Activatable Nanoparticles: Recent Advances in Redox-Sensitive Magnetic Resonance Contrast Agent Candidates Capable of Detecting Inflammation
Abstract
1. Introduction
2. Glutathione Activatable Nanoparticle-Based Contrast Agents
2.1. MR Specific Glutathione Responsive Contrast Agents
2.2. Multimodal Glutathione Responsive Contrast Agents
3. ROS Activatable Nanoparticle-Based Contrast Agents
3.1. Peroxide Sensitive Agents
3.2. Nanoparticles Sensitive to Other Species of ROS
4. Emerging Redox Environment Responsive Contrast Agents
5. Redox Activatable Combined Diagnostic and Therapeutic Agents
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Heim, B.; Krismer, F.; De Marzi, R.; Seppi, K. Magnetic resonance imaging for the diagnosis of Parkinson’s disease. J. Neural Transm. 2017, 124, 915–964. [Google Scholar] [CrossRef] [PubMed]
- Tocchio, S.; Kline-Fath, B.; Kanal, E.; Schmithorst, V.J.; Panigrahy, A. MRI evaluation and safety in the developing brain. Semin. Perinatol. 2015, 39, 73–104. [Google Scholar] [CrossRef] [PubMed]
- Grover, V.P.; Tognarelli, J.M.; Crossey, M.M.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J. Magnetic Resonance Imaging: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 246–255. [Google Scholar] [CrossRef]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef]
- Behzadi, A.H.; Farooq, Z.; Newhouse, J.H.; Prince, M.R. MRI and CT contrast media extravasation. Medicine 2018, 97, e0055. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- De León-Rodríguez, L.M.; Martins, A.F.; Pinho, M.C.; Rofsky, N.M.; Sherry, A.D. Basic MR relaxation mechanisms and contrast agent design. J. Magn. Reson. Imaging 2015, 42, 545–565. [Google Scholar] [CrossRef]
- Ding, S.; Meystre, N.R.; Campeanu, C.; Gullo, G. Contrast media extravasations in patients undergoing computerized tomography scanning. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 87–116. [Google Scholar] [CrossRef]
- Lohrke, J.; Frisk, A.-L.; Frenzel, T.; Schöckel, L.; Rosenbruch, M.; Jost, G.; Lenhard, D.C.; Sieber, M.A.; Nischwitz, V.; Küppers, A.; et al. Histology and Gadolinium Distribution in the Rodent Brain After the Administration of Cumulative High Doses of Linear and Macrocyclic Gadolinium-Based Contrast Agents. Investig. Radiol. 2017, 52, 324–333. [Google Scholar] [CrossRef]
- Functional Nanoparticles for Magnetic Resonance Imaging. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5050056/ (accessed on 19 December 2020).
