCRP.
Taken together, during the absorption phase, rhein activated BCRP to decrease the absorption of MTX, which was offset by the inhibition of BCRP due to FS, resulting in non-significant decrease in MTX absorption. On other hand, during the elimination phase, FSM decreased the elimination of MTX through inhibitions on MRP2 and BCRP, which overwhelmed the activation of BCRP by rhein. To sum up, that FSM inhibited the MRP 2- and BCRP- mediated excretion of MTX could explain the increased bioavailability of MTX in rats.
A number of clinically important anticancer drugs are verified as substrates of MRP 2 and/or BCRP, such as vinblastine, 5-fluorouracil, doxorubicine, etoposide, daunorubicin, gefitinib, imatinib, ireinotecan, mitoxantrone and sunitinib [32,33,35]. The pharmacokinetics of these MRP 2/BCRP substrates are very likely altered by concurrent use of FS. We suggest that when patients are treated with these anticancer agents, if FS is used at the same time, the adverse effects of anti-cancer drugs should be carefully monitored to ensure the safety of chemotherapy.
4. Materials and Methods
4.1. Chemicals and Reagents
The crude drug of FS was purchased from an herbal drugstore in Taichung, Taiwan and identified by Dr. Yu-Chi Hou. MTX (25 mg/mL) was obtained from Pharma B. V. (Haarlem, The Netherlands). TDxFLx methotrexate monoclonal whole blood reagent pack was obtained from Abbott Laboratories (Abbott Park, IL, USA). Sennoside A (purity 96%), aloe-emodin (purity 95%), dimethyl sulfoxide (DMSO), sodium dodecyl sulfate (SDS), Triton X-100, verapamil (purity 99 %), β-glucuronidase (Type B-1, from bovine liver) and sulfatase (type H-1 from Helix pomatia, containing 14,000 units/g of sulfatase and 498,800 units/g of β-glucuronidase) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Rhein (purity 95%) was purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). 1,8-Dihydroxyanthraquinone (purity 98%) and amyl paraben (purity 98%) were obtained from Tokyo Chemical Industry Co. (Tokyo, Japan). Methanol, acetonitrile, glacial acetic acid (99%) and ethyl acetate were LC grade and purchased from J.T. Baker, Inc. (Phillipsburg, NJ, USA). L(+)-Ascorbic acid (purity 99.7%) was obtained from RdH Laborchemikalien GmbH & Co. KG (Seelze, Germany). Other reagents were HPLC grade or analytical grade. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (purity 98%) was obtained from Alfa Aesar (Lancaster, UK). Fetal Bovine Serum (FBS) was supplied by Biological Industries Inc. (Kibbutz, Beit Haemek, Israel). Penicillin-Streptomycin-Glutamine (PSG), 5-chloromethylfluorescein diacetate (CMFDA), Dulbecco’s modified Eagle medium (DMEM), trypsin/EDTA, Hank’s buffered salt solution (HBSS) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) were purchased from Invitrogen (Carlsbad, CA, USA). MK 571, mitoxantrone (MXR, purity 97%) and Ko143 (purity 96%) were obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Milli-Q plus water (Millipore, Bedford, MA, USA) was used throughout this study.
4.2. Preparation and Characterization of FS Decoction
The preparation and quantitation of FS decoction had been published in our previous study and briefly described as follows [24]. Three hundred microliters of FS decoction (1.0 g/mL) was mixed with 700 μL of methanol. After centrifugation, the supernatant (180 μL) was mixed with 20 µL of amyl paraben methanol solution (50.0 µg/mL, as internal standard) and 20 µL was subject to HPLC analysis. The mobile phase consisted of methanol (A) – 0.1 % acetic acid (B) and eluted in a gradient manner as the timetable (min, %B):(0, 55), (5, 55), (8, 48), (12, 35), (22, 20), (30, 20), (32, 55) (35, 55). The detection wavelength was set at 270 nm and the flow rate was 1.0 mL/min.
