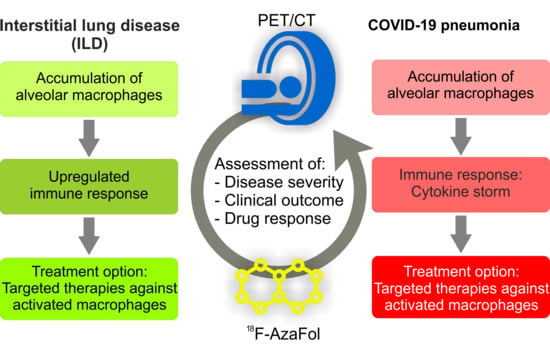
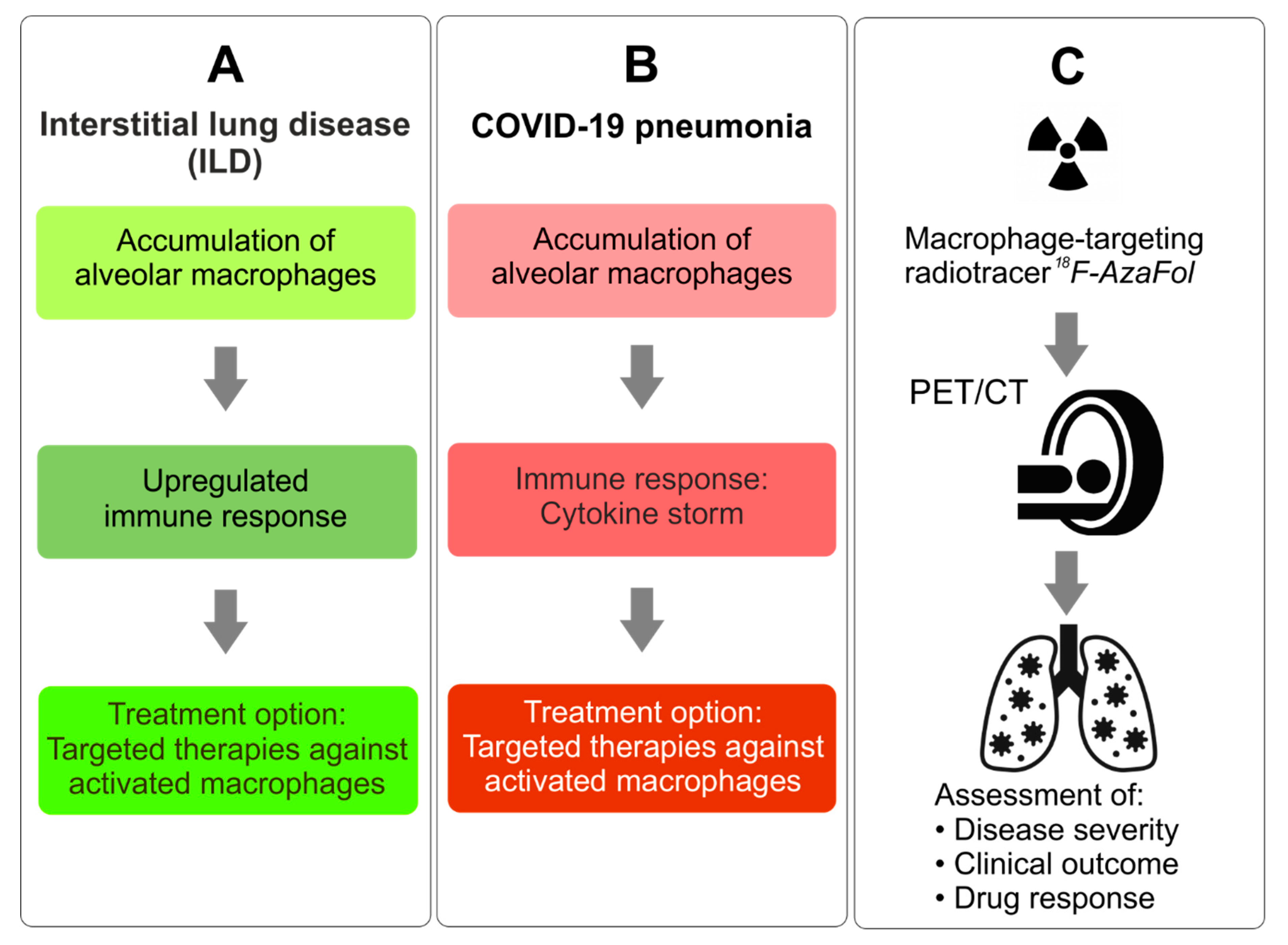
Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk?