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting Enhanced Permeability and Retention Effect Driven Delivery by Ultrafine Iron Oxide Nanoparticles with T1–T2 Switchable Magnetic Resonance Imaging Contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Van Der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef] [PubMed]
- Busquets, M.A.; Estelrich, J.; Sánchez-Martín, M.J. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Sinharay, S.; Pagel, M.D. Advances in Magnetic Resonance Imaging Contrast Agents for Biomarker Detection. Annu. Rev. Anal. Chem. 2016, 9, 95–115. [Google Scholar] [CrossRef]
- Wu, B.; Warnock, G.; Zaiss, M.; Lin, C.; Chen, M.; Zhou, Z.; Mu, L.; Nanz, D.; Tuura, R.; Delso, G. An overview of CEST MRI for non-MR physicists. EJNMMI Phys. 2016, 3, 1–21. [Google Scholar] [CrossRef]
- Hingorani, D.V.; Bernstein, A.S.; Pagel, M.D. A review of responsive MRI contrast agents: 2005-2014. Contrast Media Mol. Imaging 2014, 10, 245–265. [Google Scholar] [CrossRef]
- Carril, M. Activatable probes for diagnosis and biomarker detection by MRI. J. Mater. Chem. B 2017, 5, 4332–4347. [Google Scholar] [CrossRef]
- Tu, C.; Osborne, E.A.; Louie, A.Y. Activatable T 1 and T 2 Magnetic Resonance Imaging Contrast Agents. Ann. Biomed. Eng. 2011, 39, 1335–1348. [Google Scholar] [CrossRef]
- Hellebust, A.; Richards-Kortum, R.R. Advances in molecular imaging: Targeted optical contrast agents for cancer diagnostics. Nanomed. 2012, 7, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.B. Redox Signaling Across Cell Membranes. Antioxid. Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Chaiswing, L.; Clair, W.H.S.; Clair, D.K.S. Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer. Antioxid. Redox Signal. 2018, 29, 1237–1272. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; Del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative Stress, Redox Signaling, and Autophagy: Cell DeathVersusSurvival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Bui, A.D. Oxidation-reduction potential as a new marker for oxidative stress: Correlation to male infertility. Investig. Clin. Urol. 2017, 58, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Liemburg-Apers, D.C.; Willems, P.H.G.M.; Koopman, W.J.; Grefte, S. Interactions between mitochondrial reactive oxygen species and cellular glucose metabolism. Arch. Toxicol. 2015, 89, 1209–1226. [Google Scholar] [CrossRef]
- Yang, M.-S.; Min, K.-J.; Joe, E. Multiple mechanisms that prevent excessive brain inflammation. J. Neurosci. Res. 2007, 85, 2298–2305. [Google Scholar] [CrossRef]
- Abdulsalam, S.F.; Thowfeik, F.S.; Merino, E.J. Excessive Reactive Oxygen Species and Exotic DNA Lesions as an Exploitable Liability. Biochemistry 2016, 55, 5341–5352. [Google Scholar] [CrossRef]
- Ye, D.; Pandit, P.; Kempen, P.; Lin, J.; Xiong, L.; Sinclair, R.; Rutt, B.; Rao, J. Redox-Triggered Self-Assembly of Gadolinium-Based MRI Probes for Sensing Reducing Environment. Bioconjug. Chem. 2014, 25, 1526–1536. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- DePonte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Wu, C.; Dai, Y.; Hou, P.; Han, C.; Han, C. Activatable molecular MRI nanoprobe for tumor cell imaging based on gadolinium oxide and iron oxide nanoparticle. Biosens. Bioelectron. 2016, 86, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Son, H.-Y.; Kim, G.-Y.; Park, K.; Huh, Y.-M.; Haam, S. Redoxable heteronanocrystals functioning magnetic relaxation switch for activatable T1 and T2 dual-mode magnetic resonance imaging. Biomaterials 2016, 101, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Deng, K.; Lu, S.-T.; Zhang, M.; Ao, Y.-W.; Wang, H.; Mei, H.; Wang, C.-X.; Xu, H.; Hu, B.; et al. Reduction-active Fe3O4-loaded micelles with aggregation- enhanced MRI contrast for differential diagnosis of Neroglioma. Biomaterials 2021, 268, 120531. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Shi, H.; Hu, Y.; Feng, L.; Luo, Z.; Zhou, M.; He, J.; Zhou, Z.; Zhang, Y.; et al. Redox-Mediated Disassembly to Build Activatable Trimodal Probe for Molecular Imaging of Biothiols. ACS Nano 2016, 10, 10075–10085. [Google Scholar] [CrossRef]
- Gallo, J.; Vasimalai, N.; Fernandez-Arguelles, M.T.; Bañobre-López, M. Green synthesis of multimodal ‘OFF–ON’ activatable MRI/optical probes. Dalton Trans. 2016, 45, 17672–17680. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wen, X.; Pan, Y.; Cheng, X.; An, R.; Gao, G.; Chen, H.-Y.; Ye, D. Responsive Trimodal Probes for In Vivo Imaging of Liver Inflammation by Coassembly and GSH-Driven Disassembly. Research 2020, 2020, 4087069. Available online: https://spj.sciencemag.org/journals/research/2020/4087069/ (accessed on 9 December 2020). [CrossRef]
- Reactive Oxygen Species (ROS). Intimal Thickening, and Subclinical Atherosclerotic Disease. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688526/ (accessed on 10 October 2020).
