Disposition of Phytocannabinoids, Their Acidic Precursors and Their Metabolites in Biological Matrices of Healthy Individuals Treated with Vaporized Medical Cannabis
Abstract
1. Introduction
2. Results
2.1. Subjects and Study Design
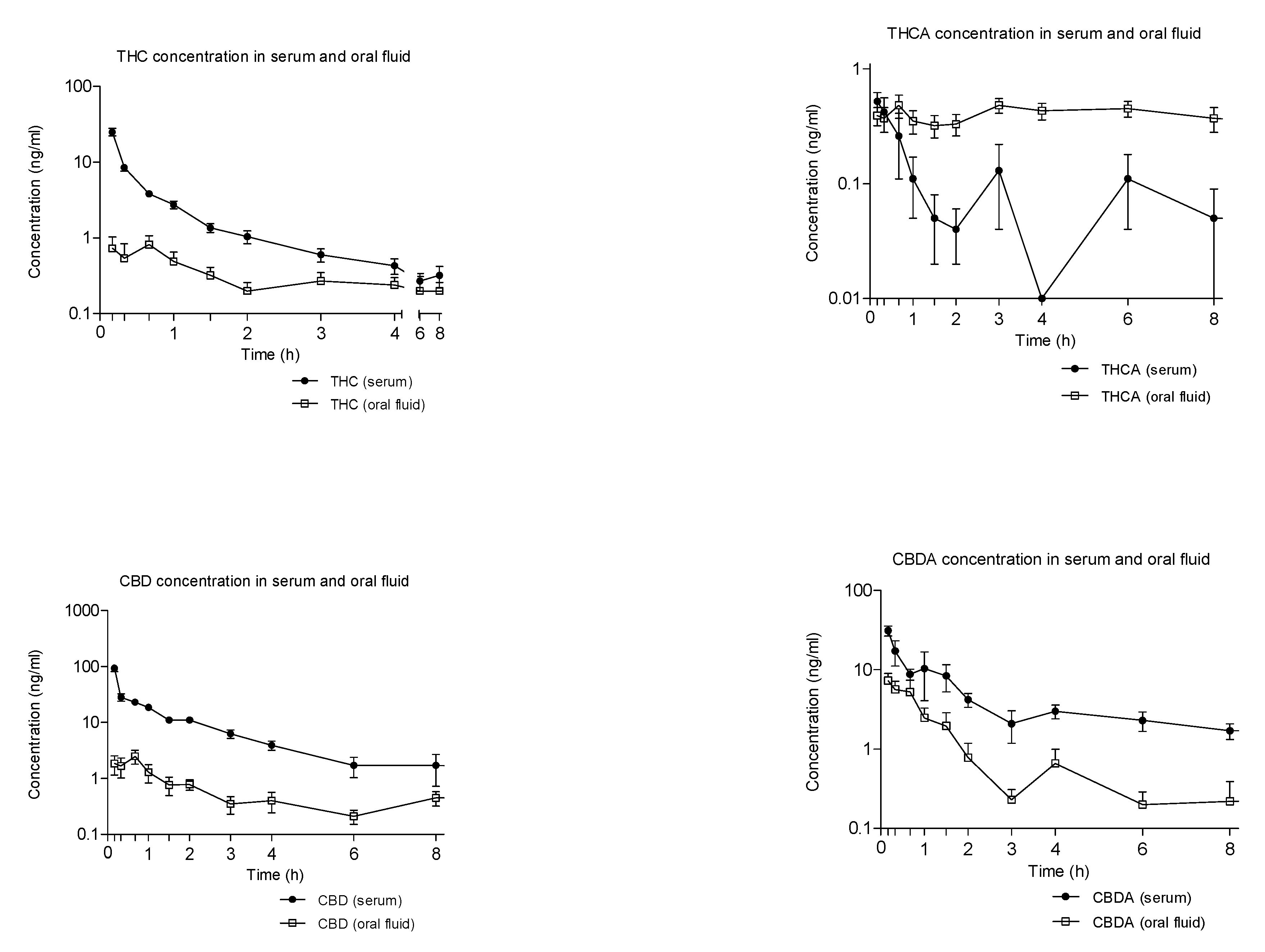
2.2. Concentration-Time Profiles and Pharmacokinetics of THC, CBD, and Their Acidic Precursors in Serum and Oral Fluid after Vaporized Cannabis Administration
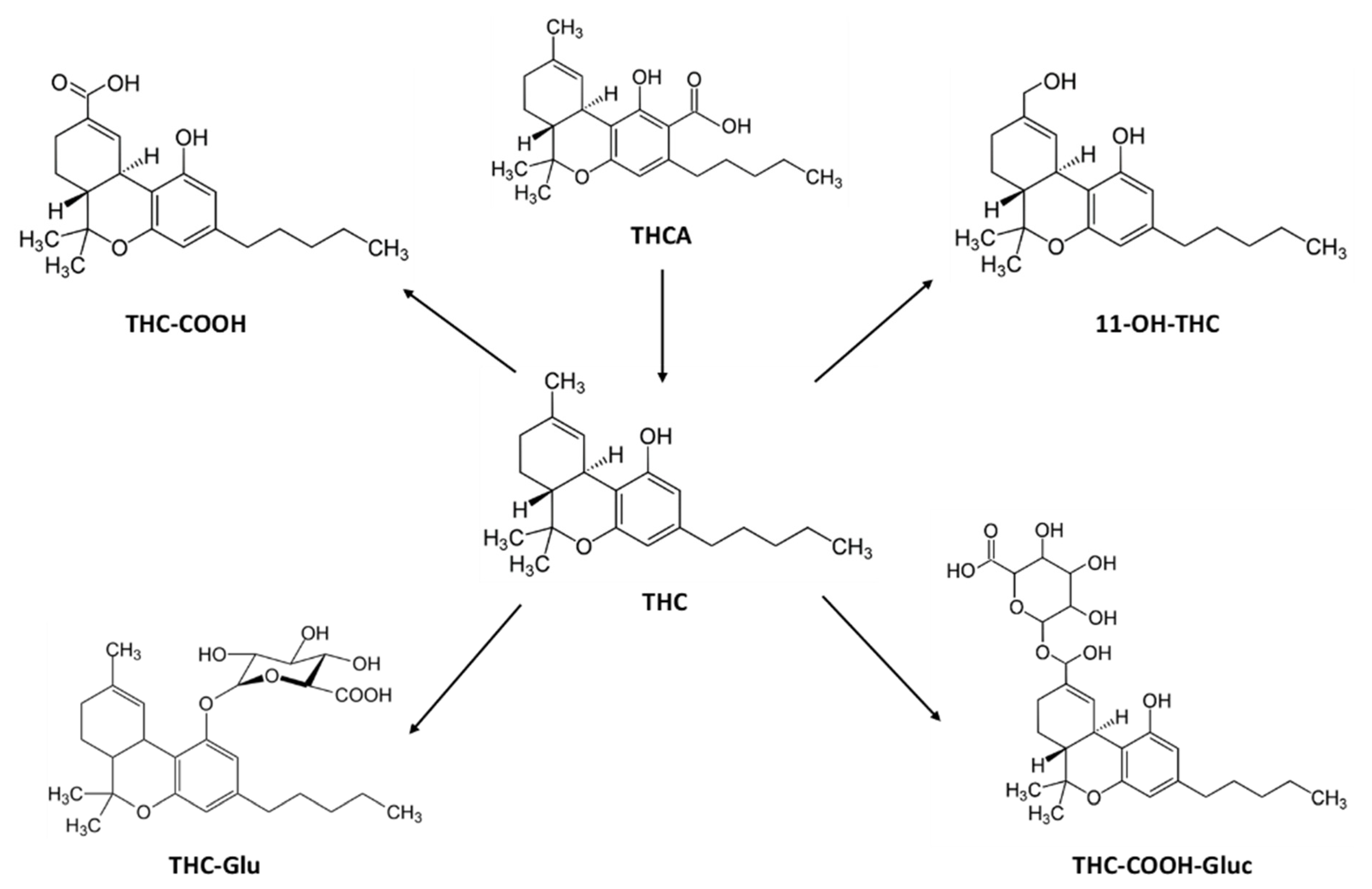
