Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity
Abstract
1. Introduction
2. Results
2.1. Antifungal Potential of Selected Flavonoids
2.2. Interference of Tested Flavonoids with C. albicans Biofilm Formation
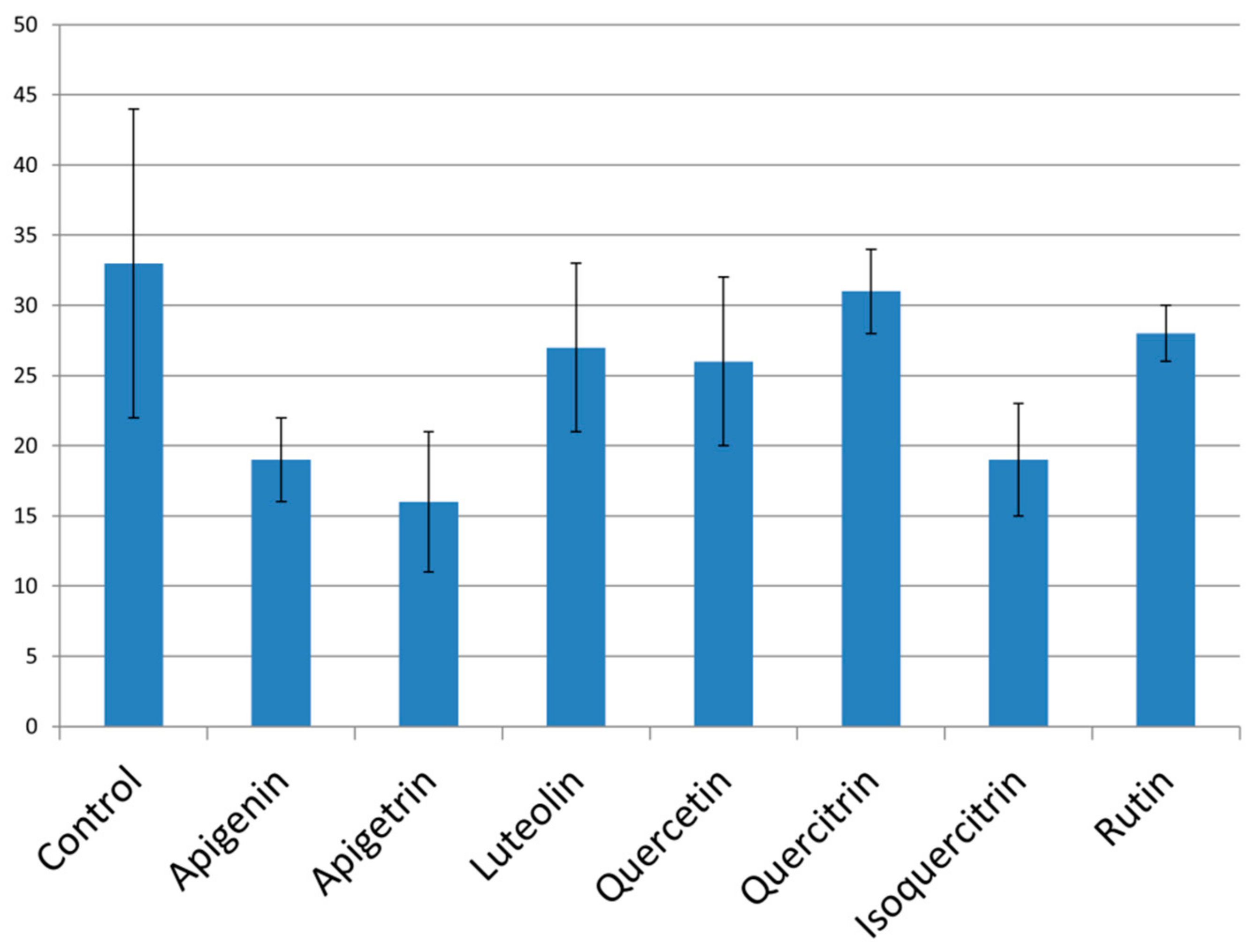
2.3. Flavonoids as Inhibitors of C. albicans Hyphal Growth
2.4. Expression of Antifungal Resistance Associated Genes after Application of Flavonoids
2.5. Cytotoxicity of Selected Compounds
3. Discussion
4. Materials and Methods
4.1. Fungal Culture Conditions
4.2. Antifungal Activity
4.3. Impact of Selected Compounds on Candida albicans Virulence Factors
4.3.1. Antibiofilm Activity
4.3.2. Anti-Hyphal Forming Activity
4.4. Interference with Expression of CDR1 and ERG11
4.4.1. RNA Isolation
4.4.2. DNAse Treatment and cDNA Synthesis
4.4.3. qPCR
4.5. Cytotoxicity of Compounds Towards Porcine Liver Primary Cells
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of Pathogenic Candida Species to Evade the Host Complement Attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Sustr, V.; Foessleitner, P.; Kiss, H.; Farr, A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J. Fungi 2020, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lara, M.F.; Ostrosky-Zeichner, L. Invasive Candidiasis. Semin. Respir. Crit. Care Med. 2020, 41, 3–12. [Google Scholar] [CrossRef]
- Dworecka-Kaszak, B.; Biegańska, M.J.; Dąbrowska, I. Occurrence of various pathogenic and opportunistic fungi in skin diseases of domestic animals: A retrospective study. BMC Vet. Res. 2020, 16, 248. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis-Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef]
- Jensen, R.H.; Astvad, K.M.; Silva, L.V.; Sanglard, D.; Jørgensen, R.; Nielsen, K.F.; Mathiasen, E.G.; Doroudian, G.; Perlin, D.S.; Arendrup, M.C. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J. Antimicrob. Chemother. 2015, 70, 2551–2555. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Houšť, J.; Spížek, J.; Havlíček, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Ćirić, A.; Petrović, J.; Glamočlija, J.; Smiljković, M.; Nikolić, M.; Stojković, D.; Soković, M. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. S. Afr. J. Bot. 2019, 120, 65–80. [Google Scholar] [CrossRef]
- Smiljković, M.; Kostić, M.; Stojković, D.; Glamočlija, J.; Soković, M. Could Flavonoids Compete with Synthetic Azoles in Diminishing Candida albicans Infections? A Comparative Review Based on In Vitro Studies. Curr. Med. Chem. 2019, 26, 2536–2554. [Google Scholar] [CrossRef] [PubMed]
- Smiljković, M.; Stanisavljević, D.; Stojković, D.; Petrović, I.; Marjanović Vićentić, J.; Popović, J.; Golič Grdadolnik, S.; Marković, D.; Sanković-Babić, S.; Glamočlija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 795–807. [Google Scholar] [PubMed]