Abstract
1. Introduction
2. Dysregulation of Immune Responses and Macrophage Activation as Prognosticators of Poor Outcome in COVID-19
3. Role of Chest Imaging in the Management of COVID-19 Patients
4. Folate-Based PET Radiotracers for Imaging of Activated Macrophages
5. 18F-AzaFol—A Clinically-Tested Folate-Based Radiotracer for PET Imaging
6. Potential Role of PET Imaging of Macrophages for the Management of COVID-19 Patients
- (a)
- Early detection of COVID-19-related (multi-)organ involvement.
- (b)
- Quantification of the extent of the disease. Since COVID-19 is a systemic disease, whole-body PET/CT may be used to visualize macrophage activity on a systemic level thereby providing a comprehensive overview of the overall disease extent and severity by visualizing the affected organs as previously proposed to be achieved with [18F]FDG [40,42,43].
- (c)
- Risk stratification and treatment guidance. Based on the correlation of 18F-AzaFol uptake in the diseased tissue with the numbers of activated, FRβ-positive macrophages [19], quantitative thresholds could be defined to stratify patients according to disease severity and outcome, including recovery time (in ARDS) and to identify patients likely to benefit from macrophage-oriented therapies [36,57].
- (d)
- Monitoring of drug response and disease course. 18F-AzaFol-PET-based imaging may represent a method to monitor the treatment responses of the numerous emerging therapies targeted at activated macrophages-related factors [36,57]. In addition, it would allow the early detection of disease sequelae or comorbidities and the differentiation of active, ongoing disease (high signal intensity) from an inactive damage state (low signal intensity or no signal) in patients with persisting compromised organ function.
7. Conclusions and Perspectives
8. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Basu, S.; Zhuang, H.; Torigian, D.A.; Rosenbaum, J.; Chen, W.; Alavi, A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin. Nucl. Med. 2009, 39, 124–145. [Google Scholar] [CrossRef]
- Czernin, J.; Allen-Auerbach, M.; Nathanson, D.; Herrmann, K. PET/CT in oncology: Current status and perspectives. Curr. Radiol. Rep. 2013, 1, 177–190. [Google Scholar] [CrossRef]
- Singh, A.S.; Radu, C.G.; Ribas, A. PET imaging of the immune system: Immune monitoring at the whole body level. Q. J. Nucl. Med. Mol. Imaging 2010, 54, 281–290. [Google Scholar] [PubMed]
- Lee, H.W.; Gangadaran, P.; Kalimuthu, S.; Ahn, B.C. Advances in molecular imaging strategies for in vivo tracking of immune cells. BioMed Res. Int. 2016, 2016, 1946585. [Google Scholar] [CrossRef]
- Venneti, S.; Lopresti, B.J.; Wiley, C.A. Molecular imaging of microglia/macrophages in the brain. Glia 2013, 61, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Vivash, L.; O’Brien, T.J. Imaging microglial activation with TSPO PET: Lighting up neurologic diseases? J. Nucl. Med. 2016, 57, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Werry, E.L.; Bright, F.M.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kril, J.J.; Kassiou, M. Recent developments in TSPO PET imaging as a biomarker of neuroinflammation in neurodegenerative disorders. Int. J. Mol. Sci. 2019, 20, 161. [Google Scholar] [CrossRef]
- Xia, W.; Hilgenbrink, A.R.; Matteson, E.L.; Lockwood, M.B.; Cheng, J.X.; Low, P.S. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood 2009, 113, 438–446. [Google Scholar] [CrossRef]
- Jager, N.A.; Westra, J.; Golestani, R.; van Dam, G.M.; Low, P.S.; Tio, R.A.; Slart, R.H.; Boersma, H.H.; Bijl, M.; Zeebregts, C.J. Folate receptor-beta imaging using 99mTc-folate to explore distribution of polarized macrophage populations in human atherosclerotic plaque. J. Nucl. Med. 2014, 55, 1945–1951. [Google Scholar] [CrossRef]
- Yi, Y.S. Folate receptor-targeted diagnostics and therapeutics for inflammatory diseases. Immune Netw. 2016, 16, 337–343. [Google Scholar] [CrossRef]
- Gibson, G.J.; Loddenkemper, R.; Lundback, B.; Sibille, Y. Respiratory health and disease in Europe: The new European Lung White Book. Eur. Respir. J. 2013, 42, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Wells, A.U.; Denton, C.P. Interstitial lung disease in connective tissue disease--mechanisms and management. Nat. Rev. Rheumatol. 2014, 10, 728–739. [Google Scholar] [CrossRef]