4.3. Animals and Drug Administration
All animal experiments were carried out in strict accordance with the recommendations by “The Guidebook for the Care and Use of Laboratory Animals” published by the Chinese Society for the Laboratory Animal Science, Taiwan. The experimental protocol had been reviewed and approved by the Insititutional Animal Care and Use Committee of China Medical University. Male Sprague-Dawley rats weighing 320–465 g were purchased from BioLASCO Taiwan Co., Ltd. (Yilan, Taiwan) and housed in a conditioned environment with 12-h light/dark cycles. Food and water were supplied ad libitum until 12 h prior to experiment. The rats fasted overnight before drug administration and food was offered 3 h after FS dosing. MTX was diluted with water to afford a concentration of 2.5 mg/mL. Three groups of rats were orally given MTX (5.0 mg/kg) alone, and with a single dose of FS (equivalent to 5.0 g/kg of FS) and seven consecutive doses of FS (equivalent to 2.5 g/kg of FS), respectively, in a parallel design. FS decoction was administered 30 min before MTX dosing via gastric gavage in the treatment groups.
4.4. Blood Collection and Determination of Serum MTX Concentration
Blood samples were collected at 15, 30, 60, 120, 240, 480, 720, 1440, 2160, 2880 min after MTX dosing. At each time point, 0.5 mL of blood was withdrawn. The blood samples were collected in microtubes and centrifuged at 10,000× g for 10 min to obtain serum, which was stored at −20 °C until analysis. The concentrations of MTX in serum were determined using a specific monoclonal fluorescence polarization immunoassay (FPIA). Validation of the calibration curve was carried out by testing three controls with low, medium and high concentrations before sample analysis. The assay was calibrated for concentrations from 0 to 1.0 μM and the lower limit of quantitation was 0.02 μM.
4.5. Cell Line and Culture Conditions
Madin-Darby canine kidney type II (MDCKII) cells expressing BCRP (MDCKII-BCRP) and MDCKII-wild type (WT) cells were kindly provided by Prof. Dr. Piet Borst (Netherlands Cancer Institute, Amsterdam, The Netherlands).
MDCKII-WT and MDCKII-BCRP cells were grown in DMEM medium supplemented with 10% FBS, 100 units/mL of penicillin, 100 μg/mL of streptomycin and 292 μg/mL of glutamine at 37 °C in a humidified incubator containing 5% CO2.
The medium was changed every other day and cells were subcultured when 80% to 90% confluency was reached.
4.6. Cell Viability Assay
The effects of tested drugs on the viability of cells described above were evaluated by MTT assay [42]. Cells were seeded into a 96-well plate. After overnight incubation, the tested agents were added into the wells and incubated for 24 h, then 15 μL of MTT (5.0 mg/mL) was added into each well and incubated for 4 h. In this period, MTT became formazan crystal by live cells. Following removal of the supernatant, SDS solution (20%) was added to dissolve the purple crystal at the end of incubation and the optical density was detected at 570 nm by a microplate reader (BioTex, Highland Park, Winooski, VT, USA).
4.7. Preparation and Characterization of Serum Metabolites of FS (FSM)
In order to mimic the molecules interacting with MRP 2 and BCRP in kidney cells in vivo, FSM was prepared from rats and characterized. Briefly, FS decoction was orally administered to rats fasted overnight. Blood was collected at 10 min after dosing. After coagulation, the serum was vortexed with 3-fold methanol. After centrifugation, the supernatant was concentrated in a rotatory evaporator under vacuum to dryness. To the residue, an appropriate volume of water was added to afford FSM solution with 10-fold serum concentration, which was divided into aliquots and stored at −80 °C for later use.
In order to characterize FSM, 100 μL of FSM solution was mixed with 50 μL of sulfatase (1000 units/mL in pH 5 acetate buffer), 50 μL of ascorbic acid (100 mg/mL), and incubated at 37 °C for 30 min. After hydrolysis, the serum was acidified with 50 μL of 0.1 N HCl and partitioned with 250 μL of ethyl acetate (containing 0.2 μg/mL of 1, 8-dihydroxyanthraquinone as internal standard). The ethyl acetate layer was evaporated under N2 to dryness and reconstituted with an appropriate volume of methanol prior to HPLC analysis, which was following a method reported previously [24]. On the other hand, blank serum was also collected from rats and processed in the same manner as FSM to prepare blank specimens as controls for comparison with correspondent specimens of FSM.