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, J.; He, L.; Chen, L. Mitochondrial superoxide/hydrogen peroxide: An emerging therapeutic target for metabolic diseases. Free Radic. Biol. Med. 2020, 152, 33–42. [Google Scholar] [CrossRef]
- Wong, H.-S.; Dighe, P.A.; Mezera, V.; Monternier, P.-A.; Brand, M.D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017, 292, 16804–16809. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Herbst, S.; Zhang, C.; Whittaker, A.K. Polymeric 19F MRI agents responsive to reactive oxygen species. Polym. Chem. 2017, 8, 4585–4595. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Shuvaev, S.; Rotile, N.; Jones, C.M.; Probst, C.K.; Ferreira, D.D.S.; Graham-O’Regan, K.; Boros, E.; Knipe, R.S.; Griffith, J.W.; et al. Peroxidase Sensitive Amplifiable Probe for Molecular Magnetic Resonance Imaging of Pulmonary Inflammation. ACS Sens. 2019, 4, 2412–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jordan, V.C.; Ramsay, I.A.; Sojoodi, M.; Fuchs, B.C.; Tanabe, K.K.; Caravan, P.; Gale, E.M. Molecular Magnetic Resonance Imaging Using a Redox-Active Iron Complex. J. Am. Chem. Soc. 2019, 141, 5916–5925. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.; Cheng, H.A.; Nardacci, L.E.; Beaman, D.J.; Drinnan, C.T.; Lee, C.; Fishbein, K.W.; Spencer, R.G.; Fisher, O.Z.; Doiron, A. Activatable interpolymer complex-superparamagnetic iron oxide