- Maher, T.M.; Wells, A.U.; Laurent, G.J. Idiopathic pulmonary fibrosis: Multiple causes and multiple mechanisms? Eur. Respir. J. 2007, 30, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, J.M.; Taroni, J.; Martyanov, V.; Wood, T.A.; Greene, C.S.; Pioli, P.A.; Hinchcliff, M.E.; Whitfield, M.L. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput. Biol. 2015, 11, e1004005. [Google Scholar] [CrossRef] [PubMed]
- Taroni, J.N.; Greene, C.S.; Martyanov, V.; Wood, T.A.; Christmann, R.B.; Farber, H.W.; Lafyatis, R.A.; Denton, C.P.; Hinchcliff, M.E.; Pioli, P.A.; et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 2017, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Thomeer, M.J.; Vansteenkiste, J.; Verbeken, E.K.; Demedts, M. Interstitial lung diseases: Characteristics at diagnosis and mortality risk assessment. Respir. Med. 2004, 98, 567–573. [Google Scholar] [CrossRef]
- Sakkas, L.I. Spotlight on tocilizumab and its potential in the treatment of systemic sclerosis. Drug Des. Dev. Ther. 2016, 10, 2723–2728. [Google Scholar] [CrossRef]
- Byrne, A.J.; Maher, T.M.; Lloyd, C.M. Pulmonary macrophages: A new therapeutic pathway in fibrosing lung disease? Trends Mol. Med. 2016, 22, 303–316. [Google Scholar] [CrossRef]
- Schniering, J.; Benesova, M.; Brunner, M.; Haller, S.; Cohrs, S.; Frauenfelder, T.; Vrugt, B.; Feghali-Bostwick, C.; Schibli, R.; Distler, O.; et al. 18F-AzaFol for detection of folate receptor-beta positive macrophages in experimental interstitial lung disease-a proof-of-concept study. Front. Immunol. 2019, 10, 2724. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Lin, C.J.F.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: Results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum Dis. 2018, 77, 212–220. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): A Phase 2, randomised, controlled trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef]
- Phelan, A.L.; Katz, R.; Gostin, L.O. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Case Rep. (Zhonghua Bing Li Xue Za Zhi) 2020, 49, 411–417. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Evaluation of activation and inflammatory activity of myeloid cells during pathogenic human coronavirus infection. Methods Mol. Biol. 2020, 2099, 195–204. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Liu, L.; Wei, Q.; Lin, Q.; Fang, J.; Wang, H.; Kwok, H.; Tang, H.; Nishiura, K.; Peng, J.; Tan, Z.; et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, H.; Lu, X.X.; Xiao, H.; Ren, J.; Zhang, F.R.; Liu, Z.S. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: A single center’s observational study. World J. Pediatr. 2020, 16, 251–259. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Li, Y.; Xia, L. Coronavirus disease 2019 (COVID-19): Role of chest CT in diagnosis and management. AJR 2020, 214, 1280–1286. [Google Scholar] [CrossRef]
- Hansell, D.M.; Goldin, J.G.; King, T.E., Jr.; Lynch, D.A.; Richeldi, L.; Wells, A.U. CT staging and monitoring of fibrotic interstitial lung diseases in clinical practice and treatment trials: A position paper from the Fleischner Society. Lancet Respir. Med. 2015, 3, 483–496. [Google Scholar] [CrossRef]
- Dai, W.C.; Zhang, H.W.; Yu, J.; Xu, H.J.; Chen, H.; Luo, S.P.; Zhang, H.; Liang, L.H.; Wu, X.L.; Lei, Y.; et al. CT imaging and differential diagnosis of COVID-19. Can. Assoc. Radiol J. 2020, 71, 195–200. [Google Scholar] [CrossRef]
- Qin, C.; Liu, F.; Yen, T.C.; Lan, X. 18F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1281–1286. [Google Scholar] [CrossRef]
- Zou, S.; Zhu, X. FDG PET/CT of COVID-19. Radiology 2020, 296, E118. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Fanti, S.; Meyer, P.T.; Allen-Auerbach, M.; Hacker, M.; Sathekge, M.; Hicks, R.; Scott, A.M.; Hatazawa, J.; Yun, M.; et al. Nuclear medicine operations in the times of COVID-19: Strategies, precautions, and experiences. J. Nucl. Med. 2020, 61, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Setti, L.; Kirienko, M.; Dalto, S.C.; Bonacina, M.; Bombardieri, E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Divband, G.; Montahaei, Z.; Dehghani, T.; Kaviani, H.; Adinehpour, Z.; Akbarian Aghdam, R.; Rezaee, A.; Vali, R. A case of COVID-19 lung infection first detected by [18F]FDG PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1771–1772. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2008, 41, 120–129. [Google Scholar] [CrossRef]