4.8. Effects of FS, FSM and Rhein on BCRP Activity
The transport assay was performed to evaluate the effects of FS and FSM on BCRP activity. MDCKII-BCRP cells were suspended at a density of 1 × 106 in each reaction tube. MXR was used as a probe for evaluating the effects of FS, FSM and rhein on BCRP-mediated transport. Ko143 was used as a positive control of the BCRP inhibitor. Before transport assay, MDCKII-BCRP cells were pre-incubated with tested agents (FS, FSM, rhein and Ko143) at 37 °C for 15 min. MXR was then added and co-incubated for another 30 min. Finally, ice-cold PBS was used to wash the cells. After centrifugation, the supernatant was removed and the cell pellet was re-suspended by ice-cold PBS. Subsequently, the fluorescence of MXR was determined by a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) equipped with a standard HeNe laser. All transport studies were performed in triplicates.
4.9. Effect of FSM and Rhein on MRP 2 Activity
The transport assay was conducted to measure the effects of FSM and rhein on MRP 2 activity as previously described [43,44,45]. Briefly, MDCKII cells (1 × 105 cells/well) were cultured in each well of a 96-well plate. After overnight incubation, the medium was removed and washed three times with ice-cold PBS buffer. The tested agents (CMFDA plus FSM or CMFDA plus rhein) were added into each well and incubated at 37 °C. After 30-min incubation, the supernatants were removed and washed for three times with ice-cold PBS. Subsequently, 100 μL of 0.1 % Triton X-100 was added to lyse the cells, and the fluorescence was measured with excitation at 485 nm and emission at 528 nm.
To quantitate the content of protein in each well, 10 μL of cell lysate was added to 200 μL of diluted protein assay reagent (Bio-Rad, Hercules, CA, USA) and the optical density was measured at 570 nm. The relative intracellular accumulation of glutathione-methylfluorescein (GSMF, a metabolite of CMFDA), a fluorescent substrate of MRP 2, was calculated by comparing it with that of control after protein correction.
4.10. Data Analysis
The serum concentration of MTX was analyzed using noncompartment model of WinNonlin (version 6.4, Pharsight Co., Cary, NC, USA). The peak serum concentration (Cmax) was obtained from experimental observation. The area under the serum concentration-time curve (AUC0–t) was calculated using trapezoidal rule to the last point. Unpaired Student’s t-test was used to analyze the difference between two groups and one-way ANOVA with Scheffe test was used for statistical comparison among three groups taking p < 0.05 as significant.
5. Conclusions
Concurrent use of FS increased the systemic exposure and MRT of MTX through inhibitions on MRP 2 and BCRP.
Author Contributions
Conceptualization, C.-P.Y., S.-P.L., and Y.-C.H.; Methodology, C.-P.Y., Y.-H.P.; Software and data analysis, C.-P.Y., Y.-H.P., C.-Y.H., Y.-W.H.; Writing—Original Draft Preparation and Review & Editing, C.-P.Y., S.-P.L. and Y.-C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by the Ministry of Science and Technology, Taiwan (MOST 108-2320-B-039-041-MY3), China Medical University, Taiwan (CMU109-S-19 and CMU110-MF-16) and China Medical University Hospital, Taiwan (DMR-106-137).
Institutional Review Board Statement
This study accordance with the Guidelines for the Care and Use of Laboratory Animals and reviewed and approved by the Insititutional Animal Care and Use Committee of China Medical University (approval number: 102-144-N).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data presented in this study will be shared on request from the corresponding author.
Conflicts of Interest
The authors have declared no conflict interest.