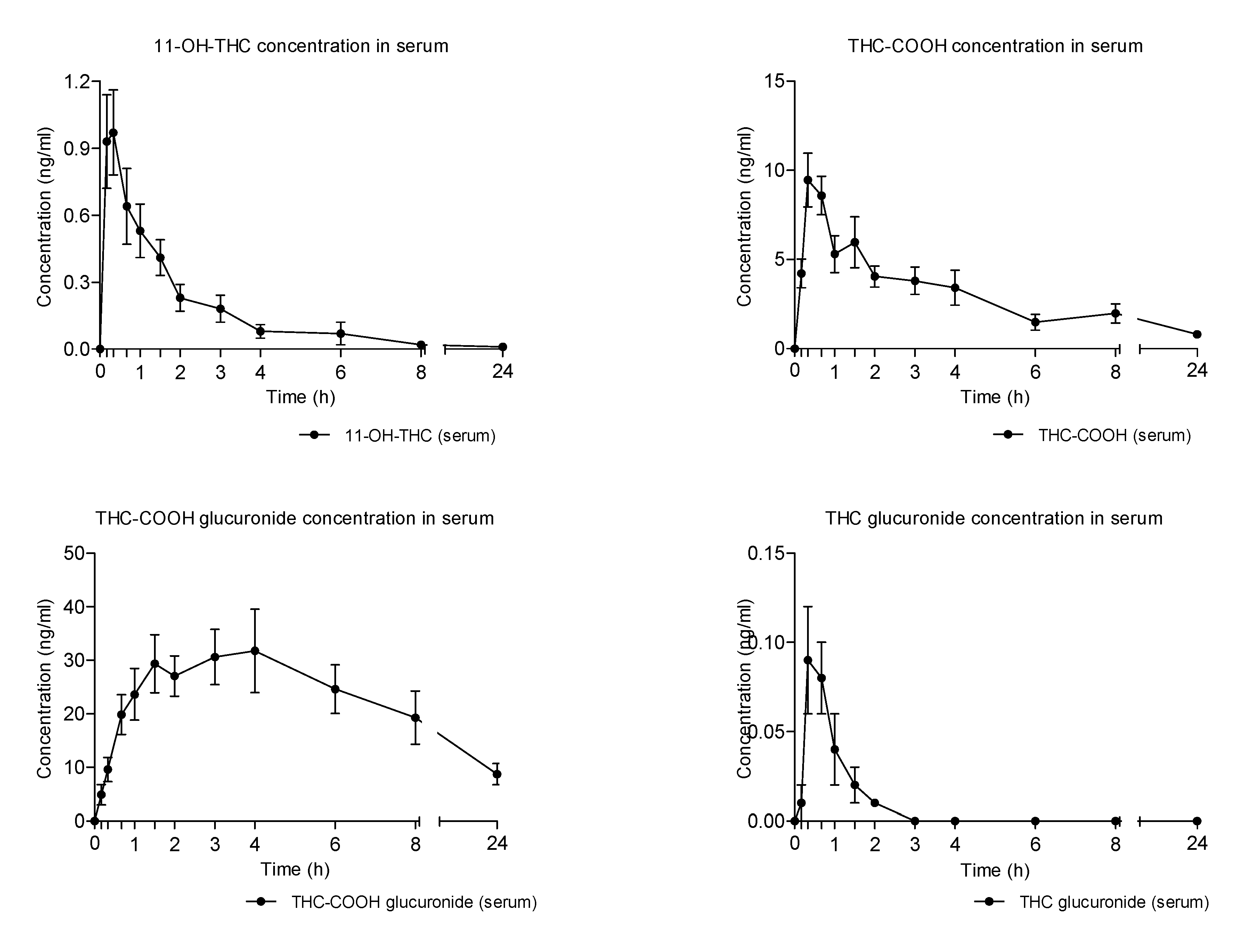
2.3. Concentration-Time Profiles and Pharmacokinetics of THC Metabolites in Seum after Vaporized Cannabis Administration
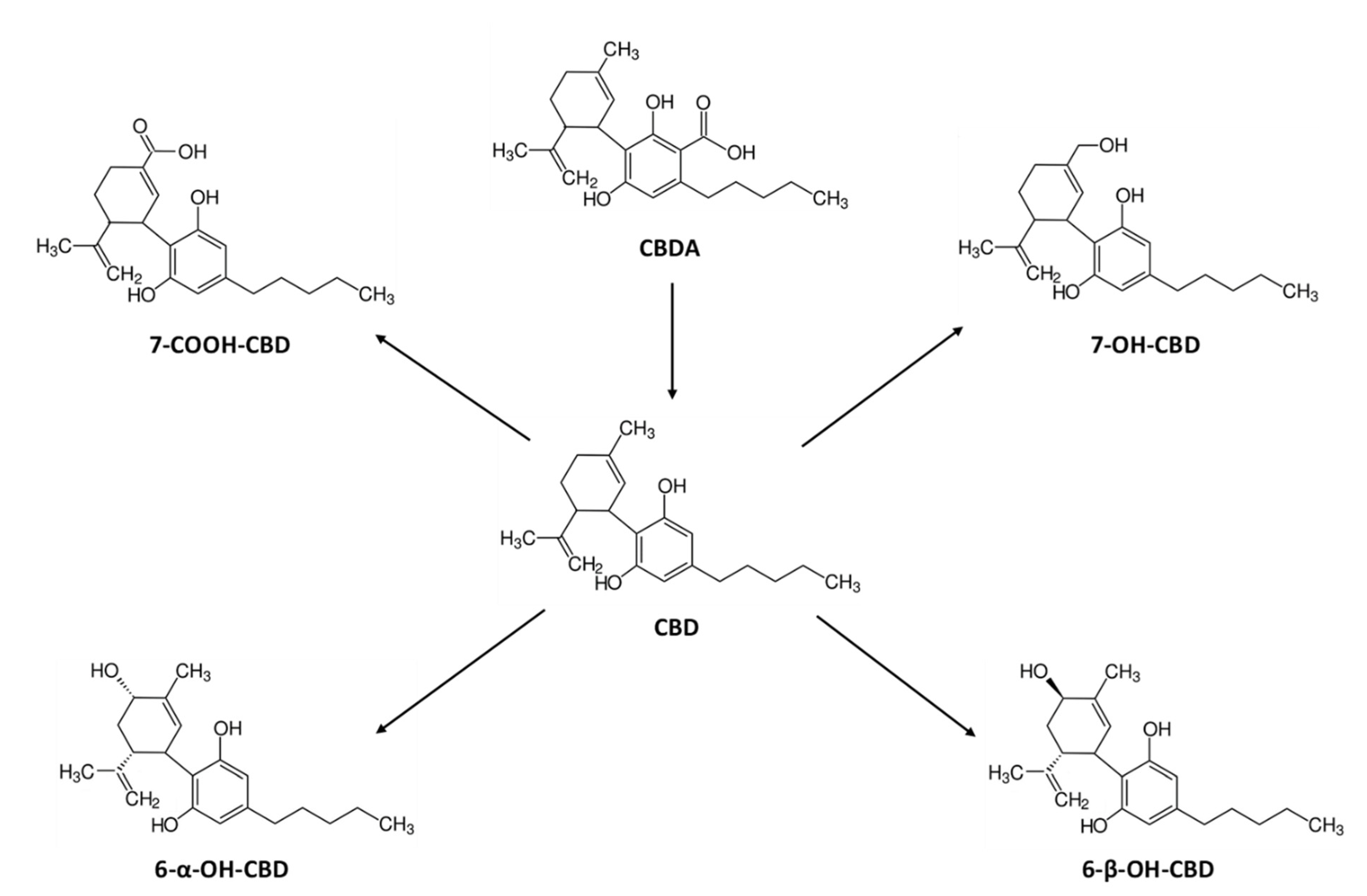
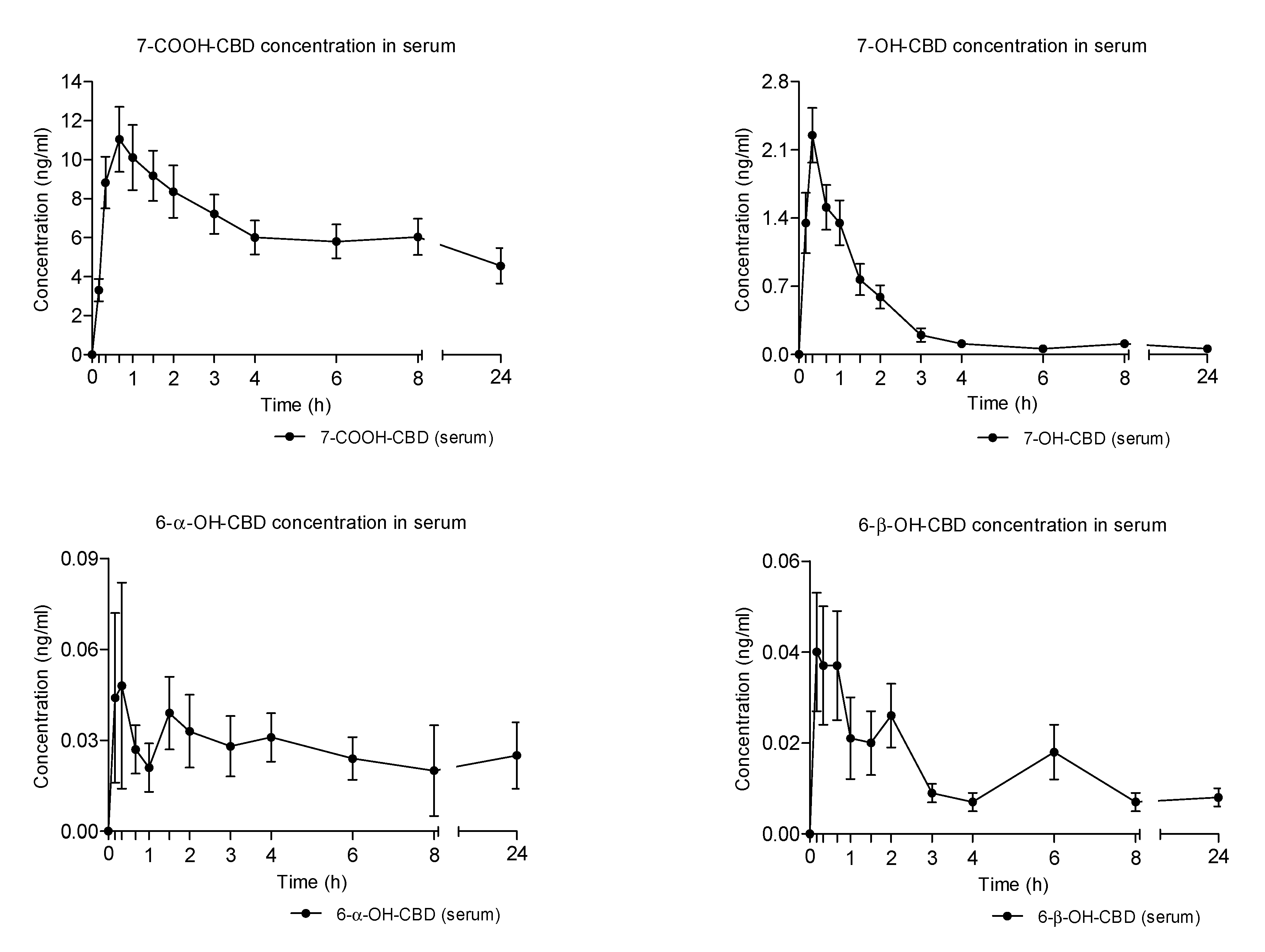
2.4. Concentration-Time Profiles and Pharmacokinetics of CBD Metabolites in Serum after Vaporized Cannabis Administration
2.5. Urinary Excretion of THC and CBD Metabolites after Vaporized Cannabis Administration
2.6. Physiological Measures and Subjective Effects
3. Discussion
- Pharmacokinetics of cannabinoids, their precursors, and their metabolites in biological fluids of healthy individuals treated with the three different formulations all showed great interindividual variability, likely due to the difficult standardization of herbal preparations in the case of decoction and oil and different inhalation rates in case of the vaporized formulation;
- A 60 times higher bioavailability and a significantly faster concentration peak of THC was observed in the case of medical cannabis inhalation vs. oral formulations, corresponding to a more than 60 times lower availability of THCA, due to acidic precursor decarboxylation during the vaporization process;
- The proportion between THC metabolites was similar in the three different preparations, with THC-COOH-GLUC being always the most formed metabolite followed by THC-CCOH.