- Ayala-Lopez, W.; Xia, W.; Varghese, B.; Low, P.S. Imaging of atherosclerosis in apoliprotein e knockout mice: Targeting of a folate-conjugated radiopharmaceutical to activated macrophages. J. Nucl. Med. 2010, 51, 768–774. [Google Scholar] [CrossRef]
- Piscaer, T.M.; Müller, C.; Mindt, T.L.; Lubberts, E.; Verhaar, J.A.; Krenning, E.P.; Schibli, R.; De Jong, M.; Weinans, H. Imaging of activated macrophages in experimental osteoarthritis using folate-targeted animal single-photon-emission computed tomography/computed tomography. Arthritis Rheumatol. 2011, 63, 1898–1907. [Google Scholar] [CrossRef]
- Shen, J.; Chelvam, V.; Cresswell, G.; Low, P.S. Use of folate-conjugated imaging agents to target alternatively activated macrophages in a murine model of asthma. Mol. Pharm. 2013, 10, 1918–1927. [Google Scholar] [CrossRef]
- Müller, A.; Beck, K.; Rancic, Z.; Müller, C.; Fischer, C.R.; Betzel, T.; Kaufmann, P.A.; Schibli, R.; Kramer, S.D.; Ametamey, S.M. Imaging atherosclerotic plaque inflammation via folate receptor targeting using a novel 18F-folate radiotracer. Mol. Imaging 2014, 13, 1–11. [Google Scholar] [CrossRef]
- Winkel, L.C.; Groen, H.C.; van Thiel, B.S.; Müller, C.; van der Steen, A.F.; Wentzel, J.J.; de Jong, M.; Van der Heiden, K. Folate receptor-targeted single-photon emission computed tomography/computed tomography to detect activated macrophages in atherosclerosis: Can it distinguish vulnerable from stable atherosclerotic plaques? Mol. Imaging 2014, 13. [Google Scholar] [CrossRef]
- Chandrupatla, D.; Jansen, G.; Vos, R.; Verlaan, M.; Chen, Q.; Low, P.S.; Windhorst, A.D.; Lammertsma, A.A.; van der Laken, C.J.; Molthoff, C.F.M. In-Vivo monitoring of anti-folate therapy in arthritic rats using [18F]fluoro-PEG-folate and positron emission tomography. Arthritis Res. Ther. 2017, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Silvola, J.M.U.; Li, X.G.; Virta, J.; Marjamaki, P.; Liljenback, H.; Hytonen, J.P.; Tarkia, M.; Saunavaara, V.; Hurme, S.; Palani, S.; et al. Aluminum fluoride-18 labeled folate enables in vivo detection of atherosclerotic plaque inflammation by positron emission tomography. Sci. Rep. 2018, 8, 9720. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Verweij, N.J.F.; Yaqub, M.; Bruijnen, S.T.G.; Pieplenbosch, S.; Ter Wee, M.M.; Jansen, G.; Chen, Q.; Low, P.S.; Windhorst, A.D.; Lammertsma, A.A.; et al. First in man study of [18F]fluoro-PEG-folate PET: A novel macrophage imaging technique to visualize rheumatoid arthritis. Sci. Rep. 2020, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Betzel, T.; Müller, C.; Groehn, V.; Müller, A.; Reber, J.; Fischer, C.R.; Kramer, S.D.; Schibli, R.; Ametamey, S.M. Radiosynthesis and preclinical evaluation of 3′-aza-2′-[18F]fluorofolic acid: A novel PET radiotracer for folate receptor targeting. Bioconjugate Chem. 2013, 24, 205–214. [Google Scholar] [CrossRef]
- Gnesin, S.; Müller, J.; Burger, I.A.; Meisel, A.; Siano, M.; Fruh, M.; Choschzick, M.; Müller, C.; Schibli, R.; Ametamey, S.M.; et al. Radiation dosimetry of 18F-AzaFol: A first in-human use of a folate receptor PET tracer. EJNMMI Res. 2020, 10, 32. [Google Scholar] [CrossRef]
- Felsenstein, S.; Herbert, J.A.; McNamara, P.S.; Hedrich, C.M. COVID-19: Immunology and treatment options. Clin. Immunol. 2020, 215, 108448. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.; Schibli, R.; Maurer, B. Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk? Pharmaceuticals 2020, 13, 238. https://doi.org/10.3390/ph13090238
Müller C, Schibli R, Maurer B. Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk? Pharmaceuticals. 2020; 13(9):238. https://doi.org/10.3390/ph13090238
Chicago/Turabian StyleMüller, Cristina, Roger Schibli, and Britta Maurer. 2020. "Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk?" Pharmaceuticals 13, no. 9: 238. https://doi.org/10.3390/ph13090238
APA StyleMüller, C., Schibli, R., & Maurer, B. (2020). Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk? Pharmaceuticals, 13(9), 238. https://doi.org/10.3390/ph13090238