References
- Mueller-Lissner, S.A.; Wald, A. Constipation in adults. BMJ Clin. Evid. 2010, 2010, 0413. [Google Scholar] [PubMed]
- Bharucha, A.E.; Wald, A. Chronic constipation. Mayo Clin. Proc. 2019, 94, 2340–2357. [Google Scholar] [CrossRef]
- Fragakis, A.; Zhou, J.; Mannan, H.; Ho, V. Association between drug usage and constipation in the elderly population of Greater Western Sydney Australia. Int. J. Environ. Res. Public Health 2018, 15, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, S.H. Constipation in long-term care. J. Am. Med. Dir. Assoc. 2007, 8, 209–218. [Google Scholar] [CrossRef]
- Alsalimy, N.; Madi, L.; Awaisu, A. Efficacy and safety of laxatives for chronic constipation in long-term care settings: A systematic review. J. Clin. Pharm. Ther. 2018, 43, 595–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Every-Palmer, S.; Newton-Howes, G.; Clarke, M.J. Pharmacological treatment for antipsychotic-related constipation. Cochrane Database Syst. Rev. 2017, 1, CD011128. [Google Scholar]
- Collamati, A.; Martone, A.M.; Poscia, A.; Brandi, V.; Celi, M.; Marzetti, E.; Cherubini, A.; Landi, F. Anticholinergic drugs and negative outcomes in the older population: From biological plausibility to clinical evidence. Aging Clin. Exp. Res. 2016, 28, 25–35. [Google Scholar] [CrossRef]
- Cirillo, C.; Capasso, R. Constipation and botanical medicines: An overview. Phytother. Res. 2015, 29, 1488–1493. [Google Scholar] [CrossRef]
- Leung, L.; Riutta, T.; Kotecha, J.; Rosser, W. Chronic constipation: An evidence-based review. J. Am. Board Fam. Med. 2011, 24, 436–451. [Google Scholar] [CrossRef]
- Garcia-Alvarez, A.; Mila-Villarroel, R.; Ribas-Barba, L.; Egan, B.; Badea, M.; Maggi, F.M.; Salmenhaara, M.; Restani, P.; Serra-Majem, L. Usage of Plant Food Supplements (PFS) for weight control in six European countries: Results from the PlantLIBRA PFS Consumer Survey 2011–2012. BMC Complement. Altern. Med. 2016, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, C.; Conquer, J.; Costa, D.; Hamilton, W.; Higdon, E.R.; Isaac, R.; Rusie, E.; Rychlik, I.; Serrano, J.M.; Tanguay-Colucci, S.; et al. An evidence-based systematic review of senna (Cassia senna) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2011, 8, 189–238. [Google Scholar] [CrossRef]
- Franz, G. The senna drug and its chemistry. Pharmacology 1993, 47 (Suppl. 1), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Krishnan, S.; Kumar, A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.J.; Ngoc, T.M.; Bae, K.; Cho, H.J.; Kim, D.D.; Chun, J.; Khan, S.; Kim, Y.S. Anti-inflammatory properties of anthraquinones and their relationship with the regulation of P-glycoprotein function and expression. Eur. J. Pharm. Sci. 2013, 48, 272–281. [Google Scholar] [CrossRef]
- Huang, Q.; Lu, G.; Shen, H.M.; Chung, M.C.; Ong, C.N. Anti-cancer properties of anthraquinones from rhubarb. Med. Res. Rev. 2007, 27, 609–630. [Google Scholar] [CrossRef]
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A Comprehensive and system review for the pharmacological mechanism of action of rhein, an active anthraquinone ingredient. Front. Pharmacol. 2016, 7, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGorter, M.K.; Xia, C.Q.; Yang, J.J.; Kim, R.B. Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 249–273. [Google Scholar] [CrossRef]
- Zha, W. Transporter-mediated natural product-drug interactions for the treatment of cardiovascular diseases. J. Food Drug Anal. 2018, 26, S32–S44. [Google Scholar] [CrossRef] [Green Version]
- Liu, X. Transporter-mediated drug-drug interactions and their significance. Adv. Exp. Med. Biol. 2019, 1141, 241–291. [Google Scholar]
- Gessner, A.; König, J.; Fromm, M.F. Clinical aspects of transporter-mediated drug-drug interactions. Clin. Pharmacol. Ther. 2019, 105, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Tamai, I. Interaction of drug or food with drug transporters in intestine and liver. Curr. Drug Metab. 2015, 16, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2015, 14, 29–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, R.E.; Jay, C.E.; Sweet, D.H. Organic solute carrier 22 (SLC22) family: Potential for interactions with food, herbal/dietary supplements, endogenous compounds, and drugs. J. Food Drug Anal. 2018, 26, S45–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.H.; Lin, S.P.; Yu, C.P.; Tsai, S.Y.; Chen, M.Y.; Hou, Y.C.; Chao, P.D. Serum concentrations of anthraquinones after intake of Folium Sennae and potential modulation on P-glycoprotein. Planta Med. 2014, 80, 1291–1297. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.S.; Yu, C.P.; Huang, C.Y.; Chao, P.L.; Lin, S.P.; Hou, Y.C. Aloe activated P-glycoprotein and CYP 3A: A study on the serum kinetics of aloe and its interaction with cyclosporine in rats. Food Funct. 2017, 8, 315–322. [Google Scholar] [CrossRef]