- Oral fluid did not appear a suitable biological matrix alternative to serum for cannabinoids monitoring following vaporized medical cannabis administration since oral fluid concentrations did not reflect the ones measured in serum.
- A more than 90 times higher bioavailability and a significantly faster concentration peak of CBD was observed in the case of medical cannabis inhalation vs. oral formulations, corresponding to a more than 3 times lower availability of CBDA in comparison with that of cannabis decoction due to acidic precursor decarboxylation during the vaporization process. Conversely, the bioavailability of CBDA in cannabis oil as similar to that of vaporized cannabis.
- Whereas minimal salivary excretion did not discriminate different methods of medical cannabis administration, urinary extraction of both THC and CBD metabolites reflected the different parent compound’s availability following the three kinds of medical cannabis formulations.
4. Materials and Method
4.1. Subjects Enrolment
4.2. Preparation and Inhalation Procedure for Vaporized Cannabis
4.3. Study Design
4.4. Physiological and Subjective Effects Measurement
4.5. Biological Samples Collection
4.6. Determination of Cannabinoids, Acidic Precursors, and Metabolites in Biological Samples
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1555–1563. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Evaluation of long-term stability of cannabinoids in standardized preparations of cannabis flowering tops and cannabis oil by ultra-high-performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2018, 56, 94–96. [Google Scholar] [CrossRef]
- Pacifici, R.; Marchei, E.; Salvatore, F.; Guandalini, L.; Busardò, F.P.; Pichini, S. Stability of cannabinoids in cannabis FM1 flowering tops and oil preparation evaluated by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2019, 57, e165–e168. [Google Scholar] [CrossRef]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, Cannabimimetic Ligands, beyond the Cannabis Plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef] [PubMed]
- Mücke, M.; Phillips, T.; Radbruch, L.; Petzke, F.; Häuser, W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD012182. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Acevedo, A.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Busardò, F.P.; Pichini, S.; et al. Disposition of cannabinoids and their metabolites in serum, oral fluid, sweat patch and urine from healthy individuals treated with pharmaceutical preparations of medical cannabis. Phyther. Res. 2020, ptr.6931. [Google Scholar] [CrossRef]
- Pérez-Acevedo, A.P.; Busardò, F.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Di Trana, A.; et al. Disposition of cannabidiol metabolites in serum and urine from healthy individuals treated with pharmaceutical preparations of medical cannabis. Pharmaceuticals 2020, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicine 2020, 56, 237. [Google Scholar]
- Baratta, F.; Simiele, M.; Pignata, I.; Ravetto Enri, L.; Torta, R.; De Luca, A.; Collino, M.; D’Avolio, A.; Brusa, P. Development of Standard Operating Protocols for the Optimization of Cannabis-Based Formulations for Medical Purposes. Front Pharmacol. 2019, 10, 701. [Google Scholar] [CrossRef]