nanoparticles as magnetic resonance contrast agents sensitive to oxidative stress. Colloids Surf. B Biointerfaces 2017, 158, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Nwasike, C.; Yoo, E.; Purr, E.; Doiron, A. Activatable superparamagnetic iron oxide nanoparticles scavenge reactive oxygen species in macrophages and endothelial cells. RSC Adv. 2020, 10, 41305–41314. [Google Scholar] [CrossRef]
- Li, A.; Tang, X.; Gong, X.; Chen, H.; Lin, H.; Gao, J. A fluorinated bihydrazide conjugate for activatable sensing and imaging of hypochlorous acid by 19F NMR/MRI. Chem. Commun. 2019, 55, 12455–12458. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, H.; Yang, W.; Wang, Z.; Lin, L.; Munasinghe, J.; Jacobson, O.; Liu, Y.; Tang, L.; Ni, Q.Q.; et al. Early stratification of radiotherapy response by activatable inflammation magnetic resonance imaging. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Dunbar, L.; Sowden, R.J.; Trotter, K.D.; Taylor, M.K.; Smith, D.; Kennedy, A.R.; Reglinski, J.; Spickett, C.M. Copper complexes as a source of redox active MRI contrast agents. BioMetals 2015, 28, 903–912. [Google Scholar] [CrossRef]
- Gale, E.M.; Jones, C.M.; Ramsay, I.; Farrar, C.T.; Caravan, P. A Janus Chelator Enables Biochemically Responsive MRI Contrast with Exceptional Dynamic Range. J. Am. Chem. Soc. 2016, 138, 15861–15864. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Nie, W.; Zhang, Q.; Wang, W.; Zhang, Y.; He, C. Multifunctional Redox-Responsive Mesoporous Silica Nanoparticles for Efficient Targeting Drug Delivery and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Xu, C.-H.; Zhao, W.; Gu, Y.; Li, X.-L.; Xu, J.-J.; Chen, H.-Y. A redox-activated theranostic nanoagent: Toward multi-mode imaging guided chemo-photothermal therapy. Chem. Sci. 2018, 9, 6749–6757. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Ding, X.; Wu, J.; Li, C.; Wang, Q.; Jiang, J. Cationic Polyelectrolyte Mediated Synthesis of MnO2 -Based Core-Shell Structures as Activatable MRI Theranostic Platform for Tumor Cell Ablation. Part. Part. Syst. Charact. 2018, 35, 1800078. [Google Scholar] [CrossRef]
- Wan, S.-S.; Cheng, Q.; Zeng, X.; Zhanga, X.-Z. A Mn(III)-Sealed Metal–Organic Framework Nanosystem for Redox-Unlocked Tumor Theranostics. ACS Nano 2019, 13, 6561–6571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bremner, D.H.; Wu, K.; Gong, X.; Fan, Q.; Xie, X.; Zhang, H.; Wu, J.; Zhu, L.-M. Platelet membrane biomimetic bufalin-loaded hollow MnO2 nanoparticles for MRI-guided chemo-chemodynamic combined therapy of cancer. Chem. Eng. J. 2020, 382, 122848. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Sun, W.; Fan, Y.; Yin, F.; Van Hest, J.C.M.; Wang, H.; Du, L.; et al. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics 2020, 10, 4349–4358. [Google Scholar] [CrossRef]