- Dai, Y.; Ma, B.L.; Zheng, M.; Shi, R.; Li, Y.Y.; Wang, T.M.; Ma, Y.M. Identification of drug transporters involved in the uptake and efflux of rhein in hepatocytes. RSC Adv. 2017, 7, 15236–15245. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Zhao, L.; Hu, H.; Qin, Y.; Bian, Y.; Jiang, H.; Zhou, H.; Yu, L.; Zeng, S. Interaction of five anthraquinones from rhubarb with human organic anion transporter 1 (SLC22A6) and 3 (SLC22A8) and drug-drug interaction in rats. J. Ethnopharmacol. 2014, 153, 864–871. [Google Scholar] [CrossRef]
- Lin, S.P.; Hou, Y.C.; Tsai, S.Y.; Wang, M.J.; Chao, P.D. Tissue distribution of naringenin conjugated metabolites following repeated dosing of naringin to rats. Biomedicine 2014, 4, 16. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Lu, L.; Li, Y.; Zeng, S.; Yang, X.; Chen, W.; Feng, Q.; Liu, W.; Tang, L.; Liu, Z. Potential role of ATP-binding cassette transporters in the intestinal transport of rhein. Food Chem. Toxicol. 2013, 58, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Deeley, R.G.; Westlake, C.; Cole, S.P. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol. Rev. 2006, 86, 849–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.K.; Wang, Y.J.; Gupta, P.; Chen, Z.S. Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J. 2015, 17, 802–812. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Mori, N. Involvement of multiple transporters-mediated transports in mizoribine and methotrexate pharmacokinetics. Pharmaceuticals 2012, 5, 802–836. [Google Scholar] [CrossRef] [Green Version]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamp, L.K.; Hazlett, J.; Highton, J.; Hessian, P.A. Expression of methotrexate transporters and metabolizing enzymes in rheumatoid synovial tissue. J. Rheumatol. 2013, 40, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Vlaming, M.L.; van Esch, A.; van de Steeg, E.; Pala, Z.; Wagenaar, E.; van Tellingen, O.; Schinkel, A.H. Impact of abcc2 [multidrug resistance-associated protein (MRP) 2], abcc3 (MRP3), and abcg2 (breast cancer resistance protein) on the oral pharmacokinetics of methotrexate and its main metabolite 7-hydroxymethotrexate. Drug Metab. Dispos. 2011, 39, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Fardel, O.; Jigorel, E.; Le Vee, M.; Payen, L. Physiological, pharmacological and clinical features of the multidrug resistance protein 2. Biomed. Pharmacother. 2005, 59, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Lemli, J.; Lemmens, L. Metabolism of sennosides and rhein in the rat. Pharmacology 1980, 20 (Suppl. 1), 50–57. [Google Scholar] [CrossRef]
- Dreessen, M.; Eyssen, H.; Lemli, J. The metabolism of sennosides A and B by the intestinal microflora: In vitro and in vivo studies on the rat and the mouse. J. Pharm. Pharmacol. 1981, 33, 679–681. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hsu, P.W.; Shia, C.S.; Wu, C.T.; Chang, N.W.; Chao, P.D.L.; Hou, Y.C. Noni increased the systemic exposure of methotrexate in rats through inhibition on multi-drug resistance protein 2 and breast cancer resistance protein (BCRP). J. Funct. Foods 2013, 5, 1414–1420. [Google Scholar] [CrossRef]
- Yang, S.Y.; Juang, S.H.; Tsai, S.Y.; Chao, P.D.; Hou, Y.C. St. John’s wort significantly increased the systemic exposure and toxicity of methotrexate in rats. Toxicol. Appl. Pharmacol. 2012, 263, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Hsieh, Y.C.; Shia, C.S.; Hsu, P.W.; Chen, J.Y.; Hou, Y.C.; Hsieh, Y.W. Increased systemic exposure of methotrexate by a polyphenol-rich herb via modulation on efflux transporters multidrug resistance-associated protein 2 and breast cancer resistance protein. J. Pharm. Sci. 2016, 105, 343–349. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).