- Carcieri, C.; Tomasello, C.; Simiele, M.; De Nicolò, A.; Avataneo, V.; Canzoneri, L.; Cusato, J.; Di Perri, G.; D’Avolio, A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm Pharmacol. 2018, 70, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Barco, S.; Fucile, C.; Manfredini, L.; De Grandis, E.; Gherzi, M.; Martelli, A.; Tripodi, G.; Mattioli, F.; Cangemi, G. A UHPLC-MS/MS method for the quantification of Δ9-tetrahydrocannabinol and cannabidiol in decoctions and in plasma samples for therapeutic monitoring of medical cannabis. Bioanalysis 2018, 10, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Licata, M.; Verri, P.; Vandelli, D.; Palazzoli, F.; Marchesi, F.; Cainazzo, M.M.; Pini, L.A.; Guerzoni, S. Pharmacokinetics and tolerability of oral cannabis preparations in patients with medication overuse headache (MOH)—A pilot study. Eur. J. Clin. Pharmacol. 2018, 74, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Gherzi, M.; Milano, G.; Fucile, C.; Calevo, M.G.; Mancardi, M.M.; Nobili, L.; Astuni, P.; Marini, V.; Barco, S.; Cangemi, G.; et al. Safety and pharmacokinetics of medical cannabis preparation in a monocentric series of young patients with drug resistant epilepsy. Complement. Ther. Med. 2020, 51, 102402. [Google Scholar] [CrossRef]
- Solowij, N.; Broyd, S.J.; van Hell, H.H.; Hazekamp, A. A protocol for the delivery of cannabidiol (CBD) and combined CBD and ∆9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol. Toxicol. 2014, 15, 58. [Google Scholar] [CrossRef]
- Aston, E.R.; Scott, B.; Farris, S.G. A qualitative analysis of cannabis vaporization among medical users. Exp. Clin. Psychopharmacol. 2019, 27, 301–308. [Google Scholar] [CrossRef]
- Lanz, C.; Mattsson, J.; Soydaner, U.; Brenneisen, R. Medicinal Cannabis: In Vitro Validation of Vaporizers for the Smoke-Free Inhalation of Cannabis. PLoS ONE 2016, 11, e0147286. [Google Scholar] [CrossRef]
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Hartman, R.L.; Brown, T.L.; Milavetz, G.; Spurgin, A.; Gorelick, D.A.; Gaffney, G.; Huestis, M.A. Controlled vaporized cannabis, with and without alcohol: Subjective effects and oral fluid-blood cannabinoid relationships. Drug Test Anal. 2016, 8, 690–701. [Google Scholar] [CrossRef]
- Wilsey, B.L.; Deutsch, R.; Samara, E.; Marcotte, T.D.; Barnes, A.J.; Huestis, M.A.; Le, D. A preliminary evaluation of the relationship of cannabinoid blood concentrations with the analgesic response to vaporized cannabis. J. Pain Res. 2016, 9, 587–598. [Google Scholar] [CrossRef]
- Swortwood, M.J.; Newmeyer, M.N.; Andersson, M.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal. 2017, 9, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Spindle, T.R.; Cone, E.J.; Schlienz, N.J.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; Hayes, E.; Vandrey, R. Urinary Excretion Profile of 11-Nor-9-Carboxy-Δ9-Tetrahydrocannabinol (THCCOOH) Following Smoked and Vaporized Cannabis Administration in Infrequent Cannabis Users. J. Anal. Toxicol. 2020, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Hazekamp, A.; Ruhaak, R.; Zuurman, L.; van Gerven, J.; Verpoorte, R. Evaluation of a vaporizing device (Volcano) for the pulmonary administration of tetrahydrocannabinol. J. Pharm. Sci. 2006, 95, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Papaseit, E.; Pérez-Mañá, C.; de Sousa Fernandes Perna, E.B.; Olesti, E.; Mateus, J.; Kuypers, K.P.; Theunissen, E.L.; Fonseca, F.; Torrens, M.; Ramaekers, J.G.; et al. Mephedrone and Alcohol Interactions in Humans. Front. Pharmacol. 2019, 10, 1588. [Google Scholar] [CrossRef]