| MR Contrast Agent Candidate | Contrast Agent | Redox Environment | Effect Detected | Multimodal | Application |
|---|---|---|---|---|---|
| GdNPs [30] | Gadolinium | Glutathione | T1 Enhancement | No | In vitro detection of breast cancer cells |
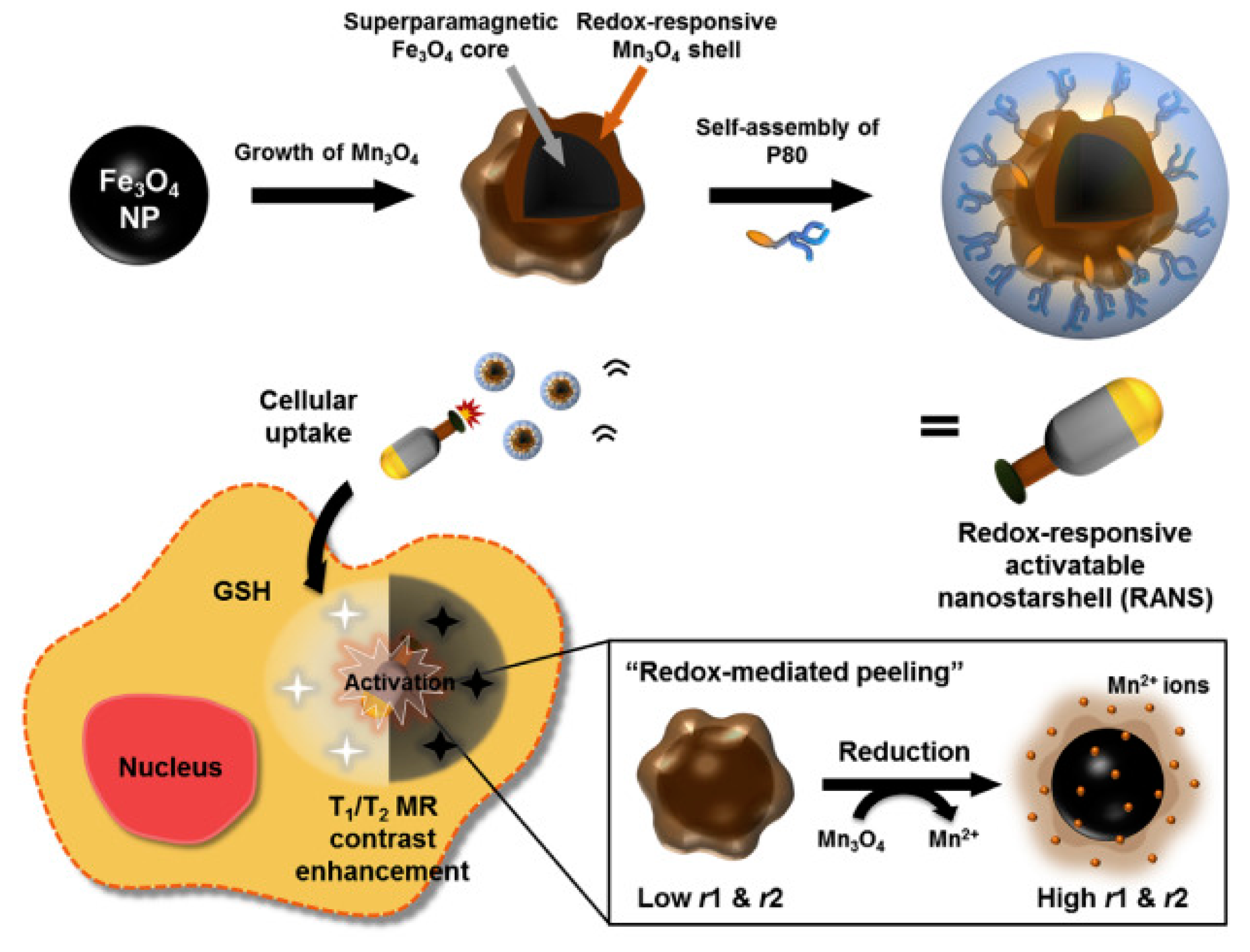
| RANS [34] | Manganese; Iron oxide | Glutathione | T1 Enhancement; T2 Contrast | No | In vitro/in vivo imaging of gastric cancer |
| Fe3O4-SS-PEG Gd2O3 [33] | Gadolinium; Iron oxide | Glutathione | T1 Enhancement | No | In vitro detection of human kidney cancer cells |
| Redox-activatable fluorescence/19F-MRS/1 H-MR triple-functional probe [35] | Gadolinium | Glutathione | T1 Enhancement | Yes; fluorescence/19F-MRS/1H-MR | In vitro imaging of HeLa |
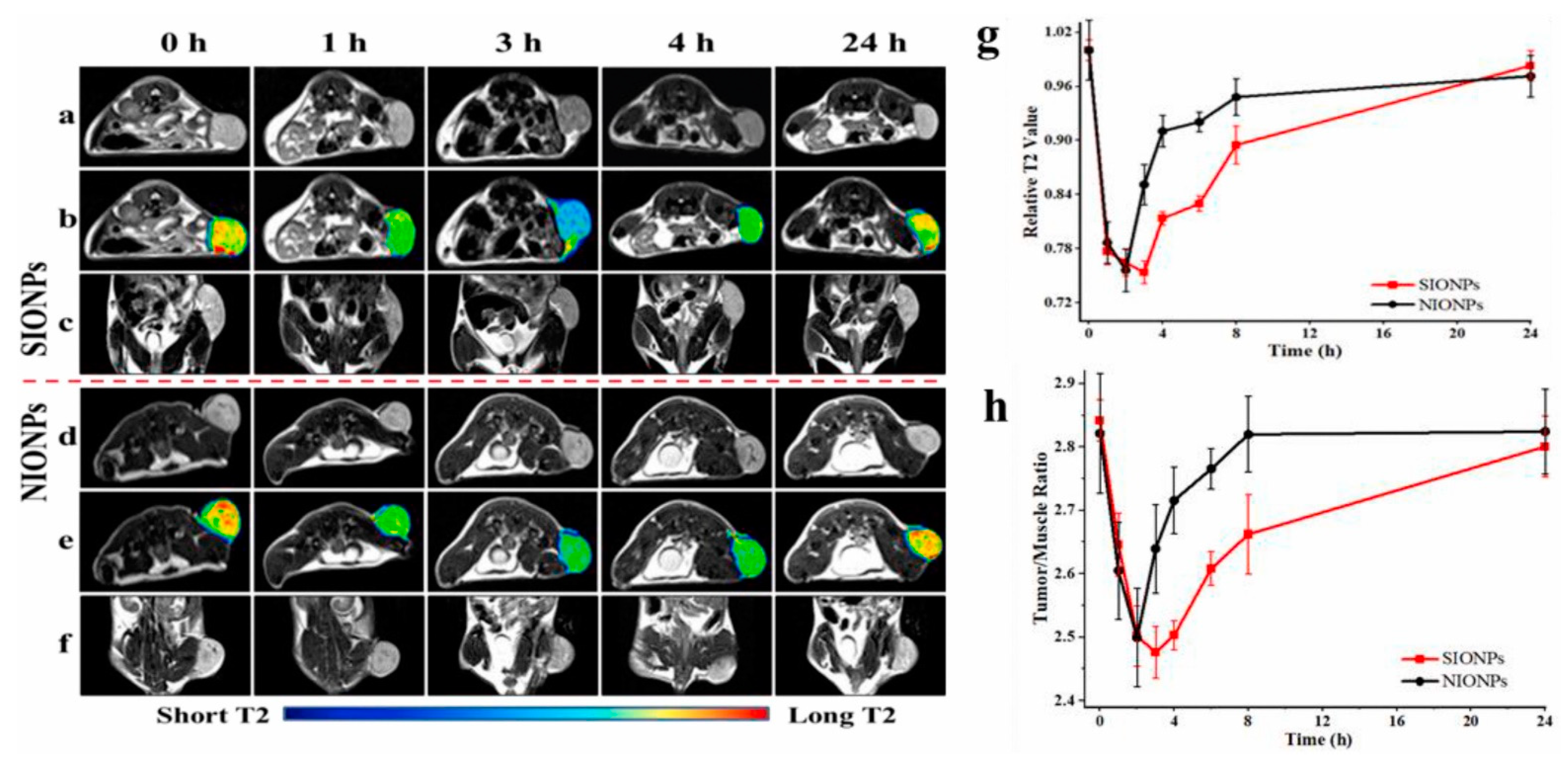
| mPEG-S-S-C16 [36] | Iron oxide | Glutathione | T2 Contrast | No | In vivo detection of neuroglioma in mice |
| MnO2_CQDs [37] | Manganese | Glutathione; Hydrogen peroxide | T1 Enhancement | Yes; MR/optical probe | In vitro detection of human epithelial cells |
| GdNPs-Gal [38] | Gadolinium | Glutathione | T1 Enhancement | Yes; 19F-MRS/1H-MR | In vivo imaging of acute hepatitis in mice |
| MR Contrast Agent Candidate | Contrast Agent | ROS | Effect Detected | Application |
|---|---|---|---|---|
| Gd-5-HT-DOTAGA [45] | Gadolinium | Hydrogen peroxide | T1 Enhancement | In vivo detection of inflammation in lungs of bleomycin-injured mice |