- Zajdel, S.M.; Graikou, K.; Głowniak, K.; Chinou, I. Chemical analysis of Penstemon campanulatus (Cav.) Willd. antimicrobial activities. Fitoterapia 2012, 83, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.M.; de Torres, N.W. Activity of polyphenolic compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar] [CrossRef]
- Mail, M.H.; Himratul-Aznita, W.H.; Musa, M.Y. Anti-hyphal properties of potential bioactive compounds for oral rinse in suppression of Candida growth. Biotechnol. Biotechnol. Equip. 2017, 31, 989–999. [Google Scholar] [CrossRef]
- Ozçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Gehrke, I.T.; Neto, A.T.; Pedroso, M.; Mostardeiro, C.P.; Da Cruz, I.B.; Silva, U.F.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J. Ethnopharmacol. 2013, 148, 486–491. [Google Scholar] [CrossRef]
- Gao, M.; Wang, H.; Zhu, L. Quercetin assists fluconazole to inhibit biofilm formations of fluconazole-resistant Candida albicans in in vitro and in vivo antifungal managements of vulvovaginal candidiasis. Cell. Physiol. Biochem. 2016, 40, 727–742. [Google Scholar] [CrossRef]
- Sekita, Y.; Murakami, K.; Yumoto, H.; Hirao, K.; Amoh, T.; Fujiwara, N.; Hirota, K.; Fujii, H.; Matsuo, T.; Miyake, Y. Antibiofilm and anti-inflammatory activities of houttuynia cordata decoction for oral care. Evid. Based Complement. Altern. Med. 2017, 2017, 2850947. [Google Scholar] [CrossRef]
- Jun, J.E.; Lee, H.; Ko, H.J.; Woo, E.R.; Lee, D.G. Fungicidal effect of isoquercitrin via inducing membrane disturbance. BBA-Biomembranes 2015, 1848, 695–701. [Google Scholar]
- Šiler, B.; Živković, S.; Banjanac, T.; Cvetković, J.; Nestorović Živković, J.; Ćirić, A.; Soković, M.; Mišić, D. Centauries as underestimated food additives: Antioxidant and antimicrobial potential. Food Chem. 2014, 147, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Han, Y. Rutin has therapeutic effect on septic arthritis caused by Candida albicans. Int. Immunopharmacol. 2009, 9, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Johann, S.; Mendes, B.G.; Missau, F.C.; de Resende, M.A.; Pizzolatti, M.G. Antifungal activity of five species of Polygala. Braz. J. Microbiol. 2011, 42, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Kytidou, K.; Artola, M.; Overkleeft, H.S.; Aerts, J. Plant Glycosides and Glycosidases: A Treasure-Trove for Therapeutics. Front. Plant Sci. 2020, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Sherry, L.; Rajendran, R.; Edwards, C.A.; Combet, E.; Ramage, G. Utilising polyphenols for the clinical management of Candida albicans biofilms. Int. J. Antimicrob. Agents 2014, 44, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Woo, E.R.; Lee, D.G. Apigenin induces cell shrinkage in Candida albicans by membrane perturbation. FEMS Yeast Res. 2018, 18, foy003. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.T.; Ferreira, I.C.; Barros, L.; Silva, S.; Azeredo, J.; Henriques, M. Antifungal activity of phenolic compounds identified in flowers from North Eastern Portugal against Candida species. Future Microbiol. 2014, 9, 139–146. [Google Scholar] [CrossRef]