- Martin-Santos, R.; Crippa, J.A.; Batalla, A.; Bhattacharyya, S.; Atakan, Z.; Borgwardt, S.; Allen, P.; Seal, M.; Langohr, K.; Farré, M.; et al. Acute effects of a single, oral dose of d9-tetrahydrocannabinol (THC) and cannabidiol (CBD) administration in healthy volunteers. Curr. Pharm. Des. 2012, 18, 4966–4979. [Google Scholar] [CrossRef]
- González, D.; Torrens, M.; Farré, M. Acute Effects of the Novel Psychoactive Drug 2C-B on Emotion. Biomed. Res. Int. 2015, 2015, 643878. [Google Scholar] [CrossRef]
- Papaseit, E.; Pérez-Mañá, C.; Mateus, J.A.; Pujadas, M.; Fonseca, F.; Torrens, M.; Olesti, E.; de la Torre, R.; Farré, M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology 2016, 41, 2704–2713. [Google Scholar] [CrossRef]
- Pichini, S.; Mannocchi, G.; Gottardi, M.; Pérez-Acevedo, A.P.; Poyatos, L.; Papaseit, E.; Pérez-Mañá, C.; Farré, M.; Pacifici, R.; Busardò, F.P. Fast and sensitive UHPLC-MS/MS analysis of cannabinoids and their acid precursors in pharmaceutical preparations of medical cannabis and their metabolites in conventional and non-conventional biological matrices of treated individual. Talanta 2020, 209, 120537. [Google Scholar] [CrossRef]
- Pichini, S.; Malaca, S.; Gottardi, M.; Pérez-Acevedo, A.P.; Papaseit, E.; Perez-Maña, C.; Farré, M.; Pacifici, R.; Tagliabracci, A.; Mannocchi, G.; et al. UHPLC-MS/MS analysis of cannabidiol metabolites in serum and urine samples. Application to an individual treated with medical cannabis. Talanta 2021, 223, 121772. [Google Scholar] [CrossRef]


| Pharmacokinetics Parameters (mean ± SD) | ||||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (hour) | AUC0–8h (ng/mL·h) | AUC0–24h (ng/mL·h) | |
| THC | 1.29 ± 1.11 | 0.67 (0.17–24.0) | 2.26 ± 2.32 | 4.90 ± 5.22 |
| THCA | 0.78 ± 0.26 | 3.50 (0.17–8.0) | 3.26 ± 1.44 | 7.79 ± 4.29 |
| CBD | 3.27 ± 2.77 | 0.67 (0.17–8.0) | 4.88 ± 3.90 | 11.36 ± 8.88 |
| CBDA | 10.22 ± 8.49 | 0.17 (0.17–1.5) | 8.79 ± 6.87 | 13.17 ± 12.60 |
| Pharmacokinetics Parameters (mean ± SD) | |||||||
|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (hour) | AUC0–8h (ng/mL·h) | AUC0–24h (ng/mL·h) | Ke (h−1) | t1/2 (hour) | MRT (hour) | |
| THC | 24.92 ± 10.74 | 0.17 | 12.21 ± 4.11 | 15.91 ± 6.76 | 0.79 ± 0.56 | 1.75 ± 1.97 | 3.59 ± 4.06 |
| THCA | 0.95 ± 0.47 | 0.33 (0.17–8.0) | 0.80 ± 0.1 | 1.49 ± 2.93 | ND | ND | 3.37 ± 2.17 |
| 11-OH-THC | 1.43 ± 0.66 | 0.33 (0.17–1.0) | 1.67 ± 0.99 | 1.91 ± 1.17 | 0.83 ± 0.79 | 1.25 ± 0.65 | 3.05 ± 2.75 |
| THC-COOH | 12.08 ± 4.76 | 0.50 (0.33–2.0) | 28.05 ± 16.71 | 50.23 ± 35.02 | ND | ND | 6.73 ± 2.16 |
| THC-COOH-GLUC | 41.85 ± 26.81 | 3.0 (1.5–8.0) | 213.83 ± 132.38 | 450.52 ± 322.36 | 0.06 ± 0.02 | 12.62 ± 3.79 | 8.55 ± 0.73 |
| THC-GLUC | 0.12 ± 0.11 | 0.5 (0.0–1.5) | 0.09 ± 0.08 | 0.10 ± 0.09 | ND | ND | 1.4 ± 1.14 |
| CBD | 93.17 ± 44.77 | 0.17 | 68.84 ± 18.25 | 88.42 ± 50.53 | 0.46 ± 0.30 | 3.45 ± 4.72 | 3.12 ± 2.68 |
| CBDA | 34.54 ± 21.54 | 0.17 (0.17–3.0) | 36.90 ± 20.52 | 63.80 ± 23.21 | 1.02 ± 1.15 | 4.75 ± 7.29 | 7.76 ± 4.18 |
| 7-COOH-CBD | 11.92 ± 6.29 | 0.67 (0.67–3.0) | 55.38 ± 28.65 | 140.17 ± 78.63 | ND | ND | 10.23 ± 1.83 |
| 7-OH-CBD | 2.36 ± 1.02 | 0.33 (0.17–1.5) | 3.28 ± 1.81 | 4.62 ± 2.82 | 0.77 ± 0.63 | 3.22 ± 5.10 | 4.71 ± 1.90 |
| 6α-OH-CBD | 0.11 ± 0.11 | 1.75 (0.17–6.0) | 0.22 ± 0.18 | 0.59 ± 0.94 | ND | ND | 1.00 ± 0.00 |
| 6β-OH-CBD | 0.08 ± 0.05 | 0.67 (0.17–24.0) | 0.13 ± 0.09 | 0.25 ± 0.14 | ND | ND | 8.80 ± 4.28 |
| Effects | Emax (mean ± SD) | Tmax (Median-Range) | AUC0–8h (mean ± SD) |