| Fe-PyC3A [46] | Iron | Hydrogen peroxide | T1 Enhancement | In vivo detection of acute and mild inflammation in mice |
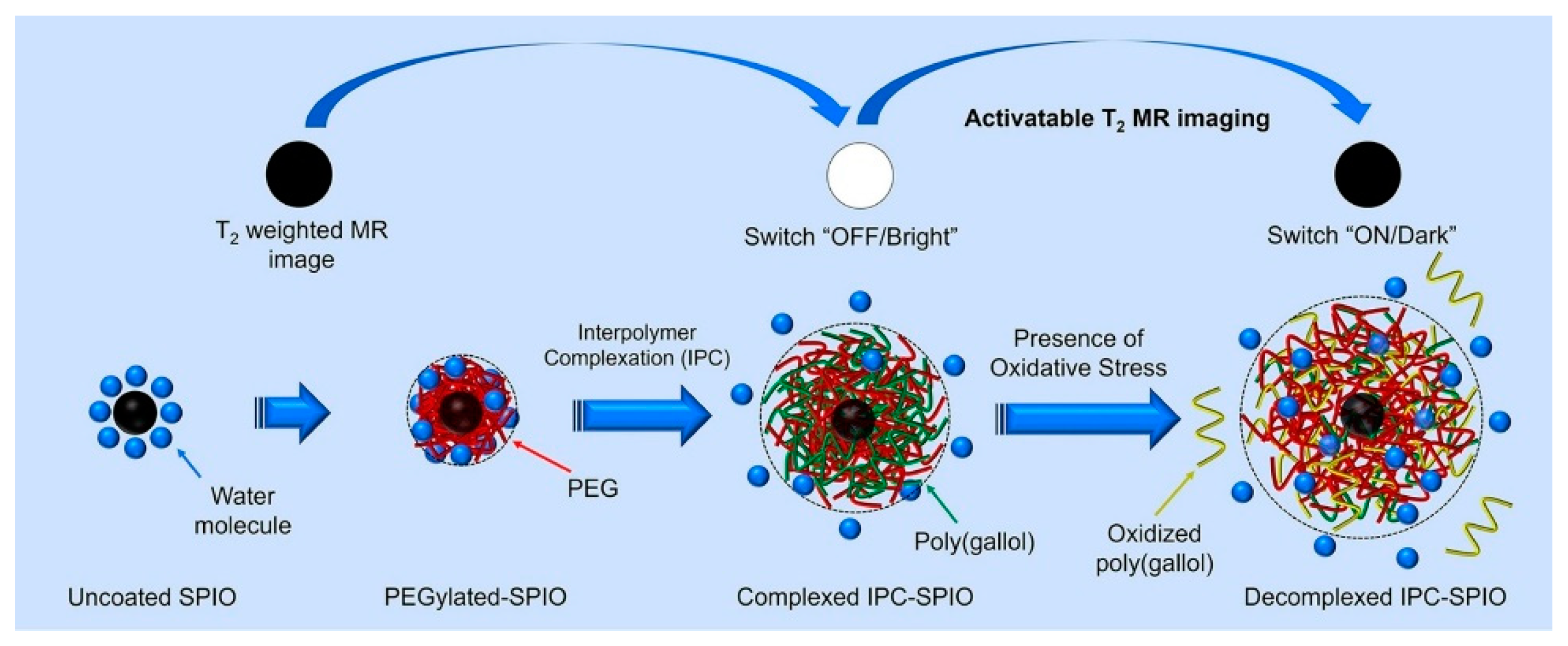
| IPC-SPIOs [47,48] | Iron oxide | Reactive oxygen species (not specific) | T2 Contrast | In vitro imaging of immune cells |
| Fluorinated bihydrazide Gd-DOTA [49] | Gadolinium | Hypochlorous acid | T1 Enhancement; T2 Contrast | In vivo detection of murine hepatocellular carcinoma |
| IO-Gd NV [50] | Iron oxide; Gadolinium | Inflammatory factors | T1 Enhancement | In vivo detection of murine epithelial cancer cells |
| MR Contrast Agent Candidate | Contrast Agent | Redox Environment | Effect Detected | Drug | Application |
|---|---|---|---|---|---|
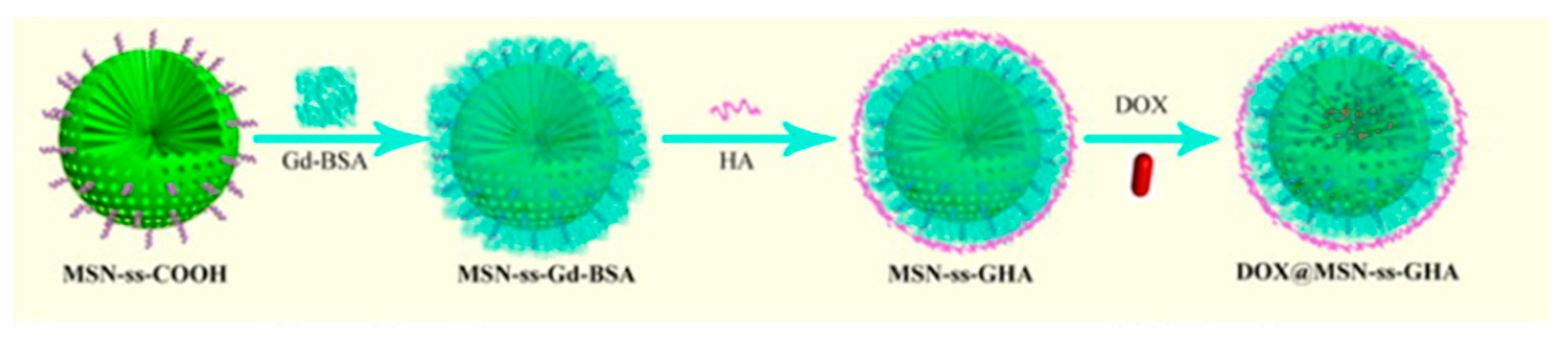
| DOX@MSN-ss-GHA [53] | Gadolinium | Glutathione | T1 Enhancement | Doxorubicin | In vitro imaging of breast cancer cells |
| SiO2@Au@MnO2–DOX/Apt [54] | Manganese | Glutathione | T1 Enhancement | Doxorubicin; Photothermal therapy | In vivo imaging of HeLa bearing mice |
| Cu2−xSe@MnO2 [55] | Manganese | Tumor reducing environment | T1 Enhancement | Photothermal therapy | In vivo detection of murine colorectal carcinoma |
| Mn(III)-TCCP MOF [56] | Manganese | Glutathione | T1 Enhancement | Photodynamic therapy | In vitro imaging of breast cancer; In vivo imaging of cancer in BALB/c mice |