- Singh, B.N.; Upreti, D.K.; Singh, B.R.; Pandey, G.; Verma, S.; Roy, S.; Naqvi, A.H.; Rawat, A.K. Quercetin sensitizes fluconazole-resistant Candida albicans to induce apoptotic cell death by modulating quorum sensing. Antimicrob. Agents Chemother. 2015, 59, 2153–2168. [Google Scholar] [CrossRef]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef]
- Zagrean-Tuza, C.; Mot, A.C.; Chmiel, T.; Bende, A.; Turcu, I. Sugar matters: Sugar moieties as reactivity-tuning factors in quercetin O-glycosides. Food Funct. 2020, 11, 5293–5307. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.; Glamočlija, J.; Golič Grdadolnik, S.; Sanglard, D.; Soković, M. Revealing the astragalin mode of anticandidal action. EXCLI J. 2020, 19, 1436–1445. [Google Scholar] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.; Schindler, B.; Kaatz, G.; Cech, N. A Mass Spectrometry-Based Assay for Improved Quantitative Measurements of Efflux Pump Inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of flavonoids toward cultured normal human cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Sayadi, S. Apigetrin inhibits adipogenesis in 3T3-L1 cells by downregulating PPARγ and CEBP-α. Lipids Health Dis. 2018, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Nakanishi, R.; Nishino, H.; Matsui, H.; Sakai, T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene 2005, 24, 7180–7189. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Predes, D.M.; Fonseca, B.F.; Cerqueira, D.M.; Reis, A.H.; Dudenhoeffer, A.C.; Borges, H.L.; Mendes, F.A.; Abreu, J.G. Isoquercitrin suppresses colon cancer cell growth in vitro by targeting the Wnt/β-catenin signaling pathway. J. Biol. Chem. 2014, 289, 35456–35467. [Google Scholar] [CrossRef]
- EUCAST (European Committee on Antibiotic Susceptibility). Method for Determination of Minimal Inhibitory Concentration (MIC) by Broth Dilution of Fermentative Yeasts; Discussion document E. Dis. 7.1; European Society of Clinical Microbiology and Infectious Diseases: Munich, Germany, 2002. [Google Scholar]
- Smiljkovic, M.; Matsoukas, M.T.; Kritsi, E.; Zelenko, U.; Grdadolnik, S.G.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sankovic-Babic, S.; Glamoclija, J.; Fotopoulou, T.; et al. Nitrate Esters of Heteroaromatic Compounds as Candida albicans CYP51 Enzyme Inhibitors. ChemMedChem 2018, 13, 251–258. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Calabrese, D.; Majcherczyk, P.A.; Bille, J. The ATP binding cassette transporter gene CgCDR1 from Candida glabrata is involved in the resistance of clinical isolates to azole antifungal agents. Antimicrob. Agents Chemother. 1999, 43, 2753–2765. [Google Scholar] [CrossRef]
- Lohberger, A.; Coste, A.T.; Sanglard, D. Distinct roles of Candida albicans drug resistance transcription factors TAC1, MRR1, and UPC2 in virulence. Eukaryot. Cell. 2014, 13, 127–142. [Google Scholar] [CrossRef]