|---|---|---|---|
| Systolic blood pressure, mm Hg | −6.57 ± 16.37 | 1.34 (0.17–6.0) | −46.14 ± 54.18 |
| Diastolic blood pressure, mm Hg | 2.93 ± 14.12 | 0.84 (0.17–6.0) | −16.04 ± 46.15 |
| Heart rate, bpm | 25.64 ± 23.23 | 0.17 (0.17–8.0) | 10.97 ± 55.62 |
| VAS Intensity, mm | 42.86 ± 22.45 | 0.17 (0.17–0.67) | 44.20 ± 35.69 |
| VAS High, mm | 43.79 ± 22.87 | 0.17 (0.17–0.33) | 42.18 ± 32.15 |
| VAS Good effects, mm | 33.00 ± 22.38 | 0.33 (0.0–1.0) | 37.34 ± 30.49 |
| VAS Bad effects, mm | 13.00 ± 19.42 | 0.17 (0.0–0.33) | 7.14 ± 12.94 |
| VAS Appetite, mm | 47.36 ± 27.42 | 2.0 (0.0–3.0) | 141.31 ± 113.40 |
| VAS Drowsiness, mm | 39.00 ± 28.25 | 1.5 (0.17–3.0) | 76.83 ± 59.86 |
| VAS Dizziness, mm | 19.64 ± 22.38 | 0.17 (0.0–1.0) | 10.06 ± 13.36 |
| VAS Confusion, mm | 11.79 ± 16.04 | 0.17 (0.0–0.67) | 4.27 ± 5.90 |
| VAS Nausea, mm | 1.57 ± 4.85 | 0.0 (0.0–0.67) | 0.80 ± 2.80 |
| VAS Vomit, mm | 1.43 ± 5.35 | 0.0 (0.0–0.33) | 0.46 ± 1.72 |
| VAS Anxiety, mm | 7.0 ± 14.82 | 0.0 (0.0–0.33) | 4.66 ± 10.86 |
| ARCI-PCAG, score | 5.71 ± 2.02 | 0.33 (0.33–3.0) | 12.25 ± 8.82 |
| ARCI-MBG, score | 4.21 ± 4.58 | 0.33 (0.0–2.0) | 8.26 ± 11.61 |
| ARCI-LSD, score | 2.14 ± 2.54 | 0.33 (0.33–1.5) | 0.81 ± 3.46 |
| ARCI-BG, score | −1.00 ± 2.57 | 0.67 (0.33–4.0) | 0.03 ± 5.54 |
| ARCI-A, score | 3.00 ± 2.39 | 0.33 (0.0–1.5) | 7.63 ± 8.45 |
| VESSPA-S, score | 0.90 ± 0.53 | 1.0 (1.0) | 2.88 ± 2.11 |
| VESSPA-SA, score | 0.69 ± 0.31 | 1.0 (1.0–2.0) | 1.86 ± 1.17 |
| VESSPA-CP, score | 0.02 ± 0.09 | 0.0 (0.0–1.0) | 0.02 ± 0.09 |
| VESSPA-PCS, score | 0.64 ± 1.08 | 1.0 (0.0–2.0) | 2.69 ± 4.75 |
| VESSPA-AE, score | 0.36 ± 0.65 | 0.0 (0.0–8.0) | 1.19 ± 2.35 |
| VESSPA-SP, score | 0.46 ± 0.75 | 1.0 (0.0–2.0) | 0.83 ± 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busardò, F.P.; Pérez-Acevedo, A.P.; Pacifici, R.; Mannocchi, G.; Gottardi, M.; Papaseit, E.; Pérez-Mañá, C.; Martin, S.; Poyatos, L.; Pichini, S.; et al. Disposition of Phytocannabinoids, Their Acidic Precursors and Their Metabolites in Biological Matrices of Healthy Individuals Treated with Vaporized Medical Cannabis. Pharmaceuticals 2021, 14, 59. https://doi.org/10.3390/ph14010059
Busardò FP, Pérez-Acevedo AP, Pacifici R, Mannocchi G, Gottardi M, Papaseit E, Pérez-Mañá C, Martin S, Poyatos L, Pichini S, et al. Disposition of Phytocannabinoids, Their Acidic Precursors and Their Metabolites in Biological Matrices of Healthy Individuals Treated with Vaporized Medical Cannabis. Pharmaceuticals. 2021; 14(1):59. https://doi.org/10.3390/ph14010059
Chicago/Turabian StyleBusardò, Francesco Paolo, Ana Pilar Pérez-Acevedo, Roberta Pacifici, Giulio Mannocchi, Massimo Gottardi, Esther Papaseit, Clara Pérez-Mañá, Soraya Martin, Lourdes Poyatos, Simona Pichini, and et al. 2021. "Disposition of Phytocannabinoids, Their Acidic Precursors and Their Metabolites in Biological Matrices of Healthy Individuals Treated with Vaporized Medical Cannabis" Pharmaceuticals 14, no. 1: 59. https://doi.org/10.3390/ph14010059
APA StyleBusardò, F. P., Pérez-Acevedo, A. P., Pacifici, R., Mannocchi, G., Gottardi, M., Papaseit, E., Pérez-Mañá, C., Martin, S., Poyatos, L., Pichini, S., & Farré, M. (2021). Disposition of Phytocannabinoids, Their Acidic Precursors and Their Metabolites in Biological Matrices of Healthy Individuals Treated with Vaporized Medical Cannabis. Pharmaceuticals, 14(1), 59. https://doi.org/10.3390/ph14010059