| PLTM-HMnO2 NPs [57] | Manganese | Glutathione | T1 Enhancement | Bufalin | In vivo detection of murine tumor |
| DOX/MnO2@PVCL NGs [58] | Manganese | Glutathione | T1 Enhancement | Doxorubicin | Early stage development |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nwasike, C.; Purr, E.; Yoo, E.; Nagi, J.S.; Doiron, A.L. Activatable Nanoparticles: Recent Advances in Redox-Sensitive Magnetic Resonance Contrast Agent Candidates Capable of Detecting Inflammation. Pharmaceuticals 2021, 14, 69. https://doi.org/10.3390/ph14010069
Nwasike C, Purr E, Yoo E, Nagi JS, Doiron AL. Activatable Nanoparticles: Recent Advances in Redox-Sensitive Magnetic Resonance Contrast Agent Candidates Capable of Detecting Inflammation. Pharmaceuticals. 2021; 14(1):69. https://doi.org/10.3390/ph14010069
Chicago/Turabian StyleNwasike, Chukwuazam, Erin Purr, Eunsoo Yoo, Jaspreet Singh Nagi, and Amber L. Doiron. 2021. "Activatable Nanoparticles: Recent Advances in Redox-Sensitive Magnetic Resonance Contrast Agent Candidates Capable of Detecting Inflammation" Pharmaceuticals 14, no. 1: 69. https://doi.org/10.3390/ph14010069
APA StyleNwasike, C., Purr, E., Yoo, E., Nagi, J. S., & Doiron, A. L. (2021). Activatable Nanoparticles: Recent Advances in Redox-Sensitive Magnetic Resonance Contrast Agent Candidates Capable of Detecting Inflammation. Pharmaceuticals, 14(1), 69. https://doi.org/10.3390/ph14010069