- Guimaraes, R.; Barros, L.; Duenas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.; Ferreira, I.C.F.R. Nutrients, phytochemicals and bioactivity of wild Roman chamomile: A comparison between the herb and its preparations. Food Chem. 2013, 136, 718–725. [Google Scholar] [CrossRef] [PubMed]



| Compounds | C. albicans 475/15 | C. albicans 527/14 | C. albicans 10/15 | C. albicans 13/15 | ||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| Luteolin | 37.5 ± 1 c | 75 ± 2 c | 37.5 ± 1 c | 75 ± 20 c | 37.5 ± 1 c | 75 ± 2 c | 37.5 ± 1 c | 75 ± 2 c |
| Quercetin | 75 ± 2 d | 150 ± 10 d | 75 ± 2 d | 150 ± 10 d | 75 ± 3 d | 150 ± 20 d | 75 ± 10 d | 150 ± 20 d |
| Quercitrin | 37.5 ± 2 c | 75 ± 3 c | 37.5 ± 2 c | 75 ± 3 c | 37.5 ± 2 c | 75 ± 3 c | 37.5 ± 1 c | 75 ± 3 c |
| Isoquercitrin | 37.5 ± 1 c | 75 ± 2 c | 37.5 ± 1 c | 75 ± 2 c | 37.5 ± 2 c | 75 ± 2 c | 37.5 ± 2 c | 75 ± 2 c |
| Rutin | 37.5 ± 1 c | 75 ± 1 c | 37.5 ± 1 c | 75 ± 1 c | 37.5 ± 1 c | 75 ± 2 c | 37.5 ± 1 c | 75 ± 2 c |
| Ketoconazole | 3.1 ± 0.1 b | 6.2 ± 0.1 b | 3.1 ± 0.1 b | 6.2 ± 0.1 b | 3.1 ± 0.1 b | 50 ± 0.1 b | 1.6 ± 0.2 b | 50 ± 0.2 b |
| Amphotericin B | 0.63 ± 0.001 a | 1.25 ± 0.002 a | 0.63 ± 0.001 a | 1.25 ± 0.002 a | 0.63 ± 0.001 a | 1.25 ± 0.002 a | 0.63 ± 0.001 a | 1.25 ± 0.002 a |
| Compound | GI50 |
|---|---|
| Apigenin | >400 |
| Apigetrin | 90 ± 1 |
| Luteolin | >400 |
| Quercetin | >400 |
| Quercitrin | 73 ± 3 |
| Isoquercitrin | >400 |
| Rutin | >400 |
| Ellipticin | 3.22 ± 0.2 |
| Primer | Sequence |
|---|---|
| CDR1-ORF-F | ATGACTCGAGATATTTTGATA |
| CDR1-ORF-R | TTAACAGCAATGGTCTTTA |
| ERG11-ORF-F | ATTGTTGAAACTGTCATTG |
| ERG11-ORF-R | CCCCTAATAATATACTGATCTG |
| ACT-ORF-F | GCATCACACTTTTTACAAT |
| ACT-ORF-R | AAACATAATTTGAGTCATCTTT |
| Probe | Sequence |
| CDR1-P2 | CATTATGAGACCTGGTGAACTTACT |
| ERG11-P2 | TTTGTCCCTTAGTGTTACACA |
| ACT1-P2 | TTGCTCCAGAAGAACATCCAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals 2021, 14, 27. https://doi.org/10.3390/ph14010027
Ivanov M, Kannan A, Stojković DS, Glamočlija J, Calhelha RC, Ferreira ICFR, Sanglard D, Soković M. Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals. 2021; 14(1):27. https://doi.org/10.3390/ph14010027
Chicago/Turabian StyleIvanov, Marija, Abhilash Kannan, Dejan S. Stojković, Jasmina Glamočlija, Ricardo C. Calhelha, Isabel C. F. R. Ferreira, Dominique Sanglard, and Marina Soković. 2021. "Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity" Pharmaceuticals 14, no. 1: 27. https://doi.org/10.3390/ph14010027
APA StyleIvanov, M., Kannan, A., Stojković, D. S., Glamočlija, J., Calhelha, R. C., Ferreira, I. C. F. R., Sanglard, D., & Soković, M. (2021). Flavones, Flavonols, and Glycosylated Derivatives—Impact on Candida albicans Growth and Virulence, Expression of CDR1 and ERG11, Cytotoxicity. Pharmaceuticals, 14(1), 27. https://doi.org/10.3390/ph14